Abstract

This Review examines the potential of breathomics in enhancing disease monitoring and diagnostic precision when integrated with artificial intelligence (AI) and electrochemical sensing techniques. It discusses breathomics’ potential for early and noninvasive disease diagnosis with a focus on chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), and lung cancer, which have been well studied in the context of VOC association with diseases. The noninvasive nature of exhaled breath analysis can be advantageous compared to traditional diagnostic methods for CKD, which often rely on blood and urine testing. VOC analysis can enhance spirometry and imaging methods used in COPD diagnosis, providing a more comprehensive picture of the disease’s progression. Breathomics could also provide a less intrusive and potentially earlier diagnostic approach for lung cancer, which is now dependent on imaging and biopsy. The combination of breathomics, electrochemical sensing, and AI could lead to more personalized and successful treatment plans for chronic illnesses using AI algorithms to decipher complicated VOC patterns. This Review assesses the viability and effectiveness of combining breathomics with electrochemical sensors and artificial intelligence by synthesizing recent research findings and technological developments.
Introduction
Chronic diseases are a major public health issue that significantly contribute to premature deaths worldwide. Characterized by their long-lasting nature, these conditions are estimated to cost $47 trillion by 2030.1 Due to the significant impact on patients’ quality of life, an increased focus has been promoted in effective detection and monitoring strategies for the global burden of chronic diseases. Among them some of the most pressing concerns are chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), and lung cancer, posing the need for early diagnosis and continuous monitoring.
Effective detection, monitoring, and management of chronic diseases are crucial for improving patient outcomes, enabling high-quality clinical decisions, and timely treatment. Traditional techniques such as immunoassays,2 mass spectrometry (MS),3 and genetic sequencing4 often rely on biomarker analysis. Despite the advanced approaches in these lab-based techniques, they show limitations, such as long wait times, high cost, procedural complexity, and the need for specialized techniques. Emerging technologies like breathomics show promise in addressing these challenges. Hence, there is a growing need for cost-effective, simpler, and real-time monitoring and enhancement of our approach to chronic disease management.
Breathomics is an emerging field focused on noninvasive disease diagnosis through exhaled breath analysis. While this technique can analyze both the liquid phase (exhaled breath condensate) and gaseous phase of breath,5 which includes volatile organic compounds (VOC), it has become popular due to its ease of access and convenient sample collection. When dealing with the gaseous phase, VOCs originated from metabolic activities can act as biochemical fingerprints, offering insight into the body’s metabolic processes.6 A schematic of how endogenously produced VOCs enter the breath is shown in Figure 1. Therefore, VOC analysis can help correlate specific profiles to certain diseases. This noninvasive approach holds significant promise for early detection and monitoring of chronic diseases, particularly CKD, COPD, and lung cancer where early diagnosis is crucial.
Figure 1.
Entrance of endogenously produced VOCs into breath via the bloodstream.
Chronic kidney disease poses a severe global health challenge. It has led to high morbidity and mortality rates, affecting millions of people. Nephrons, the functional units of the kidney, are generated only during gestation,7 making their preservation vital. Traditional diagnostic methods such as serum creatinine and blood urea nitrogen tests often detect CKD at advanced stages.8 However, breathomics has shown potential for early diagnosis. For instance, Chan et al. have studied the association of breath ammonia and impairment of renal function.9 Banga et al. have developed a ZIF-based electronic nose (ZENose) for breath ammonia detection in CKD conditions,10 associated with CKD biomarker. In another study, Saidi et al. have shown the feasibility of e-nose systems to discriminate between breath samples and correlated them to CKD conditions.11 This suggests that breath analysis could provide a valuable tool for early CKD detection, potentially improving patient outcomes.
Chronic obstructive pulmonary disease is a progressive inflammatory lung condition which is primarily diagnosed via spirometry12 and imaging techniques;13 they may not capture the early onset of the disease. Emerging research in breathomics has shown promise in identifying distinct VOC profiles associated with COPD, reflecting the underlying oxidative stress and inflammation. Cazzola et al. have leveraged e-nose technology and solid phase microextraction associated with gas chromatography–mass spectrometry to characterize COPD breath profiles.14 Gaugg et al. and Bregy et al. have employed secondary electrospray ionization mass spectrometry (SESI-MS15 and SESI-HRMS16) to distinguish between COPD breath and healthy breath samples via breath analysis. These findings show how breathomics can be utilized to build breath profiles using COPD breath data, allowing disease identification at early stages.
Lung cancer originates in epithelial cells of the respiratory tract, leading to changes in metabolic pathways.17 Breathomics offers a noninvasive alternative with studies showing its potential. For instance, Nardi-Agmon et al. demonstrated the utility of breath analysis in tracking treatment effectiveness owing to the technique’s short response time.18 Chang et al. have achieved 75% accuracy in distinguishing lung cancer patients from healthy controls using VOC analysis.19 Capuano et al. have further highlighted the preservation of VOC patterns from lung air to exhaled breath, showing the potential of breathomics as a tool.20 Bhalla et al. comprehensively reviewed the breathomics principle and its application particularly in the lung cancer domain.21 These studies underscore the promise of breathomics in overcoming existing challenges. Hence, continued research is necessary to refine this technology and incorporate it into clinical settings.
Breath VOCs indicate the disease severity. AI-enhanced electrochemical sensors detect these biomarkers, enabling the early diagnosis of conditions like lung cancer. Electronic nose and breath sensors enable rapid disease detection and monitoring. For instance, Banga et al. have developed a room temperature ionic liquid-based gas sensor to detect nitric oxide in exhaled breath for COVID-19 screening.22 Khatoon et al. have created an e-nose platform using doped SnO2 nanomaterials to identify VOCs like 1-propanol and isopropyl alcohol as lung cancer biomarkers.23 Another study by Banga et al. highlighted the role of electrochemical sensing in breathomics, particularly for diagnosing acute and chronic respiratory diseases.24 Hence, electrochemical techniques provide a method for the analysis of VOC patterns that act as breath profiles for different disease conditions.
The integration of AI with electrochemistry has gained attention for its ability to accurately analyze complex VOC data by using deep learning and machine learning algorithms. In addition, these algorithms can spot minute patterns that would be challenging or overlooked by conventional statistical analysis. Combining breathomics, electrochemical sensors, and AI enhances the identification and quantification of breath biomarkers, leading to improved diagnostics and monitoring of chronic diseases. The incorporation of breathomics, electrochemistry, and AI for the noninvasive detection of chronic diseases is shown in Figure 2.
Figure 2.
Integration of breathomics, electrochemical sensing, and artificial intelligence for the noninvasive detection of chronic diseases via breath biomarkers (VOCs).
Conventional Approaches for Detecting Biomarkers for Chronic Diseases and Potential in Breathomics Approach
While conventional biomarker detection and monitoring methods are essential, they often face challenges such as long acquisition times, high costs, the requirement for specialized personnel, and procedural complexity. These limitations highlight the need to investigate innovative approaches. Exploring the potential in breathomics provides insight into how these challenges can be addressed and how clinical practices can be improved.
Detection Techniques for Chronic Kidney Disease (CKD)
Biomarkers are essential to detecting, monitoring, and evaluating treatment effectiveness in diseases such as CKD. Thus, traditional biomarkers comprising serum creatinine, cystatin C, and urine albumin which helps evaluate estimated glomerular filtration rate (eGFR), along with urine albumin-to-creatinine ratio (ACR), continue to play a major role in evaluating kidney function along with early identification of renal injury. Hence, they improve not only diagnostic accuracy but also the patient outcomes.8 In addition, discoveries in imaging fields such as high-resolution ultrasound and magnetic resonance imaging (MRI) have also further improved the outcomes in CKD management by enabling early detection of renal pathology.25 Overall, these advances in methods for detecting biomarkers and imaging techniques significantly enhance our ability to manage CKD better.
Although the current methods that are used can lead to better patient outcomes, they also have various limitations including invasiveness and time consumption, which further increase healthcare costs. Blood-based tests are invasive. Along with them, urine tests such as ACR are time-consuming. Imaging techniques are expensive and pose a risk of radiation exposure. Therefore, breathomics, which analyzes volatile organic compounds (VOCs) present in exhaled breath, adds novelty to these detection and monitoring techniques as a noninvasive approach to diagnose and monitor various diseases. Furthermore, the real-time monitoring ability and the inexpensive nature of breathomics provide an answer to these challenges, allowing it to be incorporated along with existing methods to improve patient care and comfort. Studies in CKD have identified unique patterns of VOCs that are associated with metabolic changes which result from renal dysfunction, such as increased levels of ammonia that were studied by Meng et al.9 Their study has reported increasing levels of ammonia with the progression of kidney dysfunction with the concentrations ranging from ∼636 to ∼12781 ppb. These VOCs are associated with processes such as uremia and oxidative stress, making the case that breath analysis could complement traditional biomarkers such as serum creatinine and urine albumin in early detection and disease monitoring.
Detection Techniques for Chronic Obstructive Pulmonary Disease (COPD)
Biomarkers such as C-reactive protein (CRP) and fibrinogen play a crucial role in identifying and tracking the disease. CRP, produced by the liver as an inflammatory response, is often elevated in COPD patients, indicating ongoing inflammation.26 Fibrinogen involved in inflammation and blood clotting correlated with disease severity and the risk of exacerbations.27 Understanding these biomarkers helps clinicians monitor disease progression, predict exacerbations, and tailor treatment plans for individuals.
Due to contemporary technology, COPD detection and monitoring have greatly improved. Spirometry is a widely used test that evaluates the volume and flow of air during inhalation and exhalation to quantify lung function, aiding in the diagnosis of COPD and tracking the disease’s advancement.12 High-resolution computed tomography (HRCT) is one imaging modality that offers precise views of the lungs, making it possible to determine the degree of emphysema and structural alterations.28 Treatment options comprise corticosteroids, which lower inflammation, and bronchodilators, which relax the muscles surrounding the airways.29,30 These developments set the stage for exploring novel diagnostic modalities, such as breathomics, for the treatment of COPD. Current COPD detection and treatment methods are mostly spirometry and chest imaging; although they have benefits, they possess limitations such as extensive human effort, radiation exposure side effects, high costs, and most importantly real field accessibility. Breathomics, a noninvasive, inexpensive, and real-time testing method, could address these challenges by complementing existing technologies. In COPD, breathomics shows promise for monitoring disease progression and predicting exacerbations. Studies have identified specific VOCs linked to airway inflammation and oxidative stress such as 14 VOCs identified by Gaid at al.31 Cazzola et al. have found a positive correlation between elevated decane levels and exacerbations.14 Both of these studies focused on identifying VOCs that could be plausible breath markers in COPD conditions. However, neither study quantified the concentrations at which these VOCs are present in the breath. Further research would help bridge this gap and better establish the diagnostic potential of these VOCs. These VOCs provide real-time insights into disease activity, potentially enhancing personalized treatment strategies beyond conventional spirometry and imaging techniques.
Detection Techniques for Lung Cancer
In lung cancer, biomarkers are crucial for detection and monitoring, significantly improving diagnostic accuracy and guiding treatment decisions. Some key biomarkers include circulating tumor DNA (ctDNA),32 circulating tumor cells (CTCs),33 and protein markers like carcinoembryonic antigen (CEA) and cytokeratin 19 fragments (CYFRA 21-1).34 ctDNA and CTCs help identify specific genetic mutations such as those in EGFR and KRAS genes, which are essential for personalized therapies.32 Protein markers are used to evaluate treatment response and monitor tumor burden, providing a way to track disease progression and detect recurrence.35 These biomarkers are vital for advancing personalized medicine and improving treatment outcomes.
Advancements in lung cancer detection and treatment have significantly improved the early diagnosis and personalized care. Low dose computed tomography (LDCT) has proven effective in screening early stage lung cancer, especially in high-risk populations.36 Next generation sequencing (NGS) allows extensive profiling of the genome for molecular diagnostics, by identifying specific genetic alterations.37 Immunotherapy has shown success in boosting the immune system to fight cancer cells, improving survival rates in advanced lung cancer cases.38 Additionally targeted therapies like tyrosine kinase inhibitors (TKIs) offer personalized treatment by specifically blocking mutant proteins involved in cancer development.39 All of these technical developments lead to better patient outcomes, more precise diagnosis, and efficient therapies.
While techniques like LDCT and target therapies have advanced detection and treatment, they come with drawbacks such as high costs, long acquisition times, complex protocols, radiation exposure, and the need for trained personnel. In contrast, breathomics offers a noninvasive, low-cost route that can complement existing tools. Research has shown specific VOC signatures in lung cancer breath, indicative of underlying molecular changes and tumor metabolism. For instance, Wang et al. reported 16 VOCs and effectively differentiated lung cancer patients from healthy individuals.40 While their work identified these 16 markers as potential VOCs related to lung cancer, the work did not provide quantitative data on the levels of these VOCs in breath. These findings underscore the potential of breathomics as a noninvasive screening tool for early detection and monitoring of treatment response in lung cancer. This also leaves an important avenue for further research.
Thus, breathomics has the potential to revolutionize the treatment of a wide range of diseases as analytical technology and bioinformatics continue to progress. This is why its application in clinical practice remains so promising. In the future, investigating the incorporation of sophisticated electrochemical sensors into breathomics provides a possibility to improve the real-time measurement of VOC biomarkers as well as their sensitivity and specificity. Furthermore, as the field expands, new research is emerging incorporating artificial intelligence (AI) and machine learning (ML) in these sensing strategies. For instance, Thakur et al. have developed a predictive model using supervised ML to predict future events in CKD patients going through dialysis using data like heart rate, respiratory rate, and heart rate variability from a noncontact sensor device.41 Fernandez-Granero et al. incorporated AI in predicting exacerbations in COPD patients. The study has collected data on respiratory sounds using an electronic sensor, and a predictive model has been developed for the prediction of exacerbation with a detection accuracy of 78%.42 In another study, Binson et al. developed an AI based e-nose system that can discriminate healthy individuals from lung cancer individuals. They have been able to achieve 85.38% accuracy with a random forest model, which has been shown to be better than a logistic regression model.43 AL/ML has become essential in processing the vast data generated by these sensors, which enables more precise analysis and decision-making. Several studies have shown the benefits that would come with application in healthcare. For instance, according to Bohr et al. it is projected that AI applications could reduce annual U.S. healthcare costs by as much as $150 billion by 2026.44 The synergy of these technologies has the potential to completely transform personalized medicine by giving medical professionals insightful knowledge about the conditions of patients and how their treatments work.
Electrochemical Sensing Approaches for Endogenously Produced VOCs for Screening Chronic Disease States
Electrochemistry transforms chemical interaction into electrical signals for precise analysis, with techniques divided into Faradaic and non-Faradaic methodology. Faradaic processes are specific reactions, mainly capturing charge transfer at an electrode surface when charged particles move from one bulk phase to another across the metal–solution interface,45 while the non-Faradaic-EIS sensor offers raw measurement of the μ environment of the electrochemical interface.46 Electrochemical impedance spectroscopy (EIS) has emerged as a valuable analytical tool in this domain, facilitating nondestructive measurements and enabling in situ analysis of various analytes of medical, environmental, and industrial significance.47
Electrochemical techniques are varied based on the system’s electrochemical nature, with Faradaic sensing relying on redox activity of a Faradaic probe being used as a signal modulator to provide selective and sensitive outputs for target analytes.48 EIS is a powerful electrochemical tool which utilizes both a Faradaic and a non-Faradaic process to cater to a point of care electrochemical sensing strategy.49 Faradaic EIS utilizes the redox activity of the probe, causes a significant charge transfer phenomenon at the electrode electrolyte interface, and provides a suitable EIS signal.50 One of the important applications of EIS in health is to develop next generation point of care biosensors, which offer excellent stability and sensitivity. A Faradaic e-chem biosensor is made of suitable assays of cross-linker, antibody–antigen, and redox probe where the antibody–antigen interaction is being monitored as a function of redox charge transfer of the Faradaic probe used,51 represented in Figure 3, where a Faradaic EIS biosensing scheme has been demonstrated for the detection of retinol binding protein 4, a type 2 diabetes biomarker.
Figure 3.

Schematic representation of an EIS based sensor assay for the detection of picomolar concentration of RBP4, using a Faradaic redox principle, with Fe(II)/Fe(III) as a redox tag for signal output. The scheme depicted in the figure is reproduced with permission from ref (51), copyright Elsevier, 2019.
Redox activity results in charge transfer, which is further transduced to the electrochemical double layer, duly captured by EIS. The technique, also known as impedimetry, has been widely used to fabricate easy point of care biosensors and utilizes common Faradaic probes such as ferrocene and K3Fe(CN)6/K4Fe(CN)6.52 There are numerous applications of impedimetry where probes like DNA-complementary DNA and antigen–antibody interactions are utilized for the fabrication of biosensors. Generally, the functionalization of the electrode is done using suitable chemicals so that the biomolecule can firmly hold onto the electrode surface. There are several functionalization techniques such as formation of a self-assembled monolayer over a gold electrode surface by functionalization with the thiol group. An indium tin oxide electrode functionalized with a silane compound was extensively used. Entrapment or covalent bonding of biomolecules with the conductive polymer coating of the electrodes and covalent bonding of the biomolecules with the nonconductive electropolymerized films are also used explicitly for immobilization of biomolecules.53 Electrochemical sensing enables fast, sensitive detection using minimal sample volumes.
Other applications of Faradaic biosensing include amperometric glucose biosensing applications. The technique is governed by the Cottrell equation. Using this technique, amperometric sensors have been developed and utilized explicitly by the scientific community.55 Point of care detection of glucose from saliva or sweat with mediator free noninvasive methods had been the characteristic feature of the third-generation devices. Various authors have reviewed this timeline extensively.56,57 A fundamental concept in the development of novel biosensors and bioelectrocatalytic devices is mediator-free direct electron transfer between a redox protein’s active core and the electrode, depicted in Figure 4.54,58 The transducer plays the most important role in the sensing operation and should be aligned with the application principle, too. One of the important aspects that generally appears in biosensing operations is material toxicity, and in most cases, biomaterials are chosen as the standard transducer. Although a noninvasive application like breathomics is mainly ex situ, 2D porous materials are well chosen for this.
Figure 4.

Amperometric glucose sensor utilizing GOx as a Faradaic probe for tandem sensing applications. The figure is reproduced with permission from ref (54), copyright American Chemical Society 2018.
In contrast, non-Faradaic sensing is an electrochemical technique that detects changes in the electrical properties of a sensor’s surface without involving direct electron transfer between the analyte and the electrode.59 This method is fundamentally based on measuring variations in capacitance or impedance that occur when VOCs interact with the sensor’s surface.46 Non-Faradaic sensors enable real-time VOC monitoring without redox reactions, ideal for rapid breath analysis in noninvasive diagnostics. Chronoamperometry measures current overtime at fixed voltage, revealing how VOCs interact with electrode surfaces through adsorption and desorption. In the context of chronic disease detection, chronoamperometry offers several key benefits.60 Chronoamperometry measures VOC concentrations by tracking the electrical current over time. This is particularly important for diseases like diabetes, where the concentration of acetone in the breath can correlate with blood glucose levels.61
The diffusion of VOCs across the electrode–electrolyte interface is a critical factor influencing the performance of electrochemical sensors.62 Understanding and optimizing this diffusion process are essential for enhancing sensor sensitivity and response time. The rate of diffusion of VOCs to the electrode surface can be a limiting factor in the sensor response. Employing techniques such as microfabrication to create nanostructured electrodes can enhance mass transport and improve sensor performance by increasing the effective surface area and promoting rapid diffusion of VOCs.63 Modifying the electrode surface with nanomaterials, like gold nanoparticles, carbon nanotubes, or graphene, can enhance the diffusion of VOCs.64−66 These materials provide a high surface area and can facilitate faster electron transfer, improving the overall sensitivity and selectivity of the sensor. Zinc imidazole frameworks (mainly ZIF-8) have been extensively used in electrochemical sensing applications, especially in the field of breathomics.64 ZIF-8 stands out for its highly tunable and well-defined pore structure.67 This allows for precise control over the pore size, a crucial feature for selectively capturing molecules. This characteristic makes ZIF-8 ideal for applications such as breath analysis, where researchers aim to detect specific volatile organic compounds (VOCs) in exhaled breath. The high surface area of ZIF-8, combined with its microscopic channels, facilitates the selective adsorption of targeted breath components.68 Other materials, including room temperature ionic liquids, can be promising candidates, which also have similar throughput.69 This interaction leads to measurable changes in the electrochemical signal, enabling detection. Our research group has demonstrated the effectiveness of a custom-designed ZIF-8-based transducing element for the precise and selective identification of various VOCs and inorganic gases.24
In research done by Banga et al., the researchers incorporated ferrocene inