Abstract
Cancer diseases are a serious health problem for society, and among them cervical and prostate cancer rank high in terms of mortality. One of the reasons is the phenomenon of drug resistance and side effects accompanying conventional chemo- and radiotherapy. This requires continuous development of alternative treatment methods and searching for new compounds with anti-cancer potential. An example is quinalizarin, which was tested for its anti-cancer potential. The MTT test showed cytotoxic activity of quinalizarin against Hela and DU145 cell lines. Morphological analysis showed nuclear changes typical of apoptosis, which was confirmed by the annexin V/PE test, activation of caspases 3/7 and inhibition of Bcl-2 protein expression. Increased permeability of mitochondrial membranes and ROS generation were demonstrated. Inhibition of cell migration, blocking in the G0/G1 phase, increased number of cells with damaged DNA and an increase in markers of mitotic catastrophe, i.e. micro- and multinucleation including the presence of abnormal mitotic figures were also observed. At the same time, increased autophagy was observed, and preincubation of cells with chloroquine inhibited this process, which contributed to the increased cytotoxicity of quinalizarin towards the tested cells. Quinalizarin has a multidirectional effect based on apoptosis and alternative types of cell death.
Keywords: Quinalizarin, Apoptosis, Autophagy, Mitotic catastrophe, Oxidative stress
Subject terms: Cancer, Cell biology, Drug discovery
Introduction
Cancer is the leading cause of death and a significant barrier to increasing life expectancy in every country in the world. According to the World Health Organization (WHO), cancer is the first or second leading cause of death before the age of 701. It has been shown that 8.97 million deaths worldwide, just after ischemic heart disease, are due to cancer, and projections show that cancer is likely to become the first cause of mortality in 2060 (~ 18.63 million deaths).
In men, the most commonly diagnosed malignant tumor is prostate cancer, which is the second leading cause of death in the United States2 and the fifth worldwide3. Compared to European and American countries, this cancer occurs significantly more frequently (60% higher frequency) among African Americans, causing more than twice the mortality rate4,5.
Prostate cancer can be asymptomatic in its early stages, and the most common symptoms accompanying the disease are difficulty urinating and an increased frequency of nocturia, which is a consequence of prostate hypertrophy. In the advanced stage of the disease, urinary retention and characteristic back pain may occur, which is a consequence of bone metastases3. The most common risk factors contributing to the development of prostate cancer are: advanced age, ethnicity, family history, genetic mutations (BRCA1/2 gene mutations), insulin-like growth factors, improper diet, use of stimulants (tobacco and alcohol), obesity, and lack of physical activity. Factors that may also increase the risk of prostate cancer include environmental, occupational factors, and ionizing radiation6–8.
Treatment of prostate cancer includes, in addition to surgery, hormone therapy, radiotherapy, chemotherapy, and targeted therapy9,10. In most cases, cancer detected at an early stage allows for effective treatment. However, a significant proportion of patients are diagnosed at a late stage of the disease, when metastases to nearby or distant organs are already observed2.
Among women, the fourth leading cause of cancer deaths worldwide is cervical cancer, which, similarly to men, accounts for almost 90% of deaths in African countries11,12. The main cause of cervical cancer is chronic infection with human papillomavirus, whose genotypes HPV-16 and HPV-18 are responsible for over 70% of cancer cases13,14. Other factors that affect the incidence of cervical cancer include: geographical location, number of screening tests, socioeconomic status, access to health care, social awareness, use of oral contraceptives, smoking, and HIV infection12,15.
As in the case of prostate cancer, the methods used in treatment are surgical procedures often supplemented with radiotherapy or chemotherapy, or combined methods16–18.
Cervical chemotherapy includes the use of both natural and synthetic compounds administered in single-component and combined therapy. Such compounds include docetaxel, vinorelbine, cisplatin, gemcitabine, irinotecan, ifosfamide, mitomycin C, topotecan, and doxorubicin19,20.
In the case of prostate cancer, hormonal therapy is often supplemented with the use of chemotherapeutics such as vinorelbine, doxorubicin, cisplatin and its derivatives, mitoxantrone, or cyclophosphamide, which are often used in combined therapy21,22. However, the most common standard in the treatment of prostate cancer is docetaxel, used especially in the earlier stage of the disease, as well as in patients with metastases. It is also used in the case of advanced, castration-resistant metastatic prostate cancer, where the second-line drug is cabazitaxel from the taxane group21,23,24.
However, conventional radiotherapy and chemotherapy often cause numerous side effects25. Hence, it is very important to search for new compounds, including natural plant extracts, especially since many of them are used in medicine due to their safety. Numerous studies have shown that plant compounds with anti-cancer effects in relation to prostate cancer25 and cervical cancer16 include alkaloids, flavonoids, terpenoids, and phenols. An example of compounds with high therapeutic potential are also anthraquinones and their derivatives26, which, due to their properties, may become a new group of anticancer drugs in the future. One of them is quinalizarin, whose mechanism of action is not fully investigated, especially against cervical and prostate cancer cells, which is the subject of the studies presented in this paper.
Results
Quinalizarin induces apoptosis in HeLa and DU145 cell lines by activating caspases 3/7
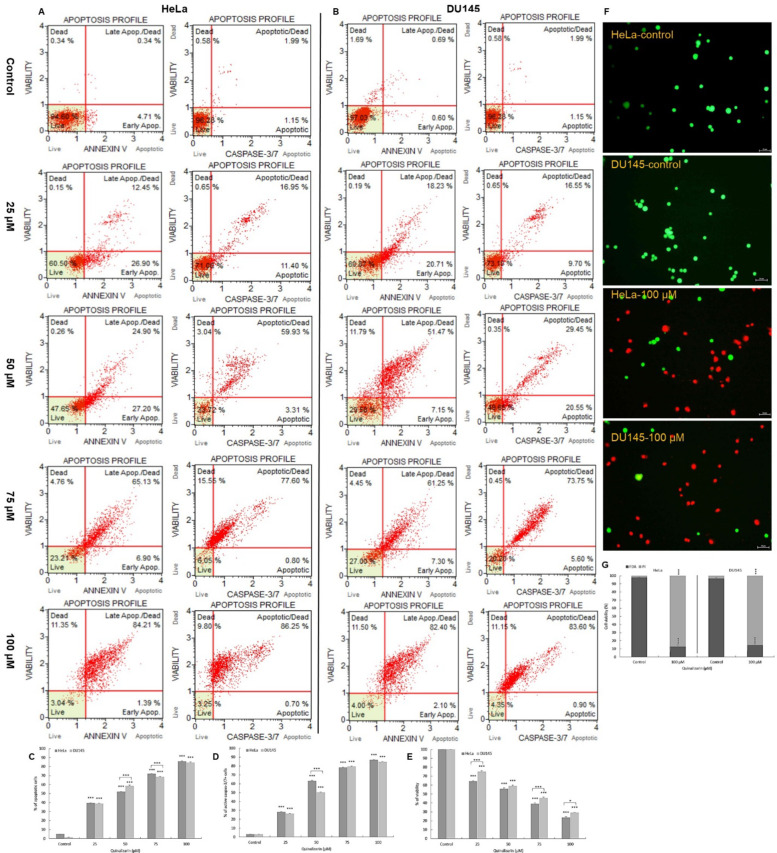
Exposure of the tested cells to quinalizarin caused an increase in the frequency of early-apoptotic (annexin V-PE+/7-AAD) and late-apoptotic (annexin V-PE+/7-AAD+) cells. At a concentration of 25 µM, apoptotic cells of the HeLa line constituted 39.4% (p ≤ 0.0001), and at 50 µM over 50% (p ≤ 0.0001) (Fig. 1A). The highest percentage of apoptotic cells was observed at subsequent concentrations (75 µM and 100 µM) up to 72% and 86% (p ≤ 0.0001), with a clear predominance of cells with a late-apoptotic phenotype.
Fig. 1.
Proapoptotic effect of quinalizarin. HeLa and DU145 cells were treated for 48 h with quinalizarin at concentrations of 25 µM, 50 µM, 75 µM and 100 µM. Representative histograms of the distribution of apoptotic and caspase-positive cells of HeLa (A) and DU145 (B) lines. The level of apoptosis was determined by annexin V-PE/7-AAD staining. Live cells (annexin V-PE-/7-AAD-), early apoptotic cells (annexin V-PE+/7-AAD-), late apoptotic cells (annexin V-PE+/7-AAD+) and dead cells (annexin V-PE-/7-AAD+). The level of caspase 3/7 activity was assessed by the Muse Caspase-3/7 Kit. Live cells (caspase 3/7-/7-AAD-), early apoptotic cells (caspase 3/7+/7-AAD-), late apoptotic cells (caspase 3/7+/7-AAD+), dead cells (caspase 3/7-/7-AAD+). Comparison of the percentage of apoptotic cells (both early and late) (C) and caspase-positive cells (D) depending on the quinalizarin concentration in HeLa and DU145 cells. Cell viability determined by the MTT assay (E). Double staining with fluorescein acetate/propidium iodide (FDA/PI) of HeLa and DU145 cells (F). Fluorescent staining indicates live cells (green fluorescence emission), while red fluorescence corresponds to PI, which binds to DNA after membrane damage. Percentage distribution of live and dead cells after double staining with FDA and PI (G). Magnification 200 ×. Representative data from three parallel experiments corresponding to mean values. SEM standard error of the mean. Statistical differences were confirmed at: *p < 0.05; **p < 0.01; ***p < 0.001.
The consequence of encubranced DU145 cells with quinalizarin at a concentration of 25 µM was a statistically significant (p ≤ 0.0001) increase in the number of apoptotic cells (38.9%). Incubation of cells with increasing concentrations of the tested compound caused further induction of apoptotic processes up to 58.6% at 50 µM and up to 68.5% (75 µM). At 100 µM, more than 84% (p ≤ 0.0001) of the pool of all cells were apoptotic cells (Fig. 1B).
In addition, it was shown that quinalizarin induces apoptosis dependent on caspase 3/7 activation. With increasing concentration of the tested anthraquinone, an increasing number of cells with active caspase 3/7 was observed, both in HeLa and DU145 cells. The highest activity of executive caspases was demonstrated for the concentration of 100 µM, where apoptotic cells constituted 86% (HeLa line) and 84% (DU145 line). The results indicate a proapoptotic effect of quinalizarin dependent on caspase 3/7 activation.
Quinalizarin inhibits the viability of HeLa and DU145 cells
The MTT test showed a statistically significant (p ≤ 0.0001) inhibition of the ability of the tested cells to reduce the MTT dye (Fig. 1E). At the lowest concentration (25 µM), the viability of HeLa cells was 64% and 75% for the DU145 line. Subsequent concentrations of 50 and 75 µM caused a significant decrease in viability, respectively, to 55% and 39% (HeLa line) and to 59% 45% (DU145 line). The lowest percentage of live cells was obtained at a 100 µM concentration of the tested compound, i.e. 23% for the HeLa line and 29% for the DU145 line. The control was assumed as 100%. Quinalizarin inhibits the metabolic activity of mitochondria depending on the concentration, which indicates mitochondrial damage.
The cytotoxic effect of quinalizarin on the tested cell lines was confirmed by fluorescent staining of FDA/PI cells. Microscopic analysis showed that 100 µM concentration of quinalizarin caused a significant increase in the number of dead cells (over 80%) in both HeLa and DU145 cell lines, as indicated by very numerous dead cells with red fluorescence resulting from labeling with propidium iodide, which binds to DNA after membrane damage. Control (live) cells were characterized by green fluorescence emission resulting from fluorescein diacetate staining (Fig. 1F).
Quinalizarin inactivates Bcl-2 protein
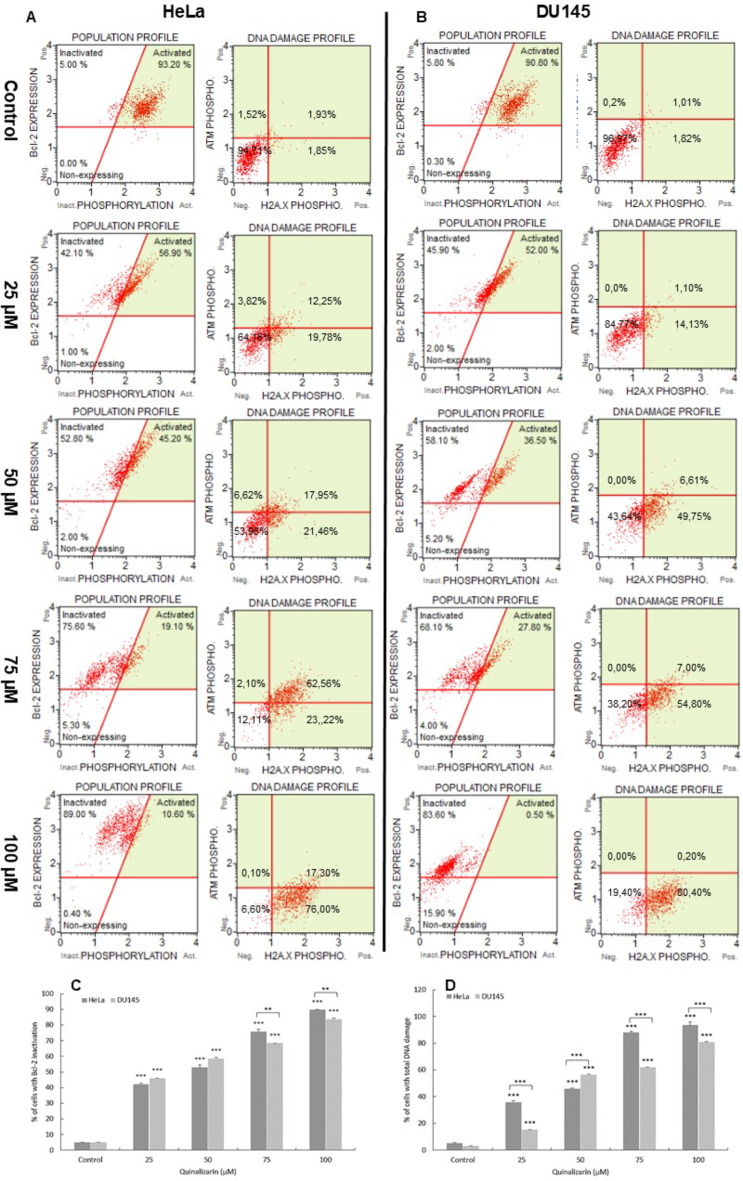
Quinalizarin induced inactivation of Bcl-2 protein in HeLa and DU145 cell lines, the expression of which determines the survival of cancer cells. With increasing concentration, gradual changes in Bcl-2 protein expression were demonstrated (Fig. 2). At concentrations of 25 and 50 µM, the level of inactivation of the antiapoptotic protein was 42.15% and 52.89%, with p ≤ 0.0001 (HeLa line) and 45.96% and 58.56%, with p ≤ 0.0001 (DU145 line). On the other hand, at a concentration of 100 µM, over 93% of HeLa cells and 80.69% of DU145 line (p ≤ 0.0001) were dephosphorylated. This confirms the inactivation of the anti-apoptotic protein Bcl-2 as a result of quinalizarin.
Fig. 2.
Inactivation of Bcl-2 protein and the degree of DNA damage in HeLa and DU145 cells exposed to 48 h of quinalizarin. Representative histograms of the distribution of cells with Bcl-2 protein inactivation and DNA damage in HeLa (A) and DU145 (B) cells. The histograms indicate over 80% dephosphorylation of Bcl-2 protein occurring in the studied cells after exposure to quinalizarin at a concentration of 100 µM. Scatter plots of the distribution of cells expressing the activation of ATM and H2A.X. The demonstrated coactivation of ATM and H2A.X (over 80%) after exposure to quinalizarin (100 µM) indicates double-stranded DNA breaks and its damage. Percentage of cells with Bcl-2 protein inactivation (C) and with double-stranded DNA breaks (dual activation of ATM and H2A.X) (D) induced by quinalizarin. Data representative of three parallel experiments correspond to mean values. SEM standard error of the mean. Statistical differences were confirmed at: *p < 0.05; **p < 0.01; ***p < 0.001.
Quinalizarin causes DNA damage
48-hour exposure to quinalizarin caused a concentration-dependent increase in phosphorylated H2A.X in response to DNA double-strand breaks (DSBs) (Fig. 2). At the highest concentration of 100 µM, cells with DBS accounted for more than 93% (p ≤ 0.0001) of HeLa cells and more than 80% (p ≤ 0.0001) of prostate cancer cells. The number of cells with damaged DNA after quinalizarin treatment correlated with the severity of apoptotic processes.
Quinalizarin generates ROS in the studied cells
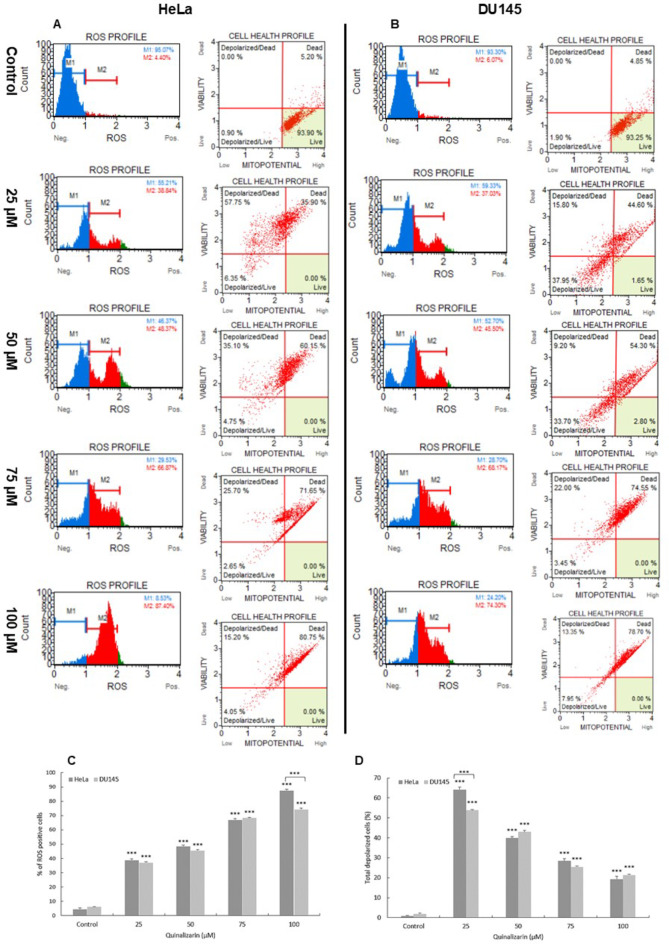
The estimation of the percentage of cervical and prostate cancer cells exposed to oxidative stress as a result of exposure to quinalizarin was based on intracellular detection of superoxide radicals. In the histograms, cells generating ROS are referred to as ROS (+), while cells that did not bind the dye are referred to as ROS (−). Flow cytometric analysis showed a statistically significant (p ≤ 0.0001) increase in the production of ROS generated as a result of quinalizarin, which was noted at all concentrations, in the case of both studied cell lines. At the concentration of 100 µM, the highest percentage of ROS (+) cells was noted, i.e. over 87% for HeLa cells and 74% for DU145, compared to control cells (HeLa = 4.45%; DU145 = 6.07%) (Fig. 3). The obtained results indicate the induction of oxidative stress in the tested cells as a result of the action of quinalizarin.
Fig. 3.
Induction of oxidative stress in HeLa and DU145 cells by quinalizarin. Generation of reactive oxygen species and changes in mitochondrial membrane potential as a result of quinalizarin in HeLa (A) and DU145 (B) cells. Percentage of ROS (+) cells (C) and cells with mitochondrial membrane depolarization (D) observed at different quinalizarin concentrations. Each sample was analyzed three times. Differences were statistically confirmed at: *p < 0.05; **p < 0.01; ***p < 0.001.
Quinalizarin induces submicroscopic changes
As a result of the use of quinalizarin at a concentration of 25 µM in HeLa cells, swollen mitochondria with a cleared matrix, swollen rough endoplasmic reticulum and numerous autophagic vacuoles were observed (Fig. 4A). As a result of encumbrancing the cells with 50 µM of the tested compound, mitochondria became further swollen. Their matrix was very cleared and mitochondrial cristae were significantly reduced. Swollen channels of the rough endoplasmic reticulum were also visible in their area. At 75 µM, mitochondria with a strongly clear matrix and damaged cristae predominated, and the cell nuclei had a changed shape. Numerous Golgi apparatuses with swollen and scattered cisternae were also observed in the cytoplasm. Numerous primary lysosomes, autophagosomes and autophagolysosomes were also shown, indicating the intensification of degradative processes. The greatest changes were observed at a concentration of 100 µM, where mitochondria were characterized by disorganized structure indicating their significant damage. Some mitochondria were characterized by leakage of matrix content into the cytoplasm. Numerous megamitochondria were also observed in the cells. The observed changes correlated with the performed morphometric measurements, because with the increase in quinalizarin concentration a progressive increase in mitochondrial size was demonstrated. At 100 µM, the size of mitochondria was 1.54 μm, which indicates a 3-fold increase compared to the control (0.57 μm).
Fig. 4.
