Abstract
An immunochromatographic test (ICT) with recombinant surface antigen 1 of Neospora caninum (NcSAG1) was developed for the rapid detection of antibodies to N. caninum in cattle. The ICT was used to clearly discriminate between immunofluorescent-antibody test (IFAT)-positive bovine sera and IFAT-negative bovine sera. Serum samples collected from cattle in Yanbian, China, were examined by the ICT. Of the 96 serum samples, 23 (24.0%) were positive by the ICT, and 19 (19.8%) samples were positive by a previously developed enzyme-linked immunosorbent assay (ELISA). Eighteen of 19 ELISA-positive samples were positive according to the ICT. A good agreement was found between the results of the ICT and the ELISA. The results presented here suggest that the ICT with recombinant truncated NcSAG1 fused to glutathione S-transferase is a useful and reliable method for the detection of antibodies to N. caninum in cattle.
Neospora caninum is an apicomplexan protozoan parasite that causes fetal abortion and neonatal mortality in cattle and neuromuscular paralysis in dogs. It occasionally causes clinical infections in horses, goats, sheep, and deer (5). Dogs are the only known definitive host for N. caninum (11). Since N. caninum was first recorded in 1984 (1), neosporosis has emerged as an economically important disease with considerable impact on the livestock industry worldwide.
As a major cause of abortion in cattle, N. caninum has provoked a great deal of attention with respect to its prevalence and diagnosis. N. caninum infection in cattle has been reported in many countries (3). Quantitative studies in the United States, New Zealand, The Netherlands, and Germany indicated that 12 to 42% of aborted fetuses from dairy cattle were infected with N. caninum (4). In cattle, transplacental transmission is the major route of transmission of N. caninum. Seropositive heifers remained clinically normal but gave birth to congenitally infected calves (4). Therefore, it is important to identify the potential cause of abortion by using a rapid and reliable serodiagnostic method.
Many serological diagnostic methods have been developed to diagnose N. caninum infection. The enzyme-linked immunosorbent assay (ELISA) with purified recombinant antigen was thought to be an effective way to diagnose N. caninum infection (8). Compared with the native antigens, recombinant antigens have an additional benefit: they are easily produced in large quantities and can be readily standardized for diagnostic assays. The diagnostic potential of surface antigen 1 of N. caninum (NcSAG1) expressed in Escherichia coli was previously evaluated, and the result indicated that the recombinant NcSAG1 could be a reliable reagent for use as an antigen in an ELISA for the serodiagnosis of N. caninum infection in cattle (2). However, in general, the ELISA is time-consuming and laborious and requires special materials and equipment, which make it unsuitable for clinical or field applications. In contrast to the ELISA, the immunochromatographic test (ICT) is a simple, rapid method, which makes it suitable for clinical or field applications. Here, we report the development and evaluation of the ICT with recombinant NcSAG1 for detection of specific antibodies to N. caninum in cattle.
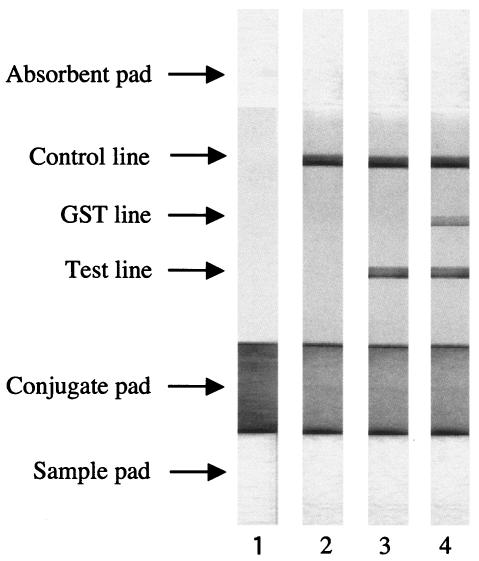
The expression and purification of recombinant truncated NcSAG1, without signal peptide and with C-terminal hydrophobic regions fused to glutathione S-transferase (GST-NcSAG1t), were performed as described previously (2). The production and preparation of mouse immunoglobulin G (IgG) against GST-NcSAG1t, as well as the preparation of ICT strips, were carried out according to a method described previously (7), with some modifications. Briefly, purified GST-NcSAG1t (200 μg/ml), GST (200 μg/ml), and anti-GST-NcSAG1t IgG (1.5 mg/ml) were jetted linearly on a nitrocellulose membrane, as test, GST, and control lines, respectively. For the preparation of the conjugated antigen, 1 ml of purified GST-NcSAG1t (100 μg/ml) was combined with 10 ml of gold colloid (British BioCell International, United Kingdom), and the conjugated antigen was then sprayed on the glass fiber. The nitrocellulose membrane with GST-NcSAG1t, GST, and IgG, as well as the conjugated pad, sample pad, and absorbent pad, was assembled on an adhesive card and cut into 3-mm-wide strips (Fig. 1, lane 1).
FIG. 1.
ICT strip assay results. The test, GST, and control lines were immobilized with GST-NcSAG1t, GST, and mouse anti-GST-NcSAG1t IgG, respectively. GST-NcSAG1t, conjugated with gold colloids, was dried on the conjugate pad. Fifty microliters of serum (1:10 diluted in phosphate-buffered saline) was spotted on the sample pad of a strip. Lane 1, pretest strip; lane 2, negative reaction exhibits no color on the test line; lane 3, positive reaction is indicated by purplish red on the test line; lane 4, nonspecific GST reaction shows purplish red on both test and GST lines. The control line shows color in all the assays.
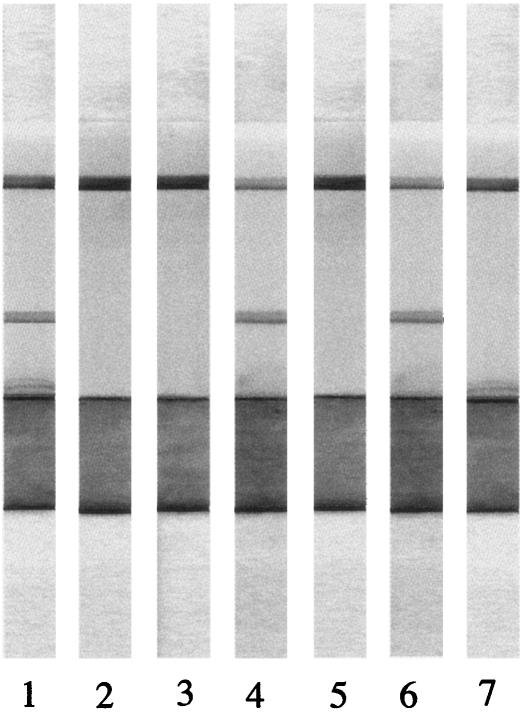
To evaluate the potential use of the ICT with GST-NcSAG1t for the detection of specific antibodies to N. caninum, serum samples collected from four mice and four dogs pre- and postexperimental infection with N. caninum were tested. All serum samples from postinfected mice (2 months postinfection) and dogs (2 months postinfection) were positive (Fig. 2), whereas all serum samples from preinfected mice and dogs were negative (Fig. 2). On the other hand, serum samples from four mice experimentally infected (2 months postinfection) with a closely related parasite, Toxoplasma gondii Beverley strain, were negative according to the ICT (Fig. 2). These results suggest that the ICT with GST-NcSAG1t not only could detect the specific antibodies to N. caninum but also could discriminate between neosporosis and toxoplasmosis, which has been thought to be important because some animals, such as dogs, cattle, sheep, and horses, can be naturally infected with both N. caninum and T. gondii (5, 10, 11, 12, 13).
FIG. 2.
ICT results with various serum samples. ICT strips reacted with N. caninum-infected mouse serum (lane 1), uninfected mouse serum (lane 2), T. gondii-infected mouse serum (lane 3), N. caninum-infected dog serum (lane 4), uninfected dog serum (lane 5), IFAT-positive (≥1:200) serum from cattle (lane 6), and IFAT-negative (<1:200) serum from cattle (lane 7).
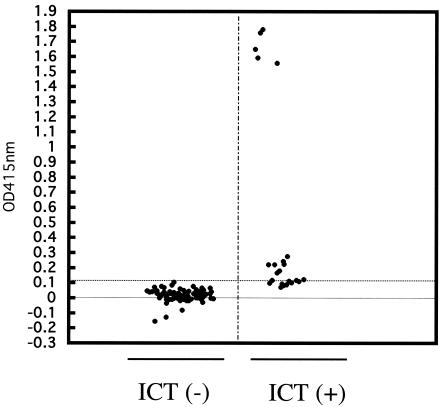
The ICT with GST-NcSAG1t was evaluated by using 20 known seropositive bovine serum samples and 20 seronegative bovine serum samples previously diagnosed by immunofluorescent-antibody test (IFAT) (14). All IFAT-positive bovine sera were positive and all IFAT-negative bovine sera were negative by the ICT. A total of 96 field serum samples collected from cattle in Yanbian, China, were then investigated by use of the ICT, and the results were compared with those of the previously developed ELISA with GST-NcSAG1t (2). As shown in Fig. 3 and Table 1, of 96 serum samples, 23 (24.0%) were positive according to the ICT, and 19 (19.8%) were positive according to the ELISA. The relative sensitivity and specificity of the ICT were 94.7% and 93.5%, respectively, when the corresponding ELISA was used as a reference. All ELISA-positive samples except one (18/19) were ICT positive. In addition, five ELISA-negative samples were ICT positive. The optical densities at 415 nm of five ELISA-negative samples that were positive by the ICT were near the cutoff point of 0.1 (ranging from 0.07 to 0.1). Western blot analysis with whole-tachyzoite lysate as an antigen was used to further evaluate the samples whose results by the ICT and ELISA were in disagreement. Four of the five samples which were negative by the ELISA but positive by the ICT showed positive reactions in Western blot analysis, and the one ELISA-positive sample that was negative by the ICT was also negative in the Western blot analysis (data not shown). The degree of agreement between the ICT and ELISA was estimated by calculating the kappa value (16). The kappa value (0.99) indicates a very good agreement between the two tests. All of these results suggested that the ICT with recombinant NcSAG1t would be reliable. The larger number of positive samples detected by the ICT could be attributed to its ability to detect all classes of immunoglobulins.
FIG. 3.
Comparison of ICT with ELISA for the detection of antibodies to N. caninum in cattle. The dotted lines represent the cutoff value for the ELISA. The ELISA was considered positive at an optical density at 415 nm of ≥0.1. Serum samples, n = 96. ICT(−), ICT-negative results; ICT(+), ICT-positive results.
TABLE 1.
Comparison of ICT with ELISA for detection of antibodies to N. caninum in cattle
| ELISAa results | ICTb test results
|
||
|---|---|---|---|
| No. (%) positive | No. (%) negative | Total (%) | |
| No. (%) positive | 18 (18.8) | 1 (1.0) | 19 (19.8) |
| No. (%) negative | 5 (5.2) | 72 (75.0) | 77 (80.2) |
| Total (%) | 23 (24.0) | 73 (76.0) | 96 (100) |
Antibodies to N. caninum were detected by ELISA with GST-NcSAG1t (2). The ELISA was considered positive when an optical density at 415 nm of ≥0.1 was observed at dilutions of 1:100 and above.
Antibodies to N. caninum were detected by ICT with GST-NcSAG1t. The ICT was considered positive when both control and test lines turned purplish red at a dilution of 1:10.
Recent studies have shown the prevalence of N. caninum infection in cattle and dogs in Asia. In Taiwan, in a dairy farm where 18 cases of abortion had been observed, up to 76.3% of cattle contained IgG and/or IgM antibodies to N. caninum (6). Kim et al. confirmed that 12.1% of cattle abortions in Korea were attributed to N. caninum (9). In Japan, dogs reared on dairy farms where cases of abortion had been observed or where the cattle were seropositive for N. caninum infection had a higher infection rate (31.3%) than dogs in urban areas (7.1%) (15). In this study, 24% of serum samples collected from cattle in Yianbian, China, were seropositive for antibodies to N. caninum, suggesting that bovine neosporosis is endemic in the area, although there was still no certain evidence for confirming that the seropositive-cattle developed active diseases. Therefore, more formally designed epidemiological studies with large sample numbers of cattle and dogs in China are needed.
In summary, the ICT with GST-NcSAG1t is simple, rapid, sensitive, and specific for the detection of antibodies to N. caninum in cattle and, probably, other animals.
Acknowledgments
This study was supported by a grant from The 21st Century COE Program (A-1), Ministry of Education, Culture, Sports, Science, and Technology, Japan, and by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Bjerkas, I., S. F. Mohn, and J. Presthus. 1984. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z. Parasitenkd. 70:271-274. [DOI] [PubMed] [Google Scholar]
- 2.Chahan, B., I. Gaturaga, X. Huang, M. Liao, S. Fukumoto, H. Hirata, Y. Nishikawa, H. Suzuki, C. Sugimoto, H. Nagasawa, K. Fujisaki, I. Igarashi, T. Mikami, and X. Xuan. 2003. Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet. Parasitol. 118:177-185. [DOI] [PubMed] [Google Scholar]
- 3.Dubey, J. P. 1999. Neosporosis in cattle: biology and economic impact. J. Am. Vet. Med. Assoc. 214:1160-1163. [PubMed] [Google Scholar]
- 4.Dubey, J. P. 2003. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 41:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubey, J. P., and D. S. Lindsay. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 67:1-59. [DOI] [PubMed] [Google Scholar]
- 6.Huang, C. C., L. J. Ting, J. R. Shiau, M. C. Chen, and H. K. Ooi. 2004. An abortion storm in cattle associated with neosporosis in Taiwan. J. Vet. Med. Sci. 66:465-467. [DOI] [PubMed] [Google Scholar]
- 7.Huang, X., X. Xuan, H. Hirata, N. Yokoyama, L. Xu, N. Suzuki, and I. Igarashi. 2004. Rapid immunochromatographic test using recombinant SAG2 for detection of antibodies against Toxoplasma gondii in cats. J. Clin. Microbiol. 42:351-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins, M. C., W. Wouda, and J. P. Dubey. 1997. Serological response over time to recombinant Neospora caninum antigens in cattle after a neosporosis-induced abortion. Clin. Diagn. Lab. Immunol. 4:270-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, J. H., J. K. Lee, B. C. Lee, B. K. Park, H. S. Yoo, W. S. Hwang, N. R. Shin, M. S. Kang, Y. H. Jean, H. J. Yoon, S. K. Kang, and D. Y. Kim. 2002. Diagnostic survey of bovine abortion in Korea: with special emphasis on Neospora caninum. J. Vet. Med. Sci. 64:1123-1127. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay, D. S., S. D. Lenz, C. C. Dykstra, B. L. Blagburn, and J. P. Dubey. 1998. Vaccination of mice with Neospora caninum: response to oral challenge with Toxoplasma gondii oocysts. J. Parasitol. 84:311-315. [PubMed] [Google Scholar]
- 11.McAllister, M. M., J. P. Dubey, D. S. Lindsay, W. R. Jolley, R. A. Wills, and A. M. McGuire. 1998. Dogs are definitive hosts of Neospora caninum. Int. J. Parasitol. 28:1473-1478. [PubMed] [Google Scholar]
- 12.McAllister, M. M., S. F. Parmley, L. M. Weiss, V. J. Welch, and A. M. McGuire. 1996. An immunohistochemical method for detecting bradyzoite antigen (BAG5) in Toxoplasma gondii-infected tissues cross-reacts with a Neospora caninum bradyzoite antigen. J. Parasitol. 82:354-355. [PubMed] [Google Scholar]
- 13.Nishikawa, Y., F. G. Claveria, K. Fujisaki, and H. Nagasawa. 2002. Studies on serological cross-reaction of Neospora caninum with Toxoplasma gondii and Hammondia heydorni. J. Vet. Med. Sci. 64:161-164. [DOI] [PubMed] [Google Scholar]
- 14.Nishikawa, Y., Y. Kousaka, K. Tragoolpua, X. Xuan, L. Makala, K. Fujisaki, T. Mikami, and H. Nagasawa. 2001. Characterization of Neospora caninum surface protein NcSRS2 based on baculovirus system system and its application for serodiagnosis of Neospora infection. J. Clin. Microbiol. 39:3987-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawada, M., C. Park, H. Kondo, T. Morita, A. Shimada, I. Yamane, and T. Umemura. 1998. Serological survey of antibody to Neospora caninum in Japanese dogs. J. Vet. Med. Sci. 60:853-854. [DOI] [PubMed] [Google Scholar]
- 16.Thomas, E. E., M. L. Puterman, E. Kawano, and M. Curran. 1988. Evaluation of seven immunoassays for detection of rotavirus in pediatric stool samples. J. Clin. Microbiol. 26:1189-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]