Abstract
Naegleria fowleri, a free-living amoeba, exists as a virulent pathogen which causes fatal primary amoebic meningoencephalitis in experimental animals and humans. Using infected and immune mouse sera, we previously cloned an nfa1 gene from a cDNA library of N. fowleri by immunoscreening. The nfa1 gene (360 bp) produced a recombinant 13.1-kDa protein, and the Nfa1 protein showed pseudopodium-specific immunolocalization on a trophozoite of N. fowleri. In this study, the role of the Nfa1 protein as a cell contact mechanism of N. fowleri cocultured with target cells was observed by an immunofluorescence assay with an anti-Nfa1 polyclonal antibody. Using confocal microscopic findings, the Nfa1 protein was located on the pseudopodia of N. fowleri trophozoites. The Nfa1 protein in N. fowleri trophozoites cocultured with CHO target cells was also located on pseudopodia, as well as in a food cup formed as a phagocytic structure in close contact with target cells. The amount of nfa1 mRNA of N. fowleri was strongly increased 6 h after coculture.
Naegleria fowleri, a free-living amoeba, is commonly found in soil and in warm bodies of fresh water, such as lakes, rivers, and hot springs. It is also found in unchlorinated swimming pools and in warm water discharge pools from industrial plants (1, 5, 10, 15). N. fowleri produces an acute lethal central nervous system disease called primary amoebic meningoencephalitis. N. fowleri trophozoites penetrate the nasal mucosa and enter the brain though the cribriform plate. The organisms are capable of multiplying in the tissues of the central nervous system and may be isolated from spinal fluid (9, 11, 14).
N. fowleri is cytopathogenic to a variety of cultured mammalian cells (12, 18, 20). The proposed mechanism(s) of the cytopathic action of this organism for mammalian cells includes active phagocytosis of cells by pseudopodium formation (11). With regard to host tissue invasion, the adherence of amoebae to host cells is the most important step in the pathogenicity mechanism of N. fowleri, when a specific pseudopodial projection, called an amoebastome, is formed (13, 14).
In a previous report, a gene (called nfa1) was cloned from a cDNA library of N. fowleri by immunoscreening using infected and immune mouse sera (17). The nfa1 gene had a coding nucleotide sequence of 360 bp, producing a recombinant protein (rNfa1) of 13.1 kDa (17). An anti-Nfa1 polyclonal antibody, obtained from mice immunized with an rNfa1 protein, was used in immunocytochemistry that showed the Nfa1 protein to be an indicator of pseudopodium-specific immunolocalization on a trophozoite of N. fowleri (3). An anti-Nfa1 antibody then has a decreasing effect on the cytotoxicity of N. fowleri trophozoites against CHO (Chinese hamster ovary) cells and the proliferation of N. fowleri trophozoites in a dose-dependent manner (8), much like the treatment of an anti-Nfa1 antibody in a coculture system (3).
In spite of the widespread use of free-living amoebae to understand protozoan pathogenicity, no information is yet fully available regarding the presence of pathogen-related proteins and their functions in these organisms. Moreover, the role of a newly cloned Nfa1 protein has not been fully carried out. In this study, by observing the localization of the Nfa1 protein in the coculture system with target cells by the immunofluorescence assay, we observed the role of the Nfa1 protein in a cell contact mechanism of N. fowleri. In addition, the transcription of nfa1 mRNA of N. fowleri during the coculture system was identified by Northern blotting with an nfa1-specific probe.
N. fowleri trophozoites (Cater NF69 strain, ATCC 30215) were axenically cultured in Nelson's medium at 37°C (20). CHO cells were cultured with Earle's minimum essential medium containing 10% fetal bovine serum (complete Earle's minimum essential medium) at 37°C in a 5% CO2 incubator, in accordance with the methods of a previous paper (8). In a cocultivating system, N. fowleri trophozoites (4 × 105) were cocultured with CHO cells (4 × 105).
To obtain an rNfa1 fusion protein, the expression of the nfa1 gene and the purification of the recombinant protein were performed according to the method of a previous paper (17). The purified DNA (5 μg/μl), obtained from the PCR-T7/NT TOPO expression vector (Invitrogen, Groningen, The Netherlands) and containing an nfa1 gene, was subsequently transformed into a BL21(DE3) pLysS Escherichia coli strain by the heat shock method. Cells were cultured at 37°C in LAC (Luria-Bertani medium containing 100 mg/ml of ampicillin and 34 mg/ml of chloramphenicol) plates for selection. A transformed colony was selected and cultured in the LAC broth at 37°C until the absorbance reached 0.5 to 0.8 at 600 nm. IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM) was then added to the medium. After 4 h of incubation, the cells were harvested by centrifugation (6,000 × g for 15 min). Cell extracts were compared with those of nontransformed BL21(DE3) pLysS by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the presence of the expressed gene product was confirmed by Western blotting.
For the production of an anti-Nfa1 polyclonal antibody, the rNfa1 protein (50 μg/200 μl/mouse) was mixed with an equal volume of complete Freund's adjuvant (Sigma) and injected intraperitoneally into 8-week-old female BALB/c mice (20 g; purchased from the Korea Institute of Science and Technology, Daejeon, Korea). The mice were intraperitoneally boosted twice a week for another 4 weeks with the rNfa1 protein (25 μg/mouse) containing an equal volume of incomplete Freund's adjuvant (Sigma). Six weeks later, an anti-Nfa1 polyclonal serum was separated from the blood of the mice by centrifugation at 2,500 × g for 30 min at 4°C. Enzyme-linked immunosorbent assay (ELISA) was performed with a purified Nfa1 protein (5 μg/ml) and with a rabbit anti-mouse whole immunoglobulin (1:10,000 dilution) conjugated with alkaline phosphate (Sigma). Western blotting was performed for the rNfa1 protein, in accordance with the method described in a previous paper (8).
For immunofluorescence studies, cultivating trophozoites of N. fowleri or CHO cells used as target cells were fixed in 10% formaldehyde for 30 min at room temperature, permeabilized in 1% ammonium hydroxide, washed in Tween 20 for 5 min, and extensively washed with 0.82% saline. N. fowleri was incubated overnight at 4°C with an anti-Nfa1 polyclonal antibody diluted serially with phosphate-buffered saline (PBS; pH 7.4). This was followed by incubation with the appropriate fluorescein isothiocyanate (FITC)-conjugated anti-mouse immunoglobulin G antibody (at a dilution of 1:100) (Sigma) for 2 h at 4°C. For healthy-mouse serum samples, N. fowleri trophozoites and CHO cells were stained with only the FITC-conjugated anti-mouse immunoglobulin G antibody. For labeling live target cells, CHO cells were incubated with 20 μM 5- (and 6)-chloromethyl SNARF-1 (red color) (Molecular Probes) for 1 h at 37°C. Dimethyl sulfoxide stock solutions (100 μl in SNARF stock) are typically diluted 1:1,000 into loading buffer (20 μl in PBS) to reduce the exposure of cells to dimethyl sulfoxide. The loading buffer should be serum free because serum often contains esterase activity. The total fluorescence of cells was visualized in an optical section produced by an Olympus FV-500 confocal microscope (Olympus, Japan). The collected images were processed using Adobe Photoshop 7.0 software.
In order to observe the change of nfa1 mRNA transcription in N. fowleri cocultured with target cells, the total RNA of N. fowleri trophozoites and CHO cells was extracted using the RNAzol B reagent (TEL-TEST; Firndswood, TX) and used for Northern blot analysis. Briefly, each cell (2 × 105) was collected in a sterile 15-ml conical tube, centrifuged at 300 × g for 5 min, and washed once with PBS. The pellet was then lysed with 500 μl of RNAzol B reagent, and RNA was extracted with 50 μl of chloroform. The tube was inverted 10 times and cooled on ice for 5 min. After centrifugation at 10,000 × g at 4°C for 20 min, the aqueous phase, which contained RNA, was transferred to a fresh tube and precipitated with 250 μl of isopropanol at 4°C for 2 h. After centrifugation at 10,000 × g at 4°C for 8 min, RNA pellets were washed in 80% ethanol, centrifuged, dried with a speed-vacutainer, and finally dissolved in 50 μl of diethyl pyrocarbonate-treated distilled water. In order to perform the Northern blot technique, total RNA (30 μg) was denatured, electrophoresed on a 1% agarose gel containing formaldehyde, and blotted onto a nylon membrane. RNA on the membrane was then hybridized with a 32P-labeled, randomly primed probe specific to the nfa1 gene.
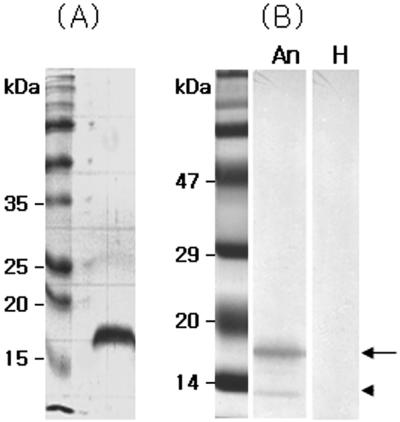
For the expression of an nfa1 gene using an E. coli expression system, nfa1 was inserted into the PCR-T7/NT TOPO vector. It was introduced into an E. coli strain, BL21, and expressed by IPTG induction. The 17-kDa (approximate) fusion protein was purified from E. coli extracts and confirmed by SDS-PAGE (Fig. 1A).
FIG. 1.
SDS-PAGE band pattern of a recombinant Nfa1 fusion protein expressed from the nfa1 gene of Naegleria fowleri (A) and its Western blotting band patterns with an anti-Nfa1 antibody (B). An, anti-Nfa1 antibody; H, normal mouse serum; arrow, uncut 17-kDa protein; arrowhead, cut 13.1-kDa protein.
To obtain a purified recombinant Nfa1 protein, a 17-kDa fusion protein was purified using a nickel resin column and enzyme digestion. Finally, the purified protein eluted from the gels had a molecular mass of 13.1 kDa, which corresponded well to the size observed in previous research (Fig. 1). Anti-Nfa1 polyclonal antibodies were collected from mice immunized with rNfa1 proteins. The specific reactivities of these against the Nfa1 protein were observed by ELISA and Western blotting (Fig. 1B). Values of anti-Nfa1 polyclonal antibodies obtained by ELISA ranged from 0.212 ± 0.021 (1:10,000 dilution, mean ± standard deviation [n = 5]) to 1.213 ± 0.024 (1:200 dilution), which showed a difference from the 0.112 ± 0.005 value (1:200 dilution) of the normal sera and also the 0.109 ± 0.005 value of the PBS control.
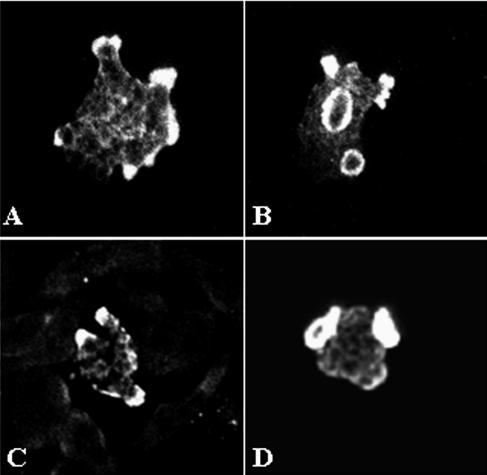
The presence of Nfa1 protein in amoebas fixed during locomotion was initially examined by staining them with anti-Nfa1 polyclonal antibodies and FITC conjugates and by recording their total fluorescence with a confocal microscope. By confocal microscopy, the Nfa1 protein of N. fowleri trophozoites in cultivation with Nelson's medium was found to be concentrated in pseudopodia (Fig. 2A) and protrusive food cups (Fig. 2B). Subsequently, in N. fowleri cocultured with CHO cells, the Nfa1 protein was shown in food cups, as well as in the pseudopods of amoebae (Fig. 2C and D). In contrast, no fluorescent signals were seen in CHO cells (Fig. 2C and D).
FIG. 2.
Confocal microscopic findings of Naegleria fowleri trophozoites immunostained with an anti-Nfa1 polyclonal antibody and an FITC conjugate. Strong fluorescent signals were shown in pseudopodia, especially in the food cups of N. fowleri trophozoites in media (A, B) and cocultured with CHO cells (C, D) (magnification, ×400).
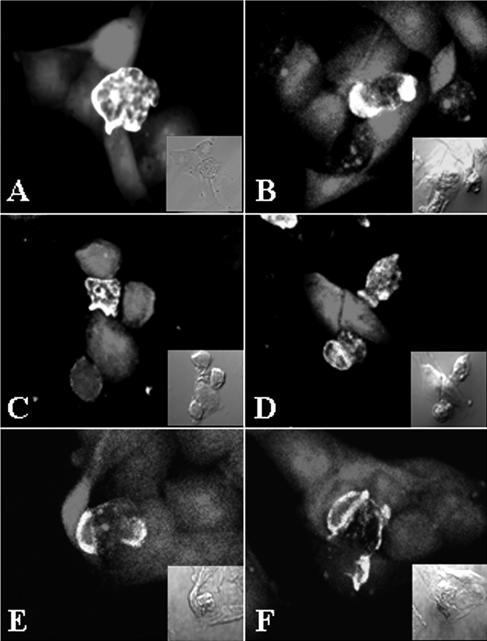
To observe the role of the Nfa1 protein of N. fowleri trophozoites cocultured with target cells, competitive counterstaining for amoebae and CHO cells was performed with an FITC conjugate and with 5 (and 6)-chloromethyl SNARF-1, respectively. After 1 h of cultivation, the Nfa1 protein of N. fowleri trophozoites was concentrated at the point of contact with CHO cells. Strong fluorescent signals were shown, especially in pseudopodia of N. fowleri trophozoites (Fig. 3A and B). The food cups of N. fowleri trophozoites have rarely been found. Instead, the Nfa1 protein was often observed on the area in contact with the target cells. After 3 h of cultivation, N. fowleri trophozoites showed continuing attachment on the surfaces of CHO cells. Strong fluorescent signals were shown in pseudopodia (Fig. 3C) and food cups (Fig. 3D) of N. fowleri trophozoites. At 6 h of cultivation, strong fluorescent signals were shown in many N. fowleri trophozoite food cups (Fig. 3E, F). Several red spots that seemed to be fragmented CHO cell debris are shown in the cytoplasm of N. fowleri trophozoites (Fig. 3F).
FIG. 3.
Immunolocalization of the Nfa1 protein in Naegleria fowleri trophozoites cocultured with CHO cells stained with 5 (and 6)-chloromethyl SNARF-1. Using a confocal microscope, strong fluorescent signals were observed in pseudopodia of N. fowleri trophozoites at 1 h (A, B) and in food cups at 3 h (C, D). At 6 h postincubation (E, F), strong fluorescent signals occurred in the food cups of the N. fowleri trophozoite, which included ingested dead CHO cells. The light microscopic findings are in each inset (magnification, ×400).
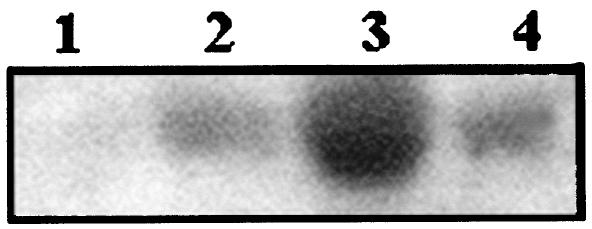
To observe the transcription of the nfa1 mRNA, total RNA was extracted from N. fowleri trophozoites and CHO cells in a coculture system (Fig. 4A). According to Northern blotting using an nfa1 gene-specific probe, the nfa1 mRNA was transcribed better in N. fowleri in the coculture system than was N. fowleri alone. Moreover, the nfa1 mRNA was not transcribed in CHO cells. In particular, the amount of nfa1 mRNA of N. fowleri was increased strongly by 6 h after coculture (Fig. 4B).
FIG. 4.
Northern blot finding of RNA extracted from Naegleria fowleri trophozoites and/or CHO cells. The nfa1 mRNA was strongly transcribed 6 h after coculture. Lane 1, CHO cells only; lane 2, N. fowleri cocultured with CHO cells for 3 h; lane 3, N. fowleri cocultured with CHO cells for 6 h; lane 4, N. fowleri only.
In the previous study, the immunolocalization study of Nfa1 protein revealed that this protein was abundant in pseudopodia and around the food vacuoles of N. fowleri trophozoites (3). This suggests that the Nfa1 protein is required for amoeba movement and food ingestion. It provides no information about N. fowleri cocultured with target cells that are specialized to different functions that comprise the mechanisms of N. fowleri pathogenic activities: cell surface contraction, frontal pseudopodium polymerization and protrusive activity, and cell anchoring to the substratum.
In this experiment, it was shown that Nfa1 protein was localized in the food cups, which play a role in the cell contact mechanism of N. fowleri. By confocal microscopy, the Nfa1 protein of N. fowleri trophozoites cocultured with CHO cells was located on pseudopodia in contact with target cells and in a food cup formed as a phagocytic structure. In addition, the amount of nfa1 mRNA of N. fowleri cocultured with CHO cells was increased 6 h after coculture as determined by Northern blotting with an nfa1-specific probe. In other words, it seemed that the Nfa1 protein played a role in the formation of food cups as a phagocytic activity.
The proposed mechanisms of cytopathogenicity for N. fowleri include phagocytosis, release of cytolytic substances, and the presence of a biologically active component (4). Phagocytosis is a basic function of this amoeba and one that causes the destruction of cells, whether in cell culture or in human tissue. It is, therefore, intimately involved in pathogenesis (12). The process has been named trogocytosis, the piecemeal engulfment of mouse embryo cells by N. fowleri (12). It is now known that trogocytosis is accomplished by amoebastomes (2), which amoebae use to engulf particles of various sizes, including cultivated mammalian cells. In addition to phagocytosis and trogocytosis, N. fowleri seems to injure cultivated mammalian cells by another contact-mediated means (19). In contrast, Pettit et al. (16) demonstrated the ingestion of whole cells or cell debris by phagocytosis but concluded that trogocytosis was not involved. In addition, sucker-like structures, termed amoebastomes and thought to be involved in the engulfment of particles, have also been demonstrated (6, 18).
In previous reports, contact-dependent killing mechanisms have been determined to be operative as well and are likely to involve the ingestion of nerve cell membranes through a food cup structure on the surface of an amoeba (4, 13). Furthermore, N. fowleri cells are highly phagocytic, and it is likely that they endocytose and also destroy nerve cells though a contact-dependent killing mechanism (7, 19).
In this study, the Nfa1 protein was located mainly at the tips of the pseudopodia of amoebae and especially at the food cups in N. fowleri trophozoites cocultured with target cells. At 6 h of cultivation, several red spots which appeared to be fragmented CHO cell debris were observed in the cytoplasm of N. fowleri trophozoites. It should be reiterated that the Nfa1 protein of free-living amoebae is necessary for locomotion and food cup formation. Consequently, trophozoites of N. fowleri destroyed several CHO cells in a time-dependent manner, while activating the Nfa1 protein expression involved in food cup formation. In another study, an anti-Nfa1 antibody caused a decreasing effect on the cytotoxicity of N. fowleri trophozoites against CHO cells (8). Finally, in this study we have elucidated that the Nfa1 protein was involved in the formation of food cups, which play an important role in phagocytic activity as a cell contact mechanism in pathogenic N. fowleri.
Acknowledgments
This study was supported by a grant (no. R05-2003-000-10314-0) from the Basic Research Program of the Korea Science & Engineering Foundation.
REFERENCES
- 1.Barnett, N. D., A. M. Kaplan, R. J. Hopkin, M. A. Saubolle, and M. F. Rudinsky. 1996. Primary amoebic meningoencephalitis with Naegleria fowleri. Pediatr. Neurol. 15:230-234. [DOI] [PubMed] [Google Scholar]
- 2.Brown, T. 1979. Observations by immunofluorescence microscopy and electron microscopy on the cytopathogenicity of Naegleria fowleri in mouse embryo-cell cultures. J. Med. Microbiol. 12:363-371. [DOI] [PubMed] [Google Scholar]
- 3.Cho, M. S., S. Y. Jung, S. Park, K. H. Kim, H. I. Kim, S. Sohn, H. J. Kim, K. I. Im, and H. J. Shin. 2003. Immunological characterizations of a cloned 13.1-kilodalton protein from pathogenic Naegleria fowleri. Clin. Diagn. Lab. Immunol. 10:954-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cline, M., R. Carchman, and F. Marciano-Cabral. 1986. Movement of Naegleria fowleri stimulated by mammalian cells in vitro. J. Protozool. 33:10-13. [DOI] [PubMed] [Google Scholar]
- 5.Culbertson, C. G. 1971. The pathogenicity of soil amoebas. Annu. Rev. Microbiol. 25:231-254. [DOI] [PubMed] [Google Scholar]
- 6.Diaz, J., A. Osuna, M. J. Rosales, J. Cifuentes, and C. Mascaro. 1991. Sucker-like structures in two strains of Acanthamoeba: scanning electron microscopy study. Int. J. Parasitol. 21:365-367. [DOI] [PubMed] [Google Scholar]
- 7.Im, K. I., and H. J. Shin. 2003. Acanthamoeba sohi, n. sp., a pathogenic Korean isolate YM-4 from a freshwater fish. Korean J. Parasitol. 41:181-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong, S. Y., S. Y. Kang, S. C. Lee, K. J. Song, K. I. Im, and H. J. Shin. 2004. Decreasing effect of an anti-Nfa1 polyclonal antibody on the in vitro cytotoxicity of pathogenic Naegleria fowleri. Korean J. Parasitol. 42:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.John, D. T. 1982. Primary amebic meningoencephalitis and the biology of Naegleria fowleri. Annu. Rev. Microbiol. 36:101-123. [DOI] [PubMed] [Google Scholar]
- 10.Kollars, T. M., and W. E. Wilhelm. 1996. The occurrence of antibodies to Naegleria species in wild mammals. J. Parasitol. 82:73-77. [PubMed] [Google Scholar]
- 11.Ma, P., G. S. Visvesvara, A. J. Martinez, F. H. Theodore, P. M. Daggett, and T. K. Sawyer. 1990. Naegleria and Acanthamoeba infections. Rev. Infect. Dis. 12:490-513. [DOI] [PubMed] [Google Scholar]
- 12.Marciano-Cabral, F., M. Patterson, D. T. John, and S. G. Bradley. 1982. Cytopathogenicity of Naegleria fowleri and Naegleria gruberi for established mammalian cell cultures. J. Parasitol. 68:1110-1116. [PubMed] [Google Scholar]
- 13.Marciano-Cabral, F., and D. T. John. 1983. Cytopathogenicity of Naegleria fowleri for rat neuroblastoma cell cultures: scanning electron microscopy study. Infect. Immun. 40:1214-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marciano-Cabral, F. 1988. Biology of Naegleria spp. Microbiol. Rev. 52:114-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page, F. C. 1967. Re-definition of the genus Acanthamoeba with descriptions of thee species. J. Protozool. 14:709-724. [DOI] [PubMed] [Google Scholar]
- 16.Pettit, D. A. D., J. Williamson, G. A. Cabral, and F. Marciano-Cabral. 1996. In vitro destruction of nerve cell cultures by Acanthamoeba spp.: a transmission and scanning electron microscopy study. J. Parasitol. 82:769-777. [PubMed] [Google Scholar]
- 17.Shin, H. J., M. S. Cho, S. Y. Jung, H. I. Kim, S. Park, H. J. Kim, and K. I. Im. 2001. Molecular cloning and characterization of a gene encoding a 13.1 kDa antigenic protein of Naegleria fowleri. J. Eukaryot. Microbiol. 48:713-717. [DOI] [PubMed] [Google Scholar]
- 18.Shin, H. J., and K. I. Im. 2004. Pathogenic free-living amoeba in Korea. Korean J. Parasitol. 42:93-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Visvesvara, G. S., and A. J. Martinez. 1999. Protozoa: free-living amebae, p. 33.1-33.6. In D. Armstrong, and S. Cohen (ed.), Infectious diseases. Mosby, London, United Kingdom.
- 20.Willaert, E. 1971. Isolement et culture in vitro des amibes de genre Naegleria. Ann. Soc. Belg. Med. Trop. 51:701-708. [PubMed] [Google Scholar]