Abstract
The study investigated the effectiveness of pre- and co-treatment with fermented yellow soybean extract (FYSE) against anti-influenza A virus (IAV) on MDCK cells. FYSE, fermented with Bacillus subtilis, was evaluated for its anti-IAV activity by inhibiting the IAV PA gene expression. Daidzein was identified as a significant contributor to FYSE's antiviral effects. Co-treatment with FYSE and daidzein during IAV infection demonstrated superior anti-IAV activity compared to their respective pre-treatment (IC50: FYSE; 8.65 vs 3.77 µg/mL, and daidzein; 6.01 vs 5.20 µg/mL). Both pre- and co-treatment with FYSE demonstrated higher therapeutic potential than daidzein (Selective index: pre-treatment; > 115.58 vs. 72.32 and co-treatment; > 265.04 vs. 83.56). Despite daidzein showing lower anti-IAV activity in both treatment methods compared to oseltamivir phosphate, it exhibited lower cytotoxicity (CC50: 434.50 vs. 395.20 µg/mL). In conclusion, co-treatment with FYSE and daidzein presents a promising anti-IAV strategy with minimal cytotoxicity in vitro, potentially offering a safer alternative for IAV treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-024-01673-2.
Keywords: Antiviral, Influenza, Daidzein, Yellow soybean
Introduction
Despite the widespread focus on the SARS-CoV-2 pandemic, influenza remains a formidable global health challenge, demanding sustained attention and approaches. Both seasonal and pandemic outbreaks of influenza are primarily driven by the influenza A virus (IAV), which can infect various avian and mammalian spp. The viral envelop of IAV contains key glycoproteins, neuraminidase (NA) and hemagglutinin (HA), both of which feature glycosylation sites critical for viral entry, release and infection (Nayak et al., 2004). Variations in the amino acid sequence of HA, a major IAV antigen, can significantly impact the effectiveness of existing vaccines (Chen et al., 2011; Ray et al., 2017). Several classes of antiviral drugs are available, with mainly three groups of antiviral drugs—viral RNA polymerase inhibitors, matrix-2 (M2) ion channel inhibitors, and NA inhibitors— approved for the treatment of influenza virus infection (Wu et al., 2017). RNA polymerase inhibitors like favipiravir have demonstrated inconsistent therapeutic efficacy (Hayden and Shindo, 2019). The effectiveness of M2 ion channel and NA inhibitors can be compromised by the emergence of resistant viral mutants (Furuse and Suzuki, 2009; Lee and Hurt, 2018). Consequently, there remains an urgent need to explore alternative therapeutics for influenza.
In recent years, phytochemical studies have been gaining interest due to the potential of bioactive compounds and secondary metabolites derived from plants. This interest has led to the identification of compounds with various biological properties, including anti-influenza activity, in extracts from soybean, licorice root, peanut skin, pomegranate, red ginseng, cocoa, ginkgo and wild watermelon juice (Mousa, 2017; Ricci and Roviello, 2023).
Among these plant extracts, soybean extract attracts attention due to its rich content of bioactive compounds, such as phenolic acids, flavonoids, isoflavones, saponins, phytosterols, and sphingolipids, all of which contribute to its health-promoting properties. Isoflavones like genistein and daidzein are well-known for their wide range of properties, including antioxidant, anti-apoptotic, neuroprotective, estrogen-like, and cholesterol-lowering capabilities (Khosravi and Razavi, 2021; Tham et al., 1998). Furthermore, isoflavones show antimicrobial, antifungal, and antiparasitic properties (Alghamdi et al., 2018), with documented antiviral effects against specific viruses such as hepatitis C virus, feline calicivirus, and murine norovirus (He et al., 2021; Seo et al., 2016). Daidzein and soyasaponin, found in soybean extract, have demonstrated viricidal effects against IAV (Hayashi et al., 1997; Horio et al., 2023; Morimoto et al., 2023). Although ethanolic and fermented soybean extracts have been investigated for their potential anti-IAV properties (Kwon et al., 2022; Sakudo and Sesoko, 2013), the efficacy of soybean extract as an antiviral agent may vary depending on factors such as treatment method, virus types, extract concentration, and methods.
Fermentation enhances the bioavailability and potential health benefits of isoflavones in soybeans by converting isoflavone glucosides to their aglycone forms, which have higher bioavailability (Piao & Eun, 2020). This conversion is mediated by β-glucosidase, which releases sugar moieties from isoflavone glycosides, producing aglycones (Rekha and Vijayalakshmi, 2010). Bacillus subtilis strains are commonly employed and dominant in soy fermentation due to their ability to produce the conversion-facilitating enzyme β-glucosidase, as well as their safety and rapid growth (Gopikrishna et al., 2021). Therefore, the objective of this study was to evaluate the anti-influenza potential of pre- and co-treatment using an extract obtained from fermented yellow soybeans (FYSE).
Materials and methods
Preparation of inoculum
Bacillus subtilis strains were isolated from fermented soybean paste and confirmed by gene sequencing, comparing their sequence with reference sequences in the GenBank database. The strain culture was prepared on a solid LB agar plate at 37 °C for 24 h. Colonies were collected and suspended in minimal media containing K2HPO4, glucose and NaCl. The inoculation amount used was 1% of the bean weight.
Preparation of fermented yellow bean extract
Fresh yellow soybeans (Glycine max (L.) Merr.) were subjected to hot air drying at 60 °C for 72 h. The dried beans were finely crushed into a powder using a grinder. The resulting powdered beans were fermented at 37 °C for 3 days with agitation at 150 rpm in an aerobic condition using Bacillus subtilis strain. The fermented product was heated at 100 °C for 15 min to arrest fermentation. The fermented product was centrifuged at 12,000 rpm for 15 min, and the supernatant obtained was then filtered through a 0.45 µm polysulfone membrane (Millipore, Bedford, USA).