Ultrastructural changes in HeLa and DU145 cell lines exposed to 48 h of quinalizarin. HeLa cells. (A) Control cell with normal morphology of the nucleus and organelles. Cells after quinalizarin treatment at a concentration of 25 µM with an increased number of swollen mitochondria. Cells treated with quinalizarin (50 µM)—numerous Golgi apparatuses with swollen and scattered cisternae, numerous autophagic vacuoles with visible double membrane and autophagolysosomes. Cells treated with a concentration of 75 µM—swollen rough reticulum, mitochondria with shortened and damaged cristae, numerous autophagolysosomes, primary and secondary lysosomes. A concentration of 100 µM quinalizarin caused severe swelling and complete electron-transparent of mitochondria, with numerous lysosomes and autophagic vacuoles visible. DU145 cells. (B) Control cell with normal morphology of the nucleus and normal structure of organelles. Cells after treatment with quinalizarin at a concentration of 25 µM with increased number of Golgi apparatuses with swollen cisternae. Cells treated with 50 µM quinalizarin–numerous Golgi apparatuses with scattered cisternae, numerous autophagic vacuoles and swollen mitochondria. Cells treated with a concentration of 75 µM—presence of mitochondria with damaged cristae, autophagic vacuoles, lysosomes and cytoskeletal elements. 100 µM concentration of quinalizarin caused strongly swollen and completely electron-transparent mitochondria with drawn into the membrane, numerous lysosomes and autophagic vacuoles. N cell nucleus, M mitochondria, VA autophagic vacuoles, AVL autophagolysosomes, AG Golgi apparatus, LP primary lysosomes, LS secondary lysosomes, RER rough endoplasmic reticulum. Magnification 11,500 ×. Quinalizarin concentration-dependent change in mitochondrial size (C). Quinalizarin modulates LC3-II protein expression. Representative histograms of HeLa (A’) and DU 145 (B’) cells after 48 h of treatment with quinalizarin at concentrations of 25–100 µM. Cells were stained with a conjugated anti-LC3/Alexa Fluor555 antibody, and fluorescence intensity was measured flow cytometrically. Percentage differences in LC3-II protein expression expressed by change in fluorescence intensity (D). Data represent mean values. SEM - standard error of the mean. Differences were statistically confirmed at the level of: *p < 0.05; **p < 0.01; ***p < 0.001.
Characteristic for the ultrastructure of DU145 cells was the intensification of autophagic processes and the presence of altered mitochondria (Fig. 4B). At a concentration of 25 µM, mitochondria with an altered shape were observed, an expanded rough endoplasmic reticulum in the form of long cisternae covered with ribosomes and the presence of primary lysosomes. Changes in the ultrastructure of the cell nucleus were also demonstrated, concerning the change of its shape. As a result of loading the cells with a concentration of 50 and 75 µM, an increased number of autophagic vacuoles filled with material for degradation and the presence of Golgi apparatus with very swollen and dispersed cisternae were demonstrated. Cell nuclei with an altered shape and with progressive fragmentation were also observed. At a concentration of 100 µM, an intensified macroautophagy was observed, which was expressed by numerous autophagosomes and autolysosomes. Different stages of vacuolar degeneration of mitochondria were also observed, which manifested itself as concentric remodeling of mitochondrial cristae with spaces between them.
Quinalizarin affects changes in LC3-II levels
In order to confirm the autophagosomes observed in the electron microscope, the LC3-II protein level was analyzed. An increase in the fluorescence intensity of the fluorochrome visible in the histograms (red area) was shown to be dependent on the quinalizarin concentration, which indicates an increase in the LC3-II protein level compared to the cells from the control group (gray area) (Fig. 4A’,B’). At 25 µM, fluorescence in HeLa cells increased to 113.87%, while at 50 µM to 143.49%. As a result of using higher concentrations of quinalizarin, a gradual reduction in dye emission was observed in labeled cells to 82.33% (75 µM) and to 64.55% at 100 µM. Also in DU145 cells, a gradual increase in fluorescence intensity was observed to 110.74% (25 µM), 152.49% (50 µM) and to 164.79% at 75 µM. At 100 µM, the emission value was 73.36% (Fig. 4D). This indicates degradation of LC3-II by lysosomal enzymes and confirms the ongoing process of macroautophagy.
Preincubation of cells with chloroquine enhances the cytotoxicity of quinalizarin
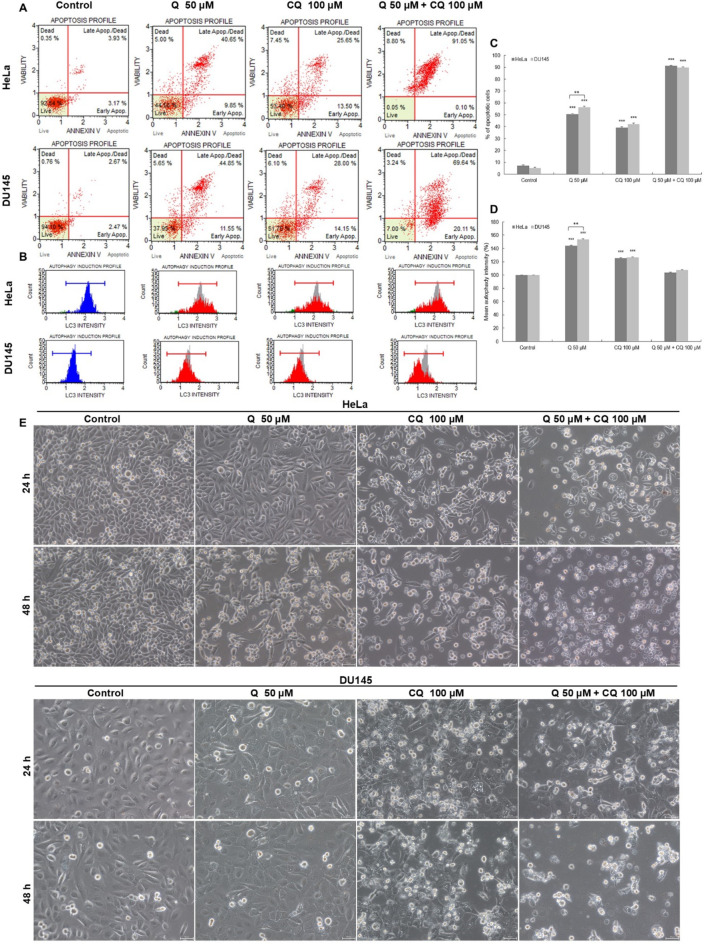
As a result of 48-hour exposure to chloroquine (CQ), the level of LC3-II protein increased to 125.29%, p ≤ 0.0001 (HeLa cells) and to 126.47%, p ≤ 0.0001 (DU145 cells) with the level of apoptosis being 39.15% and 42.15%, p ≤ 0.0001, respectively (Fig. 5). Exposure of cells to quinalizarin (50 µM) enhanced the expression of LC3-II protein in cervical cancer cells to 144.05% (p ≤ 0.0001) and the level of apoptosis to 50.5%, p ≤ 0.0001. In prostate cancer cells, the level of LC3-II was 153.52% and apoptosis 56.4%. The combined effect of chloroquine (100 µM) and quinalizarin (50 µM) was to reduce the expression of LC3-II to 103.36% (HeLa cells) and to 107.05% (DU145 cells), with a simultaneous significant increase in the level of apoptotic cells to 91.15% and 89.75%, respectively, p ≤ 0.0001. Inhibition of autophagy by chloroquine was confirmed by the presence of vacuoles in the cytoplasm, the highest number of which was observed after 24 h (Fig. 5E). On the other hand, the extension of the incubation time to 48 h was expressed by the inhibition of autophagic processes induced by quinalizarin by chloroquine and an increase in its proapoptotic effect, as indicated by the presence of numerous apoptotic cells with a reduced number of cells with cytoplasmic vacuolisation.
Fig. 5.
Effect of combined action of quinalizarin and chloroquine on apoptosis induction in HeLa and DU145 cells. Cells were treated with quinalizarin (Q-50 µM) and chloroquine (CQ-100 µM) and combined action of compounds (Q-50 µM + CQ-100 µM) for 48 h. Apoptosis level was measured using Annexin V-PE/7-AAD staining (A), and LC3-II protein expression level was measured using flow cytometry (B). Percentage of apoptotic cells (C) and LC3-II expressing cells (D). Data represent mean values. SEM standard error of the mean. Differences were statistically confirmed at the level of: *p < 0.05; **p < 0.01; ***p < 0.001. Representative images showing the morphology of HeLa and DU145 cells, which were taken using the phase contrast technique. Visible vacuoles in the cytoplasm of the tested cells after the action of chloroquine (100 µM) and quinalizarin (50 µM). The combined action of the tested compounds increased the number of cells with vacuoles, confirming the inhibition of autophagic processes by chloroquine (24 h). As a result of 48-hour incubation of cells with quinalizarin and chloroquine, the proapoptotic effect of quinalizarin was enhanced-a reduced number of cells with vacuoles and a predominance of rounded and shrunken apoptotic cells. The images were taken using the phase contrast technique. Magnification ×200.
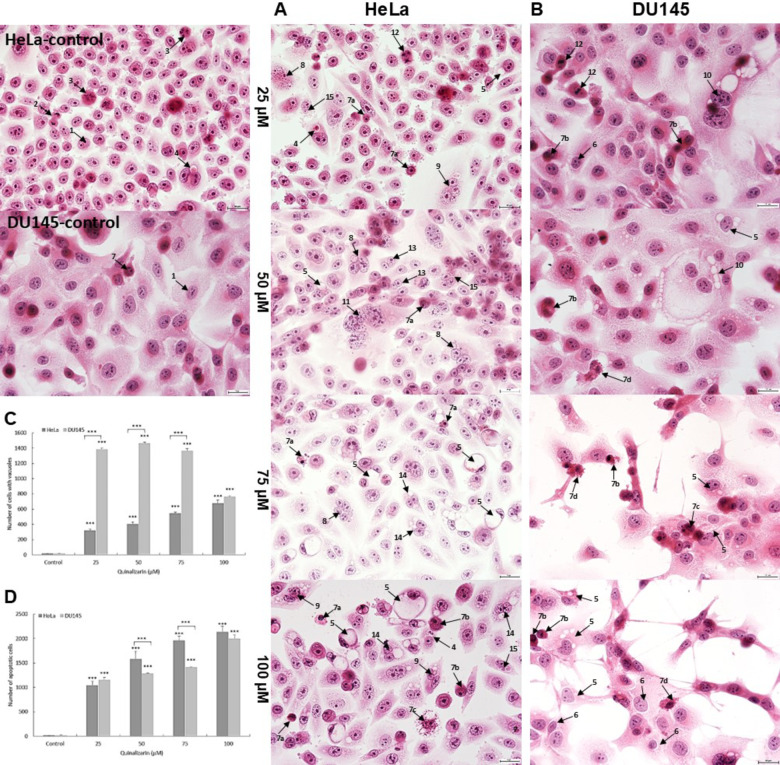
Quinalizarin increases vacuolization and apoptotic changes-assessment of morphological changes
Analysis of cells stained with the H&E technique showed a concentration-dependent progressive vacuolization of the cytoplasm and induction of apoptotic processes. Already at 25 µM, a significant increase in the number of cells with vacuoles was demonstrated (317 cells in the HeLa line and 1385 cells of the DU145 line, p ≤ 0.0001) (Fig. 6C). Morphological analysis of the HeLa line cells showed that at concentrations of 75 and 100 µM, there was a progressive increase in the number of both vacuolated and apoptotic cells, with apoptotic cells constituting over 71% of the pool of all analyzed cells (concentration of 100 µM). In the case of the DU145 line, there was a significant reduction in the number of cells with cytoplasmic vacuolization in favor of an increase in the number of apoptotic cells, which, similarly to the HeLa line at a concentration of 100 µM, constituted the highest percentage (over 66%).
Fig. 6.
Morphological changes indicating the induction of vacuolation and apoptosis in HeLa and DU145 cell lines. Representative micrographs of HeLa (A) and DU145 (B) cells stained with the hematoxylin and eosin (H&E) method. Control cells with normal morphology, including interphase cells and cells in numerous mitotic divisions. Cells treated with quinalizarin at concentrations of 25–100 µM indicating vacuolation of the cytoplasm and apoptosis. Visible cells containing strongly eosinophilic material in vacuoles destined for degradation and cells with changes typical of apoptosis (pyknotic cell nuclei, nuclei with visible chromatin condensation, cells with apoptotic bodies). Explanation of symbols: 1—interphase, 2—late telophase, 3—prometaphase, 4—cells with vacuoles filled with degradation material, 5—cells with cytoplasmic vacuolation, 6—cells with perinuclear vacuolation, 7—apoptotic cells (a—pyknotic nucleus, b—chromatin condensation, c—nuclear fragmentation, d—apoptotic bodies), 8—multinucleated cells, 9—multinucleated cells with vacuoles, 10—giant cells with vacuoles, 11—giant cells, 12—cells with abnormal chromosome segregation, 13—binucleated cells, 14—binucleated cells with vacuoles, 15—cells with micronuclei. Images were taken at 400 × magnification. Quinalizarin concentration-dependent changes in the number of vacuolated (C) and apoptotic (D) cells.
Apoptotic cells were characterized by chromatin condensation and fragmentation, shrinkage and darkly stained cytoplasm. The number of apoptotic cells shown correlated with the cytometric analysis (Annexin V test).
Quinalizarin blocks cells in the G0/G1 phase and reduces the mitotic index
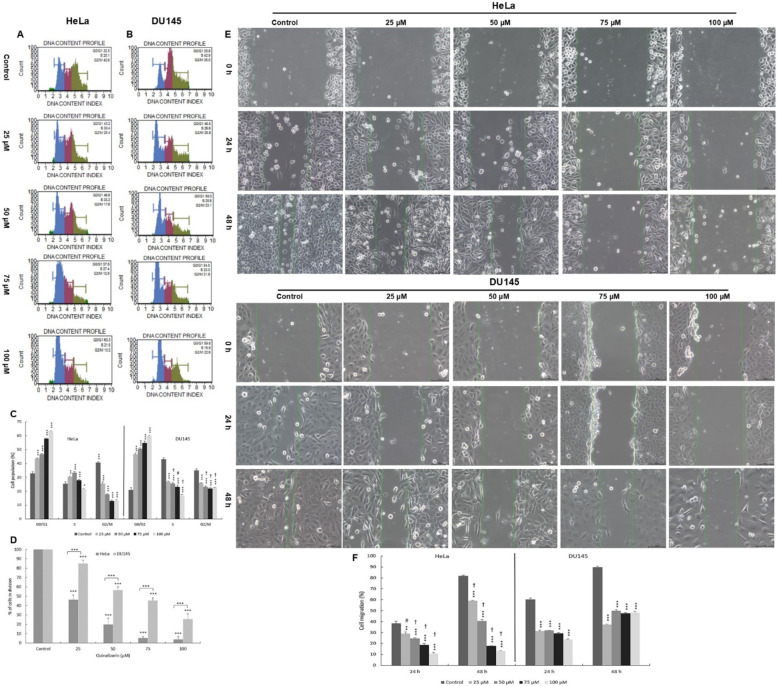
As a result of incubation of cells with quinalizarin, a statistically significant reduction in the mitotic index value occurred in relation to the control assumed as 100% (Fig. 7). At 25 µM and 50 µM, the mitotic index was 46.21% and 19.88% for the HeLa line and 85.05% and 56.54% for DU145, respectively, at p ≤ 0.0001. At the highest concentration of quinalizarin (100 µM), the lowest percentage of dividing cells was demonstrated, which was 3.92% (HeLa) and 25.65% (DU145) (p ≤ 0.0001).
Fig. 7.
Antiproliferative effect of quinalizarin on HeLa and DU145 cells. Changes in the cell cycle of HeLa (A) and DU145 (B) cells treated for 48 h with quinalizarin at concentrations of 25–100 µM (cytometric analysis). Percentage distribution of HeLa and DU145 cells in different phases of the cell cycle indicating blocking of cells in the G0/G1 phase (C). Changes in the mitotic index indicating inhibition of cell division by quinalizarin (D). The effect of quinalizarin on the migration of HeLa and DU145 cells was assessed by wound healing assay (E). Representative inverted phase contrast microscope images show the change in scratch width at different time intervals after cell migration in the control group and in the group of cells loaded with quinalizarin in the concentration range of 25–100 µM. (F) Quantification of the effect of quinalizarin on the migration potential of HeLa and DU145 cells. Magnification × 200. Results represent the mean values of three independent experiments ± SE. Statistical differences were confirmed at ***p < 0.001. The symbol # indicates a statistically significant change in wound size in the HeLa cell group (p < 0.01) for 25 µM quinalizarin compared to DU145 cells encumbranced with 25 µM quinalizarin. The symbol † indicates a statistically significant change in wound size in the HeLa cell group (p < 0.0001) for 50–100 µM quinalizarin compared to DU145 cells encumbranced with quinalizarin in the concentration range of 50–100 µM.
Flow cytometric analysis showed that quinalizarin caused a progressive blocking of cells in the G0/G1 phase of the cell cycle with increasing concentration. The highest increase in the cell population in the G0/G1 phase was observed at a concentration of 100 µM, i.e. two-fold for the HeLa line (59.85%) and three-fold for the DU145 line (63.32%), p ≤ 0.0001).
Effect of quinalizarin on HeLa cell migration
“A scratched wound” creates a cell-free space through which the rest of the cultured cells can migrate, which mimics the wound healing process.
Changes in the migration capacity of HeLa and DU145 lineage cells induced by quinalizarin were observed at two time points of 24 and 48 h (Fig. 7). The HeLa lineage cells from the control group showed an approximate 40% reduction in wound width after 24 h (270 μm), and virtually complete healing of the monolayer (more than 81%) was observed after 48 h (80 μm). The width of the “scratched wound” at point 0 was 438.3 μm. Quinalizarin showed a delay in wound healing compared to the control. The percentages of cell migration measured at time points (24, 48 h) were 28.9% (306 μm) and 59.1% (176 μm) for the 25 µM concentration and 24.47% (324 μm) and 40.55% (174 μm) for the 50 µM concentration, respectively. The greatest inhibition of cell migration was shown for concentrations of 75 and 100 µM. At 75 µM, an 18.79% (350 μm/24 hours) and 17.63% (355 μm/48 hours) reduction in wound width was shown, while for 100 μm only an average of 12% cell migration was shown at both 24 (386 μm) and 48 h (375 μm).
A lower effect of quinalizarin on cell migration was observed against cells of the DU145 line. In the control group, cells migrated and covered between 60% (364 μm) and 90% (144.8 μm) of the wound surface after 24 and 48 h.
The width of the “debrided wound” at 0 point was 364 μm. A concentration of 25 µM showed 31.3% (24 h) and 37.32% (48 h) overgrowth of the surface, and 31.8% (246 μm/24 h) and 49.86% (181 μm/48 h) at 50 µM. Exposure of cells to quinalizarin at higher concentrations induced further delay in wound healing. At 100 µM, overgrowth of the resulting scratch was found to be up to 23.67% (274 μm/24 h) and up to 48.18% (186 μm/48 h) at the measured time points. The results demonstrate the inhibitory effect of quinalizarin on the migration of the cells studied.
Quinalizarin induces mitotic death—assessment of morphological changes
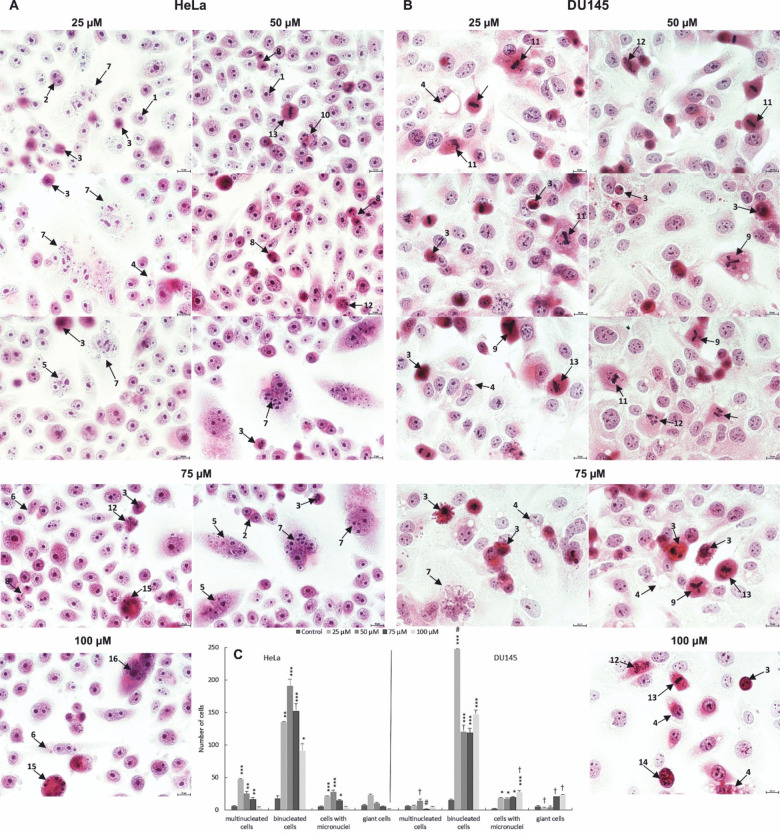
Analyzing the changes in the studied cells, there was a statistically significant increase in the number of cells that were indicators of mitotic catastrophe, i.e. binuclear cells, multinuclear cells, giant cells and cells with micronuclei (Fig. 8). Giant cells were characterized by large size, clear cytoplasm with a large surface area and an increased number of cell nuclei, diverse in size and shape. Numerous micronuclei and cytoplasmic vacuoles were also often present in the cytoplasm. The presence of abnormal mitotic figures such as: tripolar anaphase, tripolar metaphase, multipolar metaphase, anaphase bridges was also characteristic of mitotic death. It should be noted that their greatest number occurred at lower applied concentrations of quinalizarin in the range of 25–50 µM. Mitotic catastrophe marker cells were also observed, which were in the stage of apoptotic death, which is one of the most common final stages of mitotic death.
Fig. 8.
Morphological markers of mitotic catastrophe in HeLa (A) and DU145 (B) cell lines induced by 48-hour exposure to quinalizarin at concentrations of 25–100 µM. Increased mitotic changes expressed by the presence of abnormal mitotic figures, micro- and macronucleation. Quantitative changes in mitotic indices (cells with micronuclei, bi-, multinucleated cells and giant cells) after exposure to quinalizarin at concentrations of 25–100 µM (C). Differences were statistically confirmed at: *p < 0.05; **p < 0.01; ***p < 0.001. Symbol explanation: Statistically significant change (p < 0.01, symbol † and p < 0.0001, symbol #) in the number of multinucleated, binucleated, micronucleated and giant cells of the HeLa line compared to the number of the above mitotic catastrophe indicators in the DU145 cell line group loaded with quinalizarin in the concentration range of 25–100 µM. 1—interphase cells, 2—binucleated cells, 3—apoptotic cells, 4—cells with cytoplasmic vacuolation, 5—multinucleated cells, 6—cells with micronuclei, 7—giant cells, 8—chromosome bridges, 9—multipolar metaphase (bipolar, tripolar), 10—multipolar anaphase, 11—chromosome disorientation in metaphase, 12—cytoskeletal disorganization, 13—abnormal chromosome segregation, 14—apoptosis of binucleated cells, 15—apoptosis of multinucleated cells, 16—apoptosis of giant cells. Hematoxylin and eosin staining. Images were taken at 400 × magnification.
The obtained results indicate the induction of changes in the mitotic apparatus of the studied cells by quinalizarin.
Discussion
Despite advances in cancer treatment associated with the development of numerous therapies, including targeted chemotherapy, systemic chemotherapy is now the first-line treatment for many types of cancer. Often, however, after an initial response to treatment, patients become refractory to further therapy, promoting progressive tumor progression27,28. Factors influencing the development of drug resistance include incorrect dosage of cytostatics, changes in the rate of their entry into the cell, as well as changes in transport along the cell nucleus-cytoplasm pathway. Also of great importance is the ability of cancer cells to avoid the process of apoptosis associated with the overexpression of anti-apoptotic proteins and the inhibition or interference with the production of pro-apoptotic proteins. The ability of tumor cells to alter DNA repair processes should also be emphasized here, as well as the ability to actively remove cytostatic agents from the cell, involving membrane transport proteins29–31.
Currently, it is believed that chemotherapy resistance is associated with the presence of cancer stem cells, a subpopulation of cells in the tumor mass responsible for cancer initiation as well as metastasis formation27. Conventional cancer treatment methods target most cancers and are unable to target stem cells due to their high resistance, leading to metastasis and cancer relapse32. To reduce cancer mortality and morbidity, existing treatments are constantly being improved, but new, more effective anti-cancer therapies continue to be sought. Numerous studies have shown that a wide variety of natural compounds exhibit anticancer potential against various human cancer cells, which is mainly related to apoptosis induction, cell cycle modulation and antiproliferative effects33.
Interesting groups of compounds exhibiting numerous pharmacological properties include anthraquinones known for their anti-inflammatory activity, immunomodulatory34, antibacterial, antifungal35, antiviral36, hepatoprotective37, neuroprotective38, antioxidant39, and anticancer effects. The interest in these compounds also stems from the fact that many derivatives of anthraquinones are used in medicine. Such an example are the anthracyclines, whose chemical structure is based on the anthraquinone skeleton. Among the anthracyclines with a well-established position in oncology treatment, the most noteworthy include doxorubicin, daunorubicin, epirubicin, idarubicin40. Another example of anthraquinones used in medicine is diacerein, the active metabolite of which is rhein, which also has anti-cancer activity41. Diacerein is an anti-inflammatory drug currently used to treat osteoarthritis42.
Hence, in recent years, the number of studies has increased not only on natural anthraquinones isolated from plants43–50, but also on new, synthesized anthraquinone-based compounds that may become promising anticancer agents.
An anthraquinone deserving special attention is quinalizarin, whose mechanism of action is still not fully understood, especially with regard to the prostate cancer cells and cervical cancer cells that were the subject of our study. The results obtained (Figs. 1, 2, 3, 4, 5, 6, 7 and 8) may suggest that quinalizarin shows its anticancer potential through modulating effects on apoptotic processes and alternative types of cell death such as autophagy or mitotic catastrophe.
The changes seen in the cells studied in Fig. 6 indicate that quinalizarin induces apoptosis in both HeLa and DU145 line cells. In morphological analysis, characteristic changes such as chromatin aggregation, shrinking and thickening of the cytoplasm, and formation of apoptotic bodies were observed. However, at the highest concentration (100 µM) of quinalizarin, apoptotic cells constituted over 80% of all analyzed cells in both HeLa and DU145 lines. Quinalizarin-induced apoptosis in the studied cells is associated with the mitochondrial pathway, as evidenced by mitochondrial dysfunction confirmed in the MTT reduction test and mitochondrial membrane potential analysis (Fig. 1). The mechanism of quinalizarin’s proapoptotic effect may also be linked to the demonstrated activation of executive caspases 3/7, with simultaneous reduction of the expression of the antiapoptotic protein Bcl-2 (Fig. 2).
It is also worth noting that apoptosis is one of the main targets of action of numerous chemotherapeutics and is the desired death in anticancer therapy51, especially due to the lack of inflammation that arises in the necrosis process. Numerous papers show that most traditional anticancer drugs rely on BCL-2/BAX-dependent mechanisms to eliminate cancer cells52. However, this often leads to treatment failure, which is caused by a disrupted or altered mechanism of their action leading to chemoresistance. Additionally, due to defects in the apoptotic pathway in cancer cells consisting of, among others, overexpression of antiapoptotic proteins, underexpression of proapoptotic proteins, or reduced caspase function, the threshold for chemotherapy or radiotherapy is raised53,54. This leads to resistance to the therapies used, and disrupted apoptosis signaling pathways also promote resistance in the immune system55.
One of the alternative mechanisms of cell death that can counteract the phenomenon of chemoresistance is mitotic catastrophe, which is responsible for the elimination of cells that are not suitable for proper mitosis. In this process, apoptosis, necrosis, autophagy, and senescence are used to prevent proliferation of defective cells56. The induction of mitotic catastrophe is caused by extensive DNA damage, improper functioning of the mitotic machinery, and defective functioning of cell cycle checkpoints. Cells undergoing mitotic catastrophe are characterized by unique changes in the cell nucleus such as macro-, micro-, and multinucleation, which are a consequence of chromosome dispersion and breakage and karyokinesis disorders in metaphase57,58. The morphological image also shows abnormal mitotic Fig. 59.
Mitotic catastrophe of cancer cells is very important from the level of oncological treatment, because when induced by drugs, it is characterized by low dosage and, consequently, low toxicity60. This represents an important advantage in cancer treatment, as high concentrations of chemotherapeutics are often necessary for cancer cell apoptosis to occur. Therefore, the implementation of anticancer drugs in oncological therapy that induce mitotic death of cancer cells may be a new strategy for anticancer therapy60. Mitotic catastrophe was also observed in our studies, which occurred mainly at low concentrations of quinalizarin. At 25 and 50 µM, an increased number of mitotic catastrophe indicators were noted, i.e. bi- and multinucleated cells, giant cells and cells with micronuclei, among which binucleated cells predominated. Abnormal mitotic figures characteristic of mitotic catastrophe were also observed (Fig. 8). According to literature data, the above-mentioned cells are then most often directed towards apoptotic, necrotic death, and recently it was discovered that they also lead to autophagic death57. We also showed that the observed markers of mitotic death underwent apoptosis, as evidenced by the observed reduced number of them in favor of an increase in the number of apoptotic cells, especially at concentrations of 75 and 100 µM. We also observed mitotic death in HeLa cells as a result of the action of other anthraquinones such as emodin or aloe-emodin47,49. The increased levels of mitotic catastrophe may be explained by the ability to intercalate with nuclear DNA, which is caused by the flat structure of anthraquinones that allows them to penetrate the DNA helix61.
Currently, in the clinical trial phase and in oncological therapies, chemotherapeutics are used in concentrations that induce not only apoptosis (regardless of the cell cycle phase), but also, as already mentioned, are very effective in causing mitotic catastrophe at lower doses, which significantly reduces the side effects of therapy62. A good example is eribulin used in the treatment of breast cancer, the mechanism of action of which is based on the inhibition of microtubule instability in interphase cells, which results in the inhibition of the cell cycle and the induction of mitotic death63.
In addition to the induction of apoptosis, quinalizarin also shows its potential anticancer properties through antiproliferative and antimigratory activities (wound healing assay), which are very important in inhibiting metastases. Among the antiproliferative pathways, attention is paid to the antimitotic activity, which allows avoiding abnormal growth of cancer cells by disrupting continuous mitotic divisions64. Our studies show that quinalizarin is a strong inhibitor of cell division, because with increasing concentration, a decrease in the mitotic index was observed, both for HeLa and DU145 cell lines (Fig. 7). In addition to quinalizarin, this effect is also demonstrated by other anthraquinone derivatives, such as the aforementioned anthracycline antibiotics, or other plant chemotherapeutics such as vinca alkaloids (vinblastine, vincristine)64. Such effects are also demonstrated by taxanes (paclitaxel, docetaxel) and their derivatives, including cabazitaxel, which, as already mentioned, is approved for the treatment of hormone-refractory metastatic prostate cancer in patients previously treated with docetaxel65. On the other hand, the cytometric method showed a significant blocking of cell divisions in the G0/G1 phase. Numerous works also show that in the above-mentioned phase of the cell cycle, cancer cells are blocked after the action of various plant compounds, including: panduratin A (bioactive cyclohexanylchalcone)66, castricin-flavonoid extracted from Vitex rotundifolia L67, orientin, a luteolin analogue isolated from rooibos and tulsi leaves68, or ligustrazine, an active ingredient extracted from Ligusticum Chuanxiong Hort69.
Paying attention to the mechanisms of cell cycle regulation is very important because they respond to both intracellular stress factors, such as incomplete replication, and external stress factors, which include DNA damaging factors70. Checkpoints respond to specific forms of stress contributing to cell cycle arrest at a specific stage. Hence, factors such as DNA damage and antiproliferative signals in the G1 phase induce cycle arrest in the G1 phase before entering the S phase. Stress or DNA damage in the S phase affects the replication forks and slows down the progression of the aforementioned phase by activating the checkpoint. DNA damage in the G2 phase or the lack of complete decatenation of chromosomes can induce phase arrest, blocking entry into mitosis70–73. The effect of quinalizarin on the cytoskeleton and microtubules of the tested cells was also confirmed in submicroscopic analysis, where numerous Golgi apparatus vesicles were shown to be dispersed in the cytoplasm as a result of the action of the tested anthraquinone. This is due to the fact that microtubules are involved in the organization and positioning of the Golgi apparatus in the cell, and its membranes have mechanisms necessary for their nucleation, regulation and stabilization, thus acting as a secondary microtubule organizing center (MTOC)74,75. It should be noted that the above changes in the Golgi apparatus were also observed as a result of the action of emodin50, aloe-emodin47, i.e. anthraquinones with high therapeutic potential and possibilities of use in oncological treatment.
In our studies, we have shown that the multidirectional action of quinalizarin also consisted in inducing autophagic processes, which, like apoptosis, depended on the applied concentration. The confirmation of autophagy induced by quinalizarin was the presence of numerous autophagolysosomes formed as a result of the fusion of autophagosomes with primary lysosomes and the modulation of the LC3-II protein level. Comparing the effects of quinalizarin on HeLa and DU145 cell lines, we showed that greater degradative changes occurred in DU145 cells, as expressed, among other things, by the presence of a large number of autophagolysosomes and secondary lysosomes (Fig. 4). Submicroscopic analysis shows that quinalizarin-induced autophagy in DU145 cells was more selective, as it mainly involved vacuolar degradation of mitochondria. This could be explained by the quinalizarin-induced oxidative stress occurring within the mitochondria.
Autophagy is a process that maintains homeostasis not only in normal cells, but also in cancer cells, which occurs under physiological conditions and stress induced by chemotherapy or radiotherapy. It is currently believed that role of autophagy in cancer treatment is still controversial, because numerous studies show that both its stimulation and inhibition contribute to the enhancement of the effect of anticancer drugs76,77. Depending on the function that autophagy plays in cancer treatment, we distinguish cytoprotective, ensuring the survival of cancer cells, which can also protect normal cells from damage caused by anticancer drugs and reduce the side effects of their action76. Autophagy can also induce cell death by performing a cytotoxic function (which often accompanies apoptosis) and perform a cytostatic function, inhibiting cell growth76,77. Our studies also show that the progressive increase in the number of cells with cytoplasmic vacuolation at the highest concentrations (75–100 µM) was reduced in favor of the increase in the number of apoptotic cells. This indicates that quinalizarin has strong cytotoxic properties against the tested cell lines with the ability to switch autophagy into apoptosis, which may be its full mechanism of action.
In addition, we used chloroquine, an autophagy inhibitor whose mechanism of action is to block the fusion of lysosomes with autophagosomes78. We showed that in the tested cells exposed to quinalizarin at a concentration of 50 µM (48-hour incubation), there was a significant increase in the level of LC3-II protein expression, which was higher compared to the effect of chloroquine (100 µM). On the other hand, the combination of quinalizarin with chloroquine significantly reduced the level of this protein (Fig. 5). This change correlated with a significant increase in apoptotic cells, the level of which was comparable to that observed in the highest concentration of the tested anthraquinone (100 µM). This was confirmed by the analysis of morphological changes in cells, according to which the result of exposure to the combined action of quinalizarin and chloroquine was the accumulation of vacuoles (24 h) and an increase in the number of apoptotic cells as a result of extending the incubation time to 48 h.
According to literature data, inhibition of autophagic processes by using inhibitors contributes to an increase in the potency of chemotherapeutics, and the consequence of their combined action is an increase in the level of apoptosis, which is the main goal of anticancer therapy79,80.
Conclusions
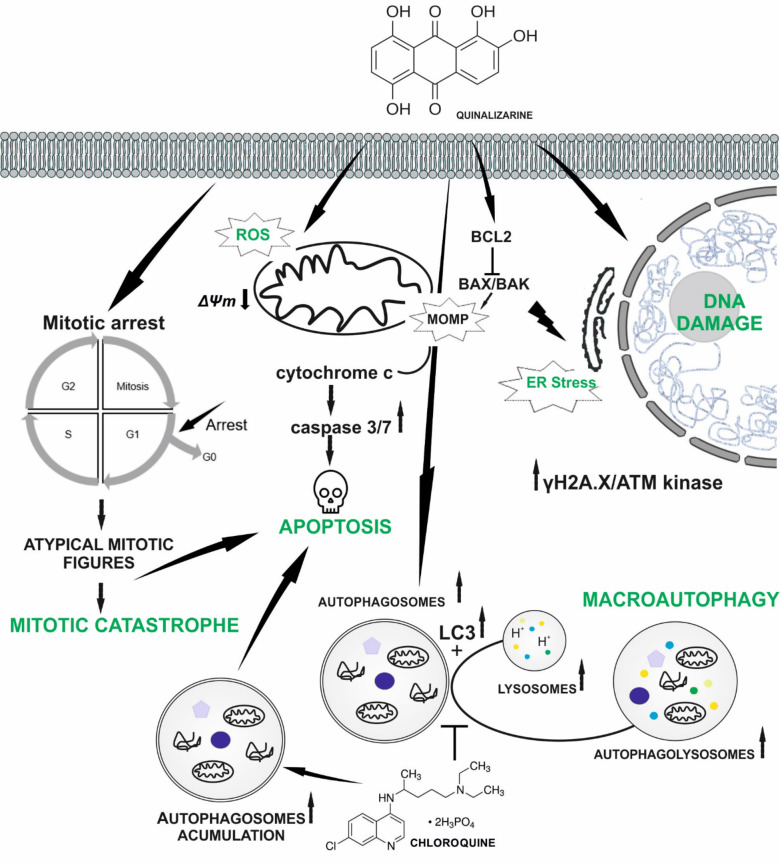
The presented studies show that quinalizarin is an anthraquinone with a multidirectional mechanism of action by inducing many types of cell death (Fig. 9), which can be used in anticancer therapy. However, further analysis of the effect of quinalizarin on other cancer cell lines is necessary.
Fig. 9.
Summary of the potential anticancer mechanism of quinalizarin against HeLa and DU145 cells. Quinalizarin induces apoptosis, autophagy, and mitotic catastrophe dependent on the applied concentration and time of action. It also inhibits cell migration and induces cell cycle arrest.
Materials and methods
In vitro culture conditions
HeLa (cervical cancer) and DU145 (prostate cancer) cells were purchased from ATCC (Rockville, MD, USA). Cells were cultured in DMEM medium (GIBCO, New York, USA) with 10% fetal bovine serum (Biowest, Nuaillé, France) and a mixture of antibiotics (amphotericin B, penicillin G, streptomycin) (Corning, Manassas, USA) in a DirectHeat CO2 incubator (Thermo Fisher Scientific, Waltham, MA, USA). Cells were incubated for 48 h with quinalizarin (C14H8O6) (Sigma-Aldrich, St. Louis, MO, USA) at concentrations of 25 µM, 50 µM, 75 µM and 100 µM.
Cell viability assessment-MTT test and FDA/PI staining
The principle of the test is based on the reduction of the yellow dye MTT to purple formazan crystals by mitochondrial dehydrogenase in living cells. After 48 h of incubation with quinalizarin, cells were incubated for 2 h with MTT solution (1 mg/ml) (3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide) (Sigma Aldrich, St. Louis, MO, USA) in 96-well plates (Falcon). The resulting formazan crystals were dissolved in DMSO (Sigma Aldrich, St. Louis, MO, USA), and the absorbance was read using a Synergy 2 multidetector microplate reader (BioTek, Winooski, VT, USA) at 570 nm.
In addition, cell viability was analyzed by fluorescence microscopy using double staining with fluorescein diacetate and propidium iodide (FDA/PI). For this purpose, cells (2 × 104) after 48-hour incubation with quinalizarin at a concentration of 100 µM were trypsinized, and the resulting suspension was subjected to centrifugation. The cell pellet was then subjected to double staining using fluorescein diacetate (1 mg/ml) and propidium iodide (1 mg/ml) (Sigma-Aldrich, St. Louis, MO, USA). The stained cells were applied to a basic slide and analyzed microscopically using a Nikon 80i epi-fluorescence microscope (Nikon Instruments, USA). The analysis was carried out on the basis of fluorescence color change-red cells (dead cells) and green fluorescence (live cells). According to the principle of the assay, fluorescein diacetate stains only living cells as a result of the conversion of fluorescein diacetate to fluorescein, while dead cells undergo staining with propidium iodide as a result of fluorochrome entering the cell through a damaged membrane and binding to DNA. The number of stained cells was counted in 30 random fields using a 20× objective, and then average values were drawn from the results. The experiment was repeated 3 times.
Apoptosis detection
The percentage of apoptotic cells was assessed using Annexin V Dead Cell Kit (Merck KGaA, Darmstadt, Germany). After 48 h of incubation with the test compound, cells were trypsinized using 0.25% trypsin-EDTA solution (Corning, Manassas, USA), followed by centrifugation and staining for 20 min in the dark at room temperature by adding 100 µl of Annexin V-PE/7-AAD. The fluorescence intensity was read on a Muse analyzer (Merck-Millipore, USA). The experiment was repeated 3 times.
Caspase 3/7 activity assay
The activity level of executive caspases was assessed with the Muse Caspase-3/7 Kit (Merck-Millipore, Guyancourt, France). After 48 h of incubation with quinalizarin, cells were trypsinized, centrifuged, and the resulting cell pellet was incubated for 30 min at 370C with 5 µl of Caspase-3/7 working solution. The percentage of caspase-positive cells was determined using the Muse analyzer (Merck-Millipore, Guyancourt, France). The experiment was repeated 3 times.
Assessment of Bcl-2 protein phosphorylation
Changes in Bcl-2 phosphorylation in HeLa and DU145 cells were assessed using the Muse Bcl-2 Activation Dual Detection Kit (Merck-Millipore, Guyancourt, France). Two directly conjugated antibodies, i.e. phospho-phospho-Bcl-2 (Ser70)-Alexa Fluor®555 and anti-Bcl-2-PECy5, were used to assess the measurement of total Bcl-2 expression levels. The degree of activation of the Bcl-2 pathway was assessed by measuring Bcl-2 phosphorylation relative to total Bcl-2 expression in the cells studied.
DNA damage assessment
After the treatment, cells were trypsinized, fixed and permeabilized using the Muse Multi-Color DNA Damage kit (Merck-Millipore, Guyancourt, France) to assess the percentage of double-stranded DNA breaks (double activation of H2A.X and ATM). Then, cells were stained with anti-phospho-Histone H2A.X (Ser139) and anti-phospho-ATM (Ser1981). The percentage of negative cells (no DNA damage), the percentage of cells with activated: ATM, H2A.X and with double DNA breaks (double activation of ATM and H2A.X) were determined using the analyzer software module and the results were presented as scatter plots.
Measurement of reactive oxygen species production
Cells after 48 h of treatment with the test compound were evaluated by determining the percentage of cells undergoing oxidative stress using the Muse Oxidative Stress Kit (Merck Millipore Guyancourt, France). Cells were treated with Muse Oxidative Stress Reagent working solution (190 µl) and then incubated for 30 min at 37 °C. The percentage of ROS (-) and ROS (+) cells was determined by cytometric analysis. The experiment was repeated 3 times.
Mitochondrial membrane potential (Δψm) measurement
The reduction in Δψm was analyzed using the Muse Mitopotential Assay kit (Merck Millipore, Guyancourt, France). Cells after quinalizarin treatment were suspended in MitoPotential working solution and incubated for 20 min (37 °C). After incubation, cells were stained with 5 µl of 7-AAD at room temperature for 5 min and analyzed on the analyzer. The experiment was repeated 3 times.
Evaluation of ultrastructural changes
Cells were fixed using 3% glutaraldehyde in 0.1 M cacodyl buffer, pH = 7.3 (Serva Electrophoresis GmbH, Germany). Secondary fixation was carried out in 2% osmium tetroxide (Spi, West Chester, PA, USA). The cells were then dehydrated in ethanol (concentration range 10-99.8%) and embedded in Epon 812 epoxy resin (Serva Electrophoresis GmbH, Germany). Polymerization was carried out successively at 40 °C and 60 °C. Ultrathin sections were cut on a Leica EM UC7 ultramicrotome (Leica Biosystems, Germany) and further contrasted with uranyl acetate and lead citrate. The cells were analyzed using a Tecnai G2 Spirit transmission electron microscope (FEI, Company USA) equipped with a Morada camera (Olympus, Soft Imagine Solutions, Münster). In addition, mitochondria were quantified and measured using TEM Imaging & Analysis 3.2 SP6 software (FEI Company, Hillsboro, OR, USA). The change in the size of mitochondria was demonstrated by measuring the organelles in 100 cells from the control group and the groups treated with different concentrations of quinalizarin. Mean values were calculated from the results.
LC3-II antibody detection
The LC3 antibody detection assay (Merck Millipore) is used to assess autophagy levels. In the assay, the cytosolic form of LC3 (LC3-I) is conjugated with phosphatidylethanolamine to form an LC3-phosphatidylethanolamine (LC3-II) conjugate that is recruited to autophagosomal membranes. LC3-II is degraded in the lumen of lysosomes. Turnover of the lysosomal autophagosomal marker LC3-II reflects autophagic activity, with levels increasing following induction of autophagosome formation but then decreasing following fusion of autophagosomes with lysosomes.
Cells plated in 96-well plates were incubated for 48 h with quinalizarin at concentrations of 25, 50, 75 and 100 µM. Then, the selective membrane permeabilization reagent (Autophagy Reagent A) in Earle’s salt solution (EBSS medium) was added to the cells and incubated for 4 h. This reagent allows for the discrimination of cytosolic and autophagic LC3, which is possible by extracting cytosolic protein while protecting LC3-II, which is translocated to autophagosomes and remains intact there. Then, the cells were washed with Hank’s salt solution (HBSS), trypsinized and centrifuged. The supernatant was removed, anti-LC3 Alexa Fluor® 555 and Autophagy Reagent B were added to the cells and incubated in the dark on ice for 30 min. Samples were then centrifuged and analyzed by flow cytometry. Positive controls included cells incubated for 4 h in serum-free medium. The experiment was performed in triplicate.
Assessment of the effects of autophagy inhibition using chloroquine inhibitor
The assessment of the intensity of apoptotic processes in the studied cells induced by quinalizarin was performed by inhibiting autophagy using a strong inhibitor, chloroquine (Sigma-Aldrich, St. Louis, MO, USA). For this purpose, cells were preincubated with chloroquine (100 µM) for 5 h, then incubated for 48 h with quinalizarin at a concentration of 50 µM. At the same time, cells were subjected to the combined action of chloroquine (100 µM) and quinalizarin (50 µM). Then, the level of LC3-II protein expression and the level of apoptosis were assessed (Annexin V-PE/7 AAD staining). Additionally, vacuolization induced by the tested compounds was assessed using a Nikon Eclipse Ti (Nikon Instruments Inc.) inverted microscope equipped with phase contrast and a cell culture system (Okolab) consisting of an incubation chamber, a humidity module and a gas mixer. The presence of vacuoles in the cytoplasm of cells was monitored after 24 and 48 h of incubation with the tested compounds.
Morphological changes assessment
Cells cultured on sterile coverslips in dishes (Falcon) were fixed in methanol, stained with Harris hematoxylin and eosin (Sigma Aldrich, St. Louis, MO, USA), dehydrated in an ascending alcohol series, and exposed in xylene. Morphological analysis was performed using a Nikon Eclipse 80i microscope with Nikon NIS Elements D 3.10 software (Nikon Instruments, Inc., NY, USA). In preparations, 3,000 cells were analyzed in three independent experiments (9,000 cells/concentration). The mitotic index was assessed by determining the number of cells in each phase of mitotic division for each concentration, and the results were expressed as a percentage. Mitotic catastrophe was determined based on morphological indices such as giant cells, multinucleated cells, cells with micronuclei, and the presence of abnormal mitotic figures.
Cell cycle analysis
Cells incubated for 48 h with quinalizarin were fixed in ice-cold 70% ethanol, followed by a cell cycle assay (Merck-Millipore, Guyancourt, France), which uses a reagent containing nuclear DNA intercalating dye, propidium iodide (PI) and RNAase A in the manufacturer’s proprietary formula. After the staining procedure, cells were analyzed using a Muse analyzer (Merck-Millipore, Guyancourt, France), determining the percentage of cells in each phase of the cycle.
Cell migration assay
The ability of quinalizarin to inhibit cell migration was assessed using a scratch wound healing assay. Cells were plated in a 6-well plate with DMEM containing 10% FBS and incubated for 24 h to form a monolayer. A vertical cut was made in each well of the plate with a sterile pipette tip with a volume of 10 µL. The wells were then washed with PBS to remove cell debris and quinalizarin was added at concentrations of 25–100 µM. The procedure was repeated in a well with control cells. After 24 and 48 h of incubation, the plate was analyzed using a Nikon Eclipse Ti inverted microscope (Nikon Instruments Inc.) equipped with phase contrast and a cell culture system (Okolab) consisting of an incubation chamber, a humidity module and a gas mixer. To determine the area of cell migration (wound area) in the control and quinalizarin-encumbranced cell groups, measurements (distance between wound edges) were taken at each time point (24 and 48 h) using a Nikon NiS Elements digital image analysis system (Nikon Instruments Inc.). The results were presented as mean values.
Statistical analysis
Analysis of results was performed using one-way analysis of variance (ANOVA), with post-hoc multiple comparisons using the Tukey test. P < 0.05 was considered statistically significant. Statistica 13.3 software (StatSoft, Poland) was used for data analysis.
Acknowledgements
The study was financed by the research project of Jan Kochanowski University of Kielce, No. SUPB.RN.23.249. This work was co-financed by the Minister of Science (Poland) under the "Regional Excellence Initiative" program (project no.: RID/SP/0015/2024/01). We would like to thank Professor Anna Lankoff from Laboratory of Cytogenetics and Genotoxicology, Centre for Radiobiology and Biological Dosimetry, Warsaw, Poland for making the cell lines available for research.
Author contributions
Wojciech Trybus: conceptualization; data collection; formal analysis; validation; methodology; visualization; writing—original draft; writing—review & editing, project administration; funding acquisition; investigation; visualisation; supervision. Ewa Trybus: conceptualization; data collection; formal analysis; research; writing—original draft; writing—review & editing; funding acquisition; investigation; methodology; Teodora Król: conceptualization (supporting); formal analysis; writing—review & editing; funding acquisition. Mateusz Obarzanowski: writing—review & editing; data collection.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. Corresponding author WT has to be contacted in case of any queries or requirement of data.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Wojciech Trybus, Email: wojciech.trybus@ujk.edu.pl.
Ewa Trybus, Email: ewa.trybus@ujk.edu.pl.
References
- 1.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71, 209–249. 10.3322/caac.21660 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Saranyutanon, S., Srivastava, S. K., Pai, S., Singh, S. & Singh, A. P. Therapies targeted to androgen receptor signaling axis in prostate cancer: Progress, challenges, and hope. Cancers (Basel). 12, 51. 10.3390/cancers12010051 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rawla, P. Epidemiology of prostate cancer. World J. Oncol.10, 63–89. 10.14740/wjon1191 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahnassy, A. A., Abdellateif, M. S. & Zekri, A. N. Cancer in Africa: is it a genetic or environmental health problem? Front. Oncol.10, 604214. 10.3389/fonc.2020.604214 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinata, N. & Fujisawa, M. Racial differences in prostate cancer characteristics and cancer-specific mortality: an overview. World J. Mens Health. 40, 217–227. 10.5534/wjmh.210070 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berenguer, C. V., Pereira, F., Camara, J. S. & Pereira, J. A. M. Underlying features of prostate cancer-statistics, risk factors, and emerging methods for its diagnosis. Curr. Oncol.30, 2300–2321. 10.3390/curroncol30020178 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergengren, O. et al. Update on prostate cancer epidemiology and risk factors—A systematic review. Eur Urol84, 191–206. 10.1016/j.eururo.2023.04.021 (2023). [DOI] [PMC free article] [PubMed]
- 8.Messina, C. et al. BRCA mutations in prostate cancer: prognostic and predictive implications. J. Oncol.2020, 4986365. 10.1155/2020/4986365 (2020). [DOI] [PMC free article] [PubMed]
- 9.Bach, C. et al. The status of surgery in the management of high-risk prostate cancer. Nat. Rev. Urol.11, 342–351. 10.1038/nrurol.2014.100 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Sekhoacha, M. et al. Prostate cancer review: Genetics, diagnosis, treatment options, and alternative approaches. Molecules27, 730. 10.3390/molecules27175730 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boon, S. S., Luk, H. Y., Xiao, C., Chen, Z. & Chan, P. K. Review of the standard and advanced screening, staging systems and treatment modalities for cervical cancer. Cancers (Basel). 14, 913. 10.3390/cancers14122913 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castle, P. E., Einstein, M. H. & Sahasrabuddhe, V. V. Cervical cancer prevention and control in women living with human immunodeficiency virus. CA Cancer J. Clin.71, 505–526. 10.3322/caac.21696 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okunade, K. S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol.40, 602–608. 10.1080/01443615.2019.1634030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pingping, S. J. et al. Clinical significance of extended high-risk human papillomavirus genotyping and viral load in cervical cancer and precancerous lesions. Gynecol. Obstet. Clin. Med.3, 22–29. 10.1016/j.gocm.2023.01.001 (2023). [Google Scholar]
- 15.Hull, R. et al. Cervical cancer in low and middle-income countries. Oncol. Lett.20, 2058–2074. 10.3892/ol.2020.11754 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou, Z. W. et al. Therapeutic effects of natural products on cervical cancer: based on inflammatory pathways. Front. Pharmacol.13, 899208. 10.3389/fphar.2022.899208 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chuang, L. T. et al. Management and care of women with invasive cervical cancer: American society of clinical oncology resource-stratified clinical practice guideline. J. Glob Oncol.2, 311–340. 10.1200/JGO.2016.003954 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burmeister, C. A. et al. Cervical cancer therapies: current challenges and future perspectives. Tumour Virus Res.13, 200238. 10.1016/j.tvr.2022.200238 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Regalado Porras, G. O., Nogueda, C., Poitevin Chacon, A. & J. & Chemotherapy and molecular therapy in cervical cancer. Rep. Pract. Oncol. Radiother. 23, 533–539. 10.1016/j.rpor.2018.09.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsvetkova, D. & Ivanova, S. Application of approved cisplatin derivatives in combination therapy against different cancer diseases. Molecules27, 466. 10.3390/molecules27082466 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nader, R., El Amm, J. & Aragon-Ching, J. B. Role of chemotherapy in prostate cancer. Asian J. Androl.20, 221–229. 10.4103/aja.aja_40_17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand, U. et al. Cancer chemotherapy and beyond: current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis.10, 1367–1401. 10.1016/j.gendis.2022.02.007 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abidi, A. & Cabazitaxel A novel taxane for metastatic castration-resistant prostate cancer-current implications and future prospects. J. Pharmacol. Pharmacother. 4, 230–237. 10.4103/0976-500X.119704 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Recine, F. & Sternberg, C. N. Hormonal therapy and chemotherapy in hormone-naive and castration resistant prostate cancer. Transl Androl. Urol.4, 355–364. 10.3978/j.issn.2223-4683.2015.04.11 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghosh, S., Hazra, J., Pal, K., Nelson, V. K. & Pal, M. Prostate cancer: therapeutic prospect with herbal medicine. Curr. Res. Pharmacol. Drug Discov. 2, 100034. 10.1016/j.crphar.2021.100034 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik, M. S. et al. Journey of anthraquinones as anticancer agents—a systematic review of recent literature. RSC Adv.11, 35806–35827. 10.1039/d1ra05686g (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaggianesi, M. et al. Messing up the cancer stem cell chemoresistance mechanisms supported by tumor microenvironment. Front. Oncol.11, 702642. 10.3389/fonc.2021.702642 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, X., Zhang, H. & Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist.2, 141–160. 10.20517/cdr.2019.10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duan, C. et al. Overcoming cancer multi-drug resistance (MDR): reasons, mechanisms, nanotherapeutic solutions, and challenges. Biomed. Pharmacother. 162, 114643. 10.1016/j.biopha.2023.114643 (2023). [DOI] [PubMed] [Google Scholar]
- 30.Talib, W. H., Alsayed, A. R., Barakat, M., Abu-Taha, M. I. & Mahmod A. I. Targeting drug chemo-resistance in cancer using natural products. Biomedicines9, 353. 10.3390/biomedicines9101353 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan, S. U., Fatima, K., Aisha, S. & Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell. Commun. Signal.22, 109. 10.1186/s12964-023-01302-1 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ayob, A. Z. & Ramasamy, T. S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci.25, 20. 10.1186/s12929-018-0426-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry, G. E., Md Akim, A., Sung, Y. Y. & Sifzizul, T. M. T. Cancer and apoptosis: the apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Front. Pharmacol.13, 842376. 10.3389/fphar.2022.842376 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin, D., Li, H., Zhou, S., Zhong, H. & Pu, W. Effects of anthraquinones on immune responses and inflammatory diseases. Molecules27. 10.3390/molecules27123831 (2022). [DOI] [PMC free article] [PubMed]
- 35.Raghuveer, D. P., Murali, V. V. & Nayak, T. S. Exploring anthraquinones as antibacterial and antifungal agents. ChemistrySelect8, 1–16 (2023). [Google Scholar]
- 36.Das, S., Singh, A. & Samanta, S. K. Naturally occurring anthraquinones as potential inhibitors of SARS-CoV-2 main protease: an integrated computational study. Biol. (Bratisl). 77, 1121–1134. 10.1007/s11756-021-01004-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruan, L. et al. Hepatotoxicity or hepatoprotection of emodin? Two sides of the same coin by (1)H-NMR metabolomics profiling. Toxicol. Appl. Pharmacol.431, 115734. 10.1016/j.taap.2021.115734 (2021). [DOI] [PubMed] [Google Scholar]
- 38.Li, X., Chu, S., Liu, Y. & Chen, N. Neuroprotective effects of anthraquinones from rhubarb in central nervous system diseases. Evid. Based Complement. Altern. Med.2019, 3790728. 10.1155/2019/3790728 (2019). [DOI] [PMC free article] [PubMed]
- 39.Trung, N. Q. et al. Radical scavenging activity of natural anthraquinones: a theoretical insight. ACS Omega. 6, 13391–13397. 10.1021/acsomega.1c01448 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marinello, J., Delcuratolo, M. & Capranico, G. Anthracyclines as topoisomerase II poisons: from early studies to new perspectives. Int. J. Mol. Sci.19, 480. 10.3390/ijms19113480 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trybus, W., Krol, T. & Trybus, E. Rhein induces changes in the lysosomal compartment of HeLa cells. J. Cell. Biochem.123, 1506–1524. 10.1002/jcb.30311 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Almezgagi, M. et al. Recent insight into pharmacological activities and molecular pathways. Biomed. Pharmacother. 131, 110594. 10.1016/j.biopha.2020.110594 (2020). Diacerein. [DOI] [PubMed] [Google Scholar]
- 43.Watroly, M. N. et al. Chemistry, biosynthesis, physicochemical and biological properties of rubiadin: a promising natural anthraquinone for new drug discovery and development. Drug Des. Dev. Ther.15, 4527–4549. 10.2147/DDDT.S338548 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trybus, W. et al. Aloe-emodin influence on the lysosomal compartment of hela cells. Asian Pac. J. Cancer Prev.18, 3273–3279. 10.22034/APJCP.2017.18.12.3273 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trybus, W. et al. Changes in the lysosomal system of cervical cancer cells induced by emodin action. Anticancer Res.37, 6087–6096. 10.21873/anticanres.12057 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Trybus, W., Krol, T., Trybus, E. & Stachurska, A. Physcion induces potential anticancer effects in cervical cancer cells. Cells10, 29. 10.3390/cells10082029 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trybus, W. et al. Induction of mitotic catastrophe in human cervical cancer cells after administration of aloe-emodin. Anticancer Res.38, 2037–2044. 10.21873/anticanres.12443 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Trybus, W., Krol, T., Trybus, E., Stachurska, A. & Krol, G. The potential antitumor effect of chrysophanol in relation to cervical cancer cells. J. Cell. Biochem.122, 639–652. 10.1002/jcb.29891 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Trybus, W. et al. Emodin induces death in human cervical cancer cells through mitotic catastrophe. Anticancer Res.39, 679–686. 10.21873/anticanres.13163 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Trybus, W., Trybus, E. & Krol, T. Emodin sensitizes cervical cancer cells to vinblastine by inducing apoptosis and mitotic death. Int. J. Mol. Sci.23, 510. 10.3390/ijms23158510 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carneiro, B. A. & El-Deiry, W. S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol.17, 395–417. 10.1038/s41571-020-0341-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yip, K. W. & Reed, J. C. Bcl-2 family proteins and cancer. Oncogene27, 6398–6406. 10.1038/onc.2008.307 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Pfeffer, C. M., Singh, A. T. K. & Apoptosis A target for anticancer therapy. Int. J. Mol. Sci.19, 448. 10.3390/ijms19020448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong, R. S. Apoptosis in cancer: from pathogenesis to treatment. J. Exp. Clin. Cancer Res.30, 87. 10.1186/1756-9966-30-87 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassan, M., Watari, H., AbuAlmaaty, A., Ohba, Y. & Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed. Res. Int.2014, 150845. 10.1155/2014/150845 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Mc Gee, M. M. Targeting the mitotic catastrophe signaling pathway in cancer. Mediat. Inflamm. 146282. 10.1155/2015/146282 (2015). [DOI] [PMC free article] [PubMed]
- 57.Sazonova, E. V., Petrichuk, S. V., Kopeina, G. S. & Zhivotovsky, B. A link between mitotic defects and mitotic catastrophe: detection and cell fate. Biol. Direct. 16, 25. 10.1186/s13062-021-00313-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vitale, I., Galluzzi, L., Castedo, M. & Kroemer, G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell. Biol.12, 385–392. 10.1038/nrm3115 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Roninson, I. B., Broude, E. V. & Chang, B. D. If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells. Drug Resist. Updat. 4, 303–313. 10.1054/drup.2001.0213 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Li, H. et al. Mitotic catastrophe and p53-dependent senescence induction in T-cell malignancies exposed to nonlethal dosage of GL-V9. Arch. Toxicol.94, 305–323. 10.1007/s00204-019-02623-2 (2020). [DOI] [PubMed] [Google Scholar]
- 61.Qiao, C. et al. Study of interactions of anthraquinones with DNA using ethidium bromide as a fluorescence probe. Spectrochim Acta Mol. Biomol. Spectrosc.70, 136–143. 10.1016/j.saa.2007.07.038 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Denisenko, T. V., Sorokina, I. V., Gogvadze, V. & Zhivotovsky, B. Mitotic catastrophe and cancer drug resistance: a link that must to be broken. Drug Resist. Updat. 24, 1–12 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Husemann, L. C. et al. The microtubule targeting agents eribulin and paclitaxel activate similar signaling pathways and induce cell death predominantly in a caspase-independent manner. Cell. Cycle. 19, 464–478. 10.1080/15384101.2020.1716144 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martino, E. et al. Vinca alkaloids and analogues as anti-cancer agents: looking back, peering ahead. Bioorg. Med. Chem. Lett.28, 2816–2826. 10.1016/j.bmcl.2018.06.044 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Azarenko, O., Smiyun, G., Mah, J., Wilson, L. & Jordan, M. A. Antiproliferative mechanism of action of the novel taxane cabazitaxel as compared with the parent compound docetaxel in MCF7 breast cancer cells. Mol. Cancer Ther.13, 2092–2103. 10.1158/1535-7163.MCT-14-0265 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Thongnuanjan, P. et al. Panduratin A derivative protects against cisplatin-induced apoptosis of renal proximal tubular cells and kidney injury in mice. Molecules26, 642. 10.3390/molecules26216642 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song, X. L. et al. Casticin induces apoptosis and G0/G1 cell cycle arrest in gallbladder cancer cells. Cancer Cell. Int.17. 10.1186/s12935-016-0377-3 (2017). [DOI] [PMC free article] [PubMed]
- 68.Thangaraj, K. et al. Orientin induces G0/G1 cell cycle arrest and mitochondria mediated intrinsic apoptosis in human colorectal carcinoma HT29 cells. Biomolecules9, 418. 10.3390/biom9090418 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bian, Y. et al. Ligustrazine induces the colorectal cancer cells apoptosis via p53-dependent mitochondrial pathway and cell cycle arrest at the G0/G1 phase. Ann. Palliat. Med.10, 1578–1588. 10.21037/apm-20-288 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Medema, R. H. & Macurek, L. Checkpoint control and cancer. Oncogene31, 2601–2613. 10.1038/onc.2011.451 (2012). [DOI] [PubMed] [Google Scholar]
- 71.Kuntz, K. & O’Connell, M. J. The G(2) DNA damage checkpoint: could this ancient regulator be the Achilles heel of cancer? Cancer Biol. Ther.8, 1433–1439. 10.4161/cbt.8.15.9081 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Ma, C. X., Janetka, J. W. & Piwnica-Worms, H. Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol. Med.17, 88–96. 10.1016/j.molmed.2010.10.009 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gabrielli, B., Brooks, K. & Pavey, S. Defective cell cycle checkpoints as targets for anti-cancer therapies. Front. Pharmacol.3, 9. 10.3389/fphar.2012.00009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rios, R. M. The centrosome-golgi apparatus nexus. Philos. Trans. R Soc. Lond. B Biol. Sci.369. 10.1098/rstb.2013.0462 (2014). [DOI] [PMC free article] [PubMed]
- 75.Sanders, A. A. & Kaverina, I. Nucleation and dynamics of golgi-derived microtubules. Front. Neurosci.9, 431. 10.3389/fnins.2015.00431 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bai, Z. et al. Autophagy and cancer treatment: four functional forms of autophagy and their therapeutic applications. J. Zhejiang Univ. Sci. B. 23, 89–101. 10.1631/jzus.B2100804 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gewirtz, D. A. The four faces of autophagy: implications for cancer therapy. Cancer Res.74, 647–651. 10.1158/0008-5472.CAN-13-2966 (2014). [DOI] [PubMed] [Google Scholar]
- 78.Mauthe, M. et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy14, 1435–1455. 10.1080/15548627.2018.1474314 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu, T., Zhang, J., Li, K., Deng, L. & Wang, H. Combination of an autophagy inducer and an autophagy inhibitor: a smarter strategy emerging in cancer therapy. Front. Pharmacol.11, 408. 10.3389/fphar.2020.00408 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pu, Y., Wang, J. & Wang, S. Role of autophagy in drug resistance and regulation of osteosarcoma (review). Mol. Clin. Oncol.16. 10.3892/mco.2022.2505 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request. Corresponding author WT has to be contacted in case of any queries or requirement of data.