MDCK cell cultivation and maintenance
The Madin-Darby canine kidney (MDCK) cell line obtained from Korean Cell Line Bank (Seoul, Korea; KCBL10034) was cultivated in growth medium comprising Dulbecco's Modified Eagle's Medium (DMEM; Welgene, Gyeongsan, South Korea) supplemented with penicillin (5 U/mL) and streptomycin (5 μg/mL), 10% fetal bovine serum (FBS, Gibco, Grand Island, USA), and 3 mM of EDTA at 37 °C and 5% CO2 in a CO2 incubator (Thermo Forma, Waltham, USA).
Frozen MDCK cells were thawed, transferred to growth medium, centrifuged at 1000 rpm for 2 min to remove the medium, and then resuspended in fresh culture medium. These cells were cultured in a 25 cm2 (T-25) culture flask at 37 °C with 5% CO2. The medium was changed every 2–3 days by washing the cells with PBS and adding fresh medium. When reaching 80% confluency, the cells were subcultured by detaching with trypsin–EDTA, centrifuging at 1200 rpm for 2 min and resuspending in fresh medium. The cells were then plated at the desired density in new culture flasks.
Virus strain and cell culture condition
In this study, a low-pathogenic human IAV H1N1 strain A/PR/8/34 (ATCC VR-1469, Manassas, USA) was utilized. The virus was cultured in a viral culture medium composed of DMEM supplemented with streptomycin (100 μg/mL), penicillin (100U/mL), and 2 μg/mL of NP-p-tosyl-L-phenylalanine chloromethyl ketone (TPCK, Sigma-Aldrich). Virus propagation was conducted in MDCK cells at 35 °C under 5% CO2 conditions.
Cytotoxicity by CCK assay
MDCK cells were seeded at a density of 2.0 × 105 cells/well in a 96-well plate. After 24 h of incubation with growth medium, the medium was removed, and the cells were washed once with 1 × PBS (Welgene). Various concentrations of FYSE (12.5 to 1000 μg/mL), non-fermented aqueous yellow soybean extract (AYSE) (12.5 to 1000 μg/mL), daidzein (2.5 to 400 μg/mL), or oseltamivir phosphate (2.5 to 400 μg/mL) were diluted in DMEM supplemented with streptomycin (100 μg/mL) and penicillin (100 U/mL). Each well was treated with 100 μL of the respective concentration and cultured at 37 °C for 48 h. Subsequently, 10 μL of CCK solution was added to each well, followed by incubation at 37 °C for an additional 2 h. Absorbance was measured at 450 nm using a microplate reader to assess cell viability.
Anti-viral activity in MDCK cells
MDCK cells were seeded into 96-well plates at a density of 2.0 × 105 cells/mL, with 100 μL of cell suspension added to each well. The cells were cultured at 37 °C for 24 h. After washing the cultured cells twice with PBS, the cells were treated with FYSE, daidzein, or oseltamivir phosphate at concentrations ranging from 25 to 200 μg/mL, 2.5 to 40 μg/mL, or 2.5 to 20 μg/mL, respectively. For the pre-treatment method, the cells were pretreated with the compounds 24 h before IAV infection, and for the co-treatment method, the cells were treated with the compounds at the same time as IAV infection. The cells were infected with IAV at a multiplicity of infection (MOI) of 0.01. After infection, the cells were cultured at 35 °C with 5% CO2 for 48 h.
IAV PA gene expression by qRT-PCR
To quantify the viral copies in cells using qRT-PCR, supernatant was collected 48 h post-infection. RNA was extracted from the collected supernatant using RNAiso Plus (Takara, Shiga, Japan), following the manufacturer’s protocol, which involved chloroform extraction, isopropanol precipitation, 75% ethanol wash and reconstitution in RNase-free water. The extracted RNA was quantified using NanoVue™ Plus Spectrophotometer (GE Healthcare, Buckinghamshire, UK). Reverse transcription was carried out with a PrimeScript™ Reverse Transcriptase (PSRT, Takara, Kyoto, Japan) following the manufacturer’s instructions. For cDNA synthesis, 10 µL of quantified RNA and 1 µL of 6-mer random primer (Takara, Kyoto, Japan) were mixed and incubated at 65 °C for 10 min. Subsequently, a mixture of 4 µL of 5 × PrimeScript® RT buffer, 2 μL of 5 × dithiothreitol, 0.5 µL of PrimeScript® RT enzyme mix, 0.5 mL RNase inhibitor, and 2 μL of deoxynucleotide triphosphate were added and incubated at 37 °C for 1 h, followed by at 95 °C for 5 min. The cDNA synthesis was performed using PCR Thermal Cycler Dice Gradient (Takara). The resulting cDNA was quantified using IAV PA gene primer set (H1N1_PA_For: 5′-GAG CCT ATG TGG ATG GAT TC-3′ and H1N1_PA_Rev: 5′-CCC ATT CGG AAG TCT AAG TG-3′) in Thermal Cycler Dice® Real Time System III (TaKaRa) with following parameters: 95 °C for 10 min initiation, 45 cycles of 95 °C for 5 s denaturation, 55 °C for 10 s annealing, 72 °C for 20 s elongation. The expression of PA gene was determined by substituting the Ct values into the PA gene standard curve. The curve was constructed using known concentrations of the PA gene and expressed as copies/mL. The analysis was replicated three times for validation.
Apoptosis assay using the muse cell analyzer
Cell apoptosis was assessed using the Muse Cell Analyzer (Merck-Millipore, Billerica, MA, USA) following the manufacturer's instructions. MDCK cells were cultured and subjected to pre- and co-treatment with FYSE (25 to 200 μg/mL) or daidzein (2.5 to 40 μg/mL) and subsequently infected with IAV for 48 h, following the protocol described before. After the infection period, the cells were collected and stained with the Muse Annexin V and Dead Cell Reagent (7-aminoactinomycin D, 7-AAD). The stained cells were incubated in the dark for 20 min at room temperature, and were then analyzed using the Muse Cell Analyzer, which quantified the percentages of dead, late apoptotic, early apoptotic, and live cells (Vaidya et al., 2016).
Chemical characterization using UPLC-MS
The chemical characterization process involved centrifuging the FYSE at 10,000 rpm for 10 min, followed by filtration using 0.22 μm hydrophilic poly(tetrafluoroethylene) syringe filters (SCAA-104, ANPEL, Shanghai, China) before analysis. Samples were prepared in triplicate to ensure reproducibility.
Chemical profiling was carried out using an ACQUITY UPLC system (Waters, Milford, USA) coupled with an Xevo G2 XS-Q-TOF mass spectrometer (Waters). An ACQUITY UPLC HSS T3 C18 column (100 Å, 2.1 mm × 100 mm × 1.8 μm; Waters) was utilized with mobile phases A (0.1% formic acid in deionized water) and B (0.1% formic acid in acetonitrile). The elution profile consisted of a gradient from 98:2 v(A)/v(B) for 2 min, followed by 65:35 v(A)/v(B) for 7 min, 20:80 v(A)/v(B) for 13 min, and 0:100 v(A)/v(B) for 16.5 min at a flow rate of 0.35 mL/min. Mass data acquisition was performed in electrospray ionization positive/negative mode with specific parameters: capillary voltage of 2.5 kV, cone voltage of 40 V, desolvation N2 flow rate of 600 L/h, desolvation temperature of 200 °C, and ion source temperature of 130 °C. Instrument tuning and mass calibration were achieved using 0.5 mM sodium formate with lock masses set at m/z 556.2771 and m/z 554.2615 for positive and negative modes, respectively.
Comparative analysis of chemical composition
Precise m/z values of precursor ions were determined, and their chemical compositions identified using Waters® MassLynx® Software. Briefly, chemicals were matched by comparing their m/z values, retention time (RT), and fragmentation patterns with standards in the ChemSpider database. Peak areas were integrated for data processing, with the analysis repeated three times for validation purposes.
Statistical analysis
GraphPad Prism for Windows, version 6 (Graph Pad Software Inc., San Diego, CA, USA) was utilized for determining 50% cytotoxic concentration for MDCK cell lines (CC50) and 50% inhibitory concentration against IAV (IC50) values, and IBM SPSS Statistics Software Version 27 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The experimental samples were analyzed in triplicate, and the results were presented as means ± SD. Statistical significance was assessed by comparing the data to the control group using ANOVA followed by Dunnett’s test with a significance level set at p-value < 0.01.
Results and discussion
Cytotoxicity of soybean extract in MDCK cells
The cell viability of MDCK cells treated with fermented yellow soybean extract (FYSE) for 48 h is illustrated in Fig. 1A. Treatment with FYSE at concentrations up to 1000 µg/mL showed no significant change in cell viability. However, non-fermented aqueous yellow soybean extract (AYSE) exhibited a decrease in the viability starting at concentrations of 600 µg/mL, with a CC50 value of 666.4 µg/mL (Supplementary Fig. 1). Similarly, non-fermented ethanolic soybean extract was reported to be toxic in Raw 264.7 and A549 cells with decreasing viability above 800 µg/mL in both cell lines (Kwon et al., 2022). Ethanolic extract of Mysore thorn showed higher toxicity in MDCK cells compared to AYSE, with a CC50 value of 152.7 µg/mL (Zhang et al., 2020). Additionally, hot water soybean extract was found to be cytotoxic in Vero, HeLa, FL, and MDCK cells with CC50 values of 3.5, 15.3, 22.3 and 14.8 mg/mL, respectively (Yamai et al., 2003). However, fermentation of black soybean with B. subtilis did not show cytotoxicity in Detroit 551 cells (Lin et al., 2012). The results suggest that the fermentation process inhibits cytotoxicity, potentially due to the production of bioactive compounds that may exert protective or antioxidant effects, thereby decreasing cytotoxicity (Kim et al., 2008).
Fig. 1.
Effects of fermented yellow soybean extract (FYSE) on cell viability and anti-IAV activity, (A) Cell viability of FYSE on MDCK cells. The extract was treated to MDCK cells at concentrations ranging from 12.5 to 1000 µg/mL. Cell viability was measured after 48 h using a CCK-8 kit and expressed as a percentage of the viability of untreated control, represented as bars. (B) Anti-IAV activity of pre-treatment and co-treatment with FYSE on MDCK cells. MDCK cells were either pre-treated with FYSE for 24 h prior to IAV infection or co-treated with IAV and FYSE, and then incubated for 48 h. Virus yield in the medium was collected, RNA was extracted, converted to cDNA, and analyzed by qRT-PCR. The anti-IAV activity is expressed as the average of IAV PA gene expression, represented as bars. An asterisk above the bars indicates a significant difference in the PA gene expression between FYSE-treated and untreated IAV-infected cells (p-value < 0.01; Dunnett’s test)
Comparison of anti-IAV activity of pre-treatment and co-treatment of fermented soybean extract
The anti-IAV activity of FYSE was assessed through pre-treatment and co-treatment methods (Fig. 1B). The results displayed a significant inhibition of the IAV PA gene in cell lines subjected to both pre-treatment and co-treatment with FYSE across all concentrations tested, as compared to the control (Dunnett’s t-test, p-value = 0.000). Previous studies consistently indicated that the ethanolic extract of soybean demonstrates concentration-dependent activity in pre- and co-treated cells, with no significant impact on post-treated cells (Kwon et al., 2022). However, the IC50 value for co-treatment (IC50; 3.77 µg/mL) was significantly lower than that for pre-treatment (IC50; 8.65 µg/mL) with FYSE, indicating that co-treatment with FYSE potentially had a higher level of effectiveness in anti-IAV activity than pre-treatment (Supplementary Table 1). This could be attributed to co-treatment having an inhibitory effect on the attachment of the virus to the cell surface. The envelope glycoprotein, HA, in IAV facilitates virion attachment to cells by binding to terminal sialic acid residues on the cell surface glycoproteins, initiating the viral infectious cycle, and plays a critical role in viral binding, fusion, and entry (Skehel and Wiley, 2000). In the case of co-treatment with FYSE, it could more effectively affect the attachment phase by interfering with the binding of viral envelope to the cell surface.
Bioactive compounds in soybean extract
In this study, a comprehensive UPLC-MS analysis of FYSE was conducted to identify bioactive compounds. The analysis revealed the presence of 12 known and unknown compounds, with L-tryptophan, naphthylamine, daidzein, and soysaponin I identified among the known compounds (Fig. 2 and Table 1). The compounds were identified based on the resulting mass spectra, which provided information on both the mass-to-charge ratio (m/z) and the fragmentation pattern of each compound. Among the identified compounds, daidzein emerged as the major constituent responsible for anti-viral activity. Notably, daidzein is the aglycone form of daidzin, a glycosidic precursor. The removal of the sugar molecule from daidzin during hydrolysis results in the active form, daidzein. Such glycosidic transformation was proposed to be facilitated by Bacillus fermentation (Pyo and Lee, 2007). During fermentation, hydrolysis of β-glucosides by microbial β-glucosidases produces the active, metabolized aglycone forms of isoflavone (Khosravi and Razavi, 2021; Lee and Chou, 2006; Setchell et al., 2002). Different isoflavone aglycones are abundant in fermented soy products such as miso, natto, koji, and tempeh, produced by Saccharomyces rouxii, Bacillus subtilis natto, Aspergillus sojae and Rhizopus oligosporus, respectively (Hu et al., 2010; Kuligowski et al., 2017; Lee and Chou, 2006; Toda et al., 1999). Additionally, B. subtilis has been confirmed to possess β-glucosidase, demonstrating the ability to hydrolyze isoflavone glucoside (Kuo and Lee, 2008). Even though daidzin is the major isoflavone in soybeans, the compound was not observed in FYSE, as a previous study documented complete deglycosylation of daidzin within 24 h, transforming it into daidzein (Kuo et al., 2006). However, our preliminary study revealed that the 3-day fermentation period inhibited the cytotoxicity of the soybean extract up to the tested concentration, which was a critical consideration for our research.
Fig. 2.
Representative chromatogram and ion mass spectra of fermented yellow soybean extract (FYSE). (A) Representative chromatogram of chemical profiling of FYSE using positive ionization mode (B) Representative chromatogram of chemical profiling of FYSE using negative ionization mode. The x-axis represents retention time, and the y-axis represents the intensity (%) of identified chemicals or metabolites. The analysis was performed using Ultra-high-performance Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry (UPLC–ESI–Q-TOFMS). The peak numbers corresponding to identified chemicals or metabolites are listed in Table 1. (C) Ion mass spectra of daidzein, a key component of FYSE. The spectra include [M + H − CO2 − H2O], [M + H − CO2], [M + H − CO], and [M + H] ions with m/z values of 181.051, 191.0758, 227.099 and 255.065 respectively
Table 1.
Predicted compounds and metabolites in fermented soybean extract with their retention time (tR) and chemical formula determined by UPLC–ESI–Q-TOFMS
| No | tR | Fragment ions (m/z) | Chemical formula | Predicted compound | Reference | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| 1 | 1.77 | 93.05([M + H]+) | – | C2H8O3 | Glycerin | Pubchem |
| 2 | 3.59 | 103.0371([M + H]+) | – | C2H8O3Na | Unknown | – |
| 3 | 4.56 | 205.097 ([M + H]+) | C11H12N2O2 | L-tryptophan | Pubchem | |
| 4 | 4.89 | 144.08([M + H]+), 127.05, 117.06, 115.06, 91.05, 77.04 | – | C10H9N | Naphthylamine | Massbank |
| 5 | 5.77 | - | 131.07([M-H]−) | C6H12O3 | Unknown | – |
| 6 | 7.69 | 255.07([M + H]+), 237.06, 227.07, 199.08, 137.02 | 253.06([M-H]−), 223.04, 208.05, 195.05, 180.06, 132.02 | C15H10O4 | Daidzein | Massbank |
| 7 | 8.18 | 454.28([M + Na]+) | 430.28([M-H]−) | C22H41NO7 | Unknown | – |
| 8 | 8.29 | 386.2604 ([M + H]+) | 384.2474 ([M-H]−) | C13H33N9O3Na | Unknown | – |
| 9 | 9.55 | 239.1 ([M + H]+) | – | C11H15N2O4 | Unknown | – |
| 10 | 10.55 | 331.1 ([M + H]+) | – | C14H19O9 | Unknown | – |
| 11 | 11.68 | 943.54([M + H]+), 797.48, 635.42, 599.40, 441.37, 423.36 | 941.52([M-H]−) | C48H78O18 | Soyasaponin I | Massbank |
| 12 | 11.97 | 441.373([M + H]+), 441.3754 | – | C30H49O2 | Unknown | – |
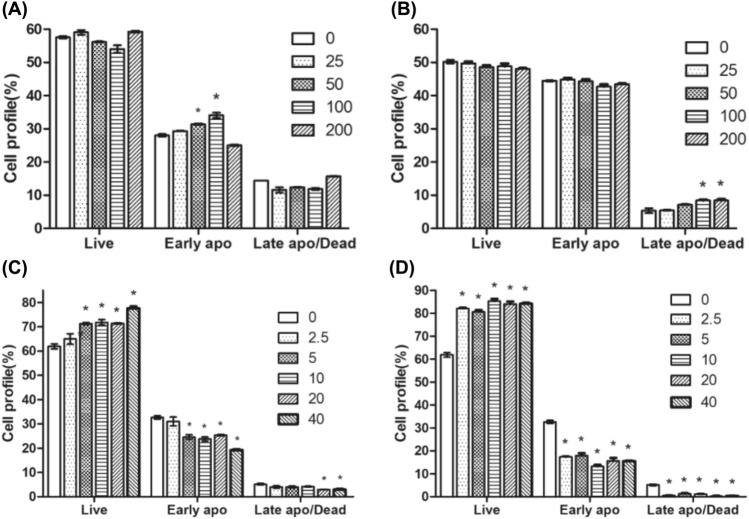
Effect of fermented yellow soybean extract on apoptosis in infected cells
The apoptosis of pre-treated and co-treated cells with FYSE or daidzein was evaluated in IAV-infected cells by assessing the percentage of live and apoptotic cells using Annexin V and dead cell staining, as depicted in Fig. 3. The percentage of live cells in pre-treated and co-treated FYSE did not show a significant difference compared to the IAV-infected sample (Dunnett’s test, p-value > 0.01) (Fig. 3A and B). Moreover, the percentage of late apoptotic and dead cells in the co-treated FYSE group was slightly higher than in the IAV-infected cells. However, pre-treatment with FYSE did not alter apoptosis in IAV-infected cells. In contrast, both pre- and co-treatment of daidzein significantly increased the percentage of live cells at almost all concentrations and significantly reduced early and late apoptosis and dead cells (Fig. 3C and D). The results indicated that daidzein treatment reduced the apoptosis of IAV-infected cells. By inducing apoptosis in cells, IAV could facilitate the progress of pathogenesis (Elbim et al., 2009). However, the treatment with daidzein during IAV exposure may create a protective environment within cells, enabling them to better resist apoptosis induced by the viral infection. This finding is consistent with a previous study that emphasized the role of soy isoflavones in effectively mitigating apoptosis induced by various stimuli, such as HIV-1 Tat (Adams et al., 2012). Furthermore, this observation aligns with the report by Yi et al. (2020) on the anti-apoptosis effects of biopeptides from soybean in cells, which was reported to involve the activation of PI3K-AKT pathway or the indirect binding to Fas and FasL receptors. In the context of soybean, daidzein has been reported as an active isoflavone that inhibits apoptosis by regulating the Bcl-2/Bax apoptotic pathway (Mao et al., 2007), and Rivera et al. (2013) indicated the capability of daidzein for lowering apoptosis by reducing caspase-3 activity.
Fig. 3.
Effect of pre-treatment (A) and co-treatment (B) with fermented yellow soybean extract (FYSE), and pre-treatment (C) and co-treatment (D) with daidzein, on apoptosis in IAV-infected MDCK cells. The effect of FYSE or daidzein on apoptosis in IAV-infected cells was analyzed by the Muse Annexin V and Dead Cell Assay. Different cell samples were stained with 7-aminoactinomycin D. Live, early apoptotic (Early apo), late apoptotic (Late apo), and dead cells were quantified using the Muse Cell Analyzer. The bars represent the mean ± SD, and an asterisk above the bars indicates a significant difference from untreated IAV-infected cells (p-value < 0.01; Dunnett’s test)
The lower effectiveness of daidzein in FYSE at inhibiting IAV-induced apoptosis compared to pure daidzein may be attributed to several factors: differences in concentration, the complex composition of FYSE, possible interactions between daidzein and other components in the extract, and variations in cellular pathways and interactions with viral proteins. Further research is needed to elucidate the specific reasons underlying these differences.
Cytotoxicity of daidzein in MDCK cells
The cytotoxicity of daidzein in MDCK cells was analyzed and compared with oseltamivir phosphate, serving as a control drug, to elucidate their safety profiles for potential therapeutic applications. The cytotoxicity was investigated over a 48-h period at concentrations ranging from 2.5 to 400 µg/mL for daidzein and oseltamivir phosphate (Fig. 4). Neither daidzein nor oseltamivir phosphate induced changes in cell viability at concentrations up to 60 µg/mL and 40 µg/mL, respectively. A comparison of CC50 values demonstrated a significantly higher value for daidzein compared to oseltamivir phosphate (CC50: 434.50 vs. 395.20 µg/mL), indicating a lower cytotoxicity effect of daidzein on MDCK cell lines (Supplementary Table 1). This suggests a favorable safety profile for daidzein in comparison to the control drug. Consistent with the findings, existing studies have reported the absence of cytotoxic activity of daidzein towards various cell lines, including A549, HeLa, HepG-2 and MG-63 cell lines (Han et al., 2015). Moreover, Guru et al. (2022) highlighted the protective effects of daidzen in gentamicin-induced cytotoxicity in MDCK cells, reinforcing its biocompatibility. A relevant connection was established in a previous study, associating the role of daidzein with the enhancement of hippocampal cell proliferation through reductions in estrogen receptor alpha and caspase-3 expression (Rivera et al., 2013). This provides insights into the mechanism of action of daidzein, contributing to its potential beneficial effects on cells. Consistent with the cytotoxicity of oseltamivir phosphate, the findings align with previous reports indicating low cytotoxicity (569.1 µg/mL) of oseltamivir in MDCK cells (Enkhtaivan et al., 2017), supporting its safety profile.
Fig. 4.
Cell viability of (A) daidzein and (B) oseltamivir phosphate on MDCK cells. MDCK cells were treated with daidzein or oseltamivir phosphate at concentrations ranging from 2.5 to 400 µg/mL. Cell viability was measured after 48 h using a CCK-8 kit and expressed as a percentage of the viability of untreated control. An asterisk above the bars indicates a significant difference from the untreated control (p-value < 0.01; Dunnett’s test)
Anti-IAV activity of daidzein through pre-treatment and co-treatment
To validate daidzein's efficiency as an anti-IAV agent, concentration-dependent effects were measured and compared to oseltamivir phosphate, a standard control drug. The anti-IAV activity of daidzein was assessed through pre- and co-treatment in MDCK cells infected with IAV by inhibiting the IAV PA gene expression after a 48-h period (Fig. 5). The result showed a significant inhibition of the PA gene in cell lines subjected to both pre- and co-treatment with daidzein at concentrations ranging from 10 to 40 µg/mL, compared to the control (Dunnett’s t-test, p-value = 0.000). Consistent with the lower IC50 value of inhibition of PA gene by co-treatment than pre-treatment of FYSE, the IC50 value of the PA gene expression by co-treatment of daidzein was lower than pre-treatment (IC50; 6.01 vs. 5.20 µg/mL). The result indicated that co-treatment of both FYSE and daidzein showed more effective anti-IAV activity than their respective pre-treatments. A previous study reported the potential of daidzein for inhibition of IAV replication in vitro by activating 5-lipoxygenase (Horio et al., 2023).
Fig. 5.
Anti-IAV activity of pre- and co-treatment with (A) daidzein and (B) oseltamivir phosphate on MDCK cells. MDCK cells were either pre-treated with daidzein or oseltamivir phosphate for 24 h prior to IAV infection or co-treated with IAV. After infection, the cells were incubated for 48 h and assessed for IAV PA gene expression. Virus yield in the medium was collected, RNA was extracted, converted to cDNA and analyzed by qRT-PCR. The anti-IAV activity is expressed as the average of IAV PA gene expression and is represented as bars. An asterisk above bars indicates a significant difference in PA gene expression in daidzein- or oseltamivir phosphate-treated IAV-infected cells compared to untreated IAV-infected cells (p-value < 0.01; Dunnett’s test)
Consistently, a significant inhibition of the IAV PA gene was observed in the cell lines subjected to both pre-and co-treatment with oseltamivir phosphate at concentrations ranging from 5 to 20 µg/mL, compared to the control (Dunnett’s t-test, p-value = 0.000). The IC50 values for both pre- and co-treatment of oseltamivir phosphate (3.55 and 1.91 µg/mL, respectively) were comparatively lower than those for daidzein, indicating that the effectiveness of oseltamivir phosphate against IAV is higher than daidzein in vitro (Supplementary Table 1). Oseltamivir phosphate also showed a higher effectiveness of PA gene inhibition by co-treatment than pre-treatment. More importantly, despite FYSE showing a higher IC50 value, its low CC50 value resulted in a higher selective index (SI) for both pre- and co-treatment of FYSE (> 115.58 and > 265.04, respectively) compared to both daidzein (SI for pre- and co-treatment 72.32 and 83.56, respectively) and oseltamivir phosphate (SI for pre- and co-treatment 111.32 and 207.35, respectively) (Supplementary Table 1). This suggests that FYSE showed greater selectivity in inhibiting IAV PA gene with less toxicity to the cells compared to other two compounds. A previous study indicated that various extracts from plants, such as seabuckthorn, demonstrated stronger anti-IAV activity compared to oseltamivir (Enkhtaivan et al., 2017). Additionally, wild watermelon showed activity against oseltamivir-resistant IAV strains (Morimoto et al., 2021). The results obtained in this study demonstrate the potential anti-IAV role of FYSE, likely attributable to the presence of daidzein, with a higher efficacy observed in the co-treatment method.
As a limitation of the study, the complex composition of FYSE suggests the presence of additional compounds and mechanisms that may contribute to its observed IAV activity, beyond those specifically analyzed. The detection of unidentified compounds in the HPLC chromatogram emphasizes the need for further research to comprehensively identify and elucidate the mechanisms of all potentially active bioactive compounds present in FYSE.
In conclusion, the study investigated the cytotoxicity, anti-IAV activity of FYSE and its major constituent, daidzein, in MDCK cells. FYSE and daidzein demonstrated significant anti-IAV activity in both pre- and co-treatment methods, with co-treatment being more effective. FYSE showed greater selectivity in inhibiting IAV PA gene with less toxicity to the cells compared to daidzein. Overall, the study suggests that FYSE, particularly daidzein, could be a promising candidate for further development as an anti-IAV agent, potentially offering a safer alternative to existing drugs.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant No. 2021R1I1A3059219 to DWK)
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adams SM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Soy isoflavones genistein and daidzein exert anti-apoptotic actions via a selective ER-mediated mechanism in neurons following HIV-1 Tat1–86 exposure. PLoS One. 7, e37540 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi SS, Khan MA, El-Harty EH, Ammar MH, Farooq M, Migdadi HM. Comparative phytochemical profiling of different soybean (Glycine max (L.) Merr) genotypes using GC–MS. Saudi Journal of Biological Sciences. 25, 15-21 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Ma C, Wong CH. Vaccine design of hemagglutinin glycoprotein against influenza. Trends in Biotechnology. 29, 426-434 (2011) [DOI] [PubMed] [Google Scholar]
- Elbim C, Katsikis PD, Estaquier J. Neutrophil apoptosis during viral infections. The Open Virology Journal. 3, 52-59. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkhtaivan G, Maria John KM, Pandurangan M, Hur JH, Leutou AS, Kim DH. Extreme effects of Seabuckthorn extracts on influenza viruses and human cancer cells and correlation between flavonol glycosides and biological activities of extracts. Saudi Journal of Biological Sciences. 24, 1646-1656 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse, Y, Suzuki AO, Hitoshi. Large-scale sequence analysis of M Gene of influenza A viruses from different species: Mechanisms for emergence and spread of amantadine resistance. Antimicrobial Agents and Chemotherapy. 53, 4457-4463 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopikrishna T, Suresh Kumar HK, Perumal K, Elangovan E. Impact of Bacillus in fermented soybean foods on human health. Annals of Microbiology. 71, 30 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guru A, Sudhakaran G, Velayutham M, Murugan R, Pachaiappan R, Mothana RA, Noman OM, Juliet A, Arockiara J. Daidzein normalized gentamicin-induced nephrotoxicity and associated pro-inflammatory cytokines in MDCK and zebrafish: Possible mechanism of nephroprotection. Comparative Biochemistry and Physiology. Part C, Pharmacology, Toxicology & Endocrinology. 258, 109364. (2022) [DOI] [PubMed] [Google Scholar]
- Han BJ, Li W, Jiang GB, Lai SH, Zhang C, Zeng CC, Liu YJ, Effects of daidzein in regards to cytotoxicity in vitro, apoptosis, reactive oxygen species level, cell cycle arrest and the expression of caspase and Bcl-2 family proteins. Oncology Reports. 34, 1115-1120 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Hayashi H, Hiraoka N, Ikeshiro Y. Inhibitory activity of soyasaponin II on virus replication in vitro. Planta Medica. 63, 102-105 (1997) [DOI] [PubMed] [Google Scholar]
- Hayden FG, Shindo N. Influenza virus polymerase inhibitors in clinical development. Current Opinion in Infectious Diseases. 32, 176 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Huang M, Tang C, Yue Y, Liu X, Zheng Z, Dong H, Liu D. Dietary daidzein inhibits hepatitis C virus replication by decreasing microRNA-122 levels. Virus Research. 298, 198404 (2021) [DOI] [PubMed] [Google Scholar]
- Horio Y, Isegawa Y, Shichiri M. Daidzein phosphorylates and activates 5-lipoxygenase via the MEK/ERK pathway: a mechanism for inducing the production of 5-lipoxygenase metabolite that inhibit influenza virus intracellular replication. The Journal of Nutritional Biochemistry. 114, 109276 (2023) [DOI] [PubMed] [Google Scholar]
- Hu Y, Ge C, Yuan W, Zhu R, Zhang W, Du L, Xue J. Characterization of fermented black soybean natto inoculated with Bacillus natto during fermentation. Journal of the Science of Food and Agriculture. 90, 1194-1202 (2010) [DOI] [PubMed] [Google Scholar]
- Khosravi A, Razavi SH. Therapeutic effects of polyphenols in fermented soybean and black soybean products. Journal of Functional Foods. 81, 104467 (2021) [Google Scholar]
- Kim NY, Song EJ, Kwon DY, Kim HP, Heo MY. Antioxidant and antigenotoxic activities of Korean fermented soybean. Food and Chemical Toxicology. 46, 1184-1189 (2008) [DOI] [PubMed] [Google Scholar]
- Kuligowski M, Pawłowska K, Jasińska-Kuligowska I, Nowak J. Isoflavone composition, polyphenols content and antioxidative activity of soybean seeds during tempeh fermentation. CYTA: Journal of Food. 15, 27-33 (2017) [Google Scholar]
- Kuo LC, Lee KT. Cloning, expression, and characterization of two β-glucosidases from isoflavone glycoside-hydrolyzing Bacillus subtilis natto. Journal of Agricultural and Food Chemistry. 56, 119-125 (2008) [DOI] [PubMed] [Google Scholar]
- Kuo LC, Cheng WY, Wu RY, Huang CJ, Lee KT. Hydrolysis of black soybean isoflavone glycosides by Bacillus subtilis natto. Applied Microbiology and Biotechnology. 73, 314-320 (2006) [DOI] [PubMed] [Google Scholar]
- Kwon EB, Kim YS, Hwang YH, Kim B, Lee SB, Park SK, Choi MS, Ha H, Choi JG. Antiviral activity of soybean GL 2626/96 (Glycine max) ethanolic extract against influenza A virus in vitro and in vivo. Biomedicine & Pharmacotherapy. 156, 113780 (2022) [DOI] [PubMed] [Google Scholar]
- Lee IH, Chou CC. Distribution profiles of isoflavone isomers in black bean kojis prepared with various filamentous fungi. Journal of Agricultural and Food Chemistry. 54, 1309-1314 (2006) [DOI] [PubMed] [Google Scholar]
- Lee N, Hurt, A.C. Neuraminidase inhibitor resistance in influenza: a clinical perspective. Current Opinion in Infectious Diseases. 31, 520-526 (2018) [DOI] [PubMed] [Google Scholar]
- Lin CC, Wu PS, Liang DW, Kwan CC, Chen YS. Quality, antioxidative ability, and cell proliferation-enhancing activity of fermented black soybean broths with various supplemental culture medium. Journal of Food Science. 77, C95-C101 (2012) [DOI] [PubMed] [Google Scholar]
- Mao Z, Zheng YL, Zhang YQ, Han BP, Zhu XW, Chang Q, Hu XB. The anti-apoptosis effects of daidzein in the brain of D-galactose treated mice. Molecules. 12, 1455-1470 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R, Yoshioka K, Nakayama M, Nagai E, Okuno Y, Nakashima A, Ogawa T, Suzuki K, Enomoto T, Isegawa Y. Juice of Citrullus lanatus var. citroides (wild watermelon) inhibits the entry and propagation of influenza viruses in vitro and in vivo. Food Science & Nutrition. 9, 544-552 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto R, Hanada A, Matsubara C, Horio Y, Sumitani H, Ogata T, Isegawa Y. Anti-influenza A virus activity of flavonoids in vitro: a structure–activity relationship. Journal of Natural Medicines. 77, 219-227 (2023) [DOI] [PubMed] [Google Scholar]
- Mousa HA. Prevention and treatment of influenza, influenza-like illness, and common cold by herbal, complementary, and natural therapies. Journal of Evidence-Based Complementary & Alternative Medicine. 22, 166-174 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak DP, Hui EKW, Barman S. Assembly and budding of influenza virus. Virus Research. 106, 147-165 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao YZ, Eun JB. Physicochemical characteristics and isoflavones content during manufacture of short-time fermented soybean product (cheonggukjang). Journal of Food Science and Technology. 57, 2190-2197 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo YH, Lee TC. The potential antioxidant capacity and angiotensin I-converting enzyme inhibitory activity of monascus-fermented soybean extracts: Evaluation of Monascus-fermented soybean extracts as multifunctional food additives. Journal of Food Science. 72, S218-S223 (2007) [DOI] [PubMed] [Google Scholar]
- Ray R, Dos Santos G, Buck PO, Claeys C, Matias G, Innis BL, Bekkat-Berkani R. A review of the value of quadrivalent influenza vaccines and their potential contribution to influenza control. Human Vaccines & Immunotherapeutics. 13, 1640-1652 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekha CR, Vijayalakshmi G. Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. Journal of Applied Microbiology. 109, 1198-208 (2010) [DOI] [PubMed] [Google Scholar]
- Ricci A, Roviello GN. Exploring the protective effect of food drugs against viral diseases: Interaction of functional food ingredients and SARS-CoV-2, influenza virus, and HSV. Life. 13 (2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera, P, Pérez-Martín M, Pavón FJ, Serrano A, Crespillo A, Cifuentes M, López-Ávalos MD, Grondona JM, Vida M, Fernández-Llebrez P, de Fonseca FR, Suárez J. Pharmacological administration of the isoflavone daidzein enhances cell proliferation and reduces high fat diet-induced apoptosis and gliosis in the rat hippocampus. PLoS One. 8, e64750 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakudo A, Sesoko M. Tofuyo (fermented soybean food) extract prolongs the survival of mice infected with influenza virus. Biomedical Reports. 1, 80-84 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DJ, Jeon SB, Oh H, Lee BH, Lee SY, Oh SH, Jung JY, Choi C. Comparison of the antiviral activity of flavonoids against murine norovirus and feline calicivirus. Food Control. 60, 25-30 (2016) [Google Scholar]
- Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol—A clue to the effectiveness of soy and its isoflavones. The Journal of Nutrition. 132, 3577-3584 (2002) [DOI] [PubMed] [Google Scholar]
- Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annual Review of Biochemistry. 69, 531-569 (2000) [DOI] [PubMed] [Google Scholar]
- Tham DM, Gardner CD, Haskell WL. Potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. The Journal of Clinical Endocrinology and Metabolism. 83, 2223-2235 (1998) [DOI] [PubMed] [Google Scholar]
- Toda T, Uesugi T, Hirai K, Nukaya H, Tsuji K, Ishida H. New 6-O-acyl isoflavone glycosides from soybeans fermented with Bacillus subtilis (natto). I. 6-O-succinylated isoflavone glycosides and their preventive effects on bone loss in ovariectomized rats fed a calcium-deficient diet. Biological & Pharmaceutical Bulletin. 22, 1193-1201 (1999) [DOI] [PubMed] [Google Scholar]
- Vaidya B, Cho SY, Oh KS, Kim SH, Kim YO, Jeong EH, Nguyen TT, Kim SH, Kim IS, Kwon J, Kim D. Effectiveness of periodic treatment of quercetin against influenza A virus H1N1 through modulation of protein expression. Journal of Agricultural and Food Chemistry. 64, 4416-4425 (2016) [DOI] [PubMed] [Google Scholar]
- Wu X, Wu X, Sun Q, Zhang C, Yang S, Li L, Jia Z. Progress of small molecular inhibitors in the development of anti-influenza virus agents. Theranostics 7, 826-845 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamai M, Tsumura K, Kimura M, Fukuda S, Murakami T, Kimura Y. Antiviral activity of a hot water extract of black soybean against a human respiratory illness virus. Bioscience, Biotechnology, and Biochemistry. 67, 1071-1079 (2003) [DOI] [PubMed] [Google Scholar]
- Yi G, Li H, Li Y, Zhao F, Ying Z, Liu M, Zhang J, Liu X. The protective effect of soybean protein-derived peptides on apoptosis via the activation of PI3K-AKT and inhibition on apoptosis pathway. Food Science & Nutrition. 8, 4591-4600 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Chen J, Ke C, Zhang H, Zhang S, Tang W, Liu C, Liu G, Chen S, Hu A. Ethanol extract of Caesalpinia decapetala inhibits influenza virus infection in vitro and in vivo. Viruses. 12, 557 (2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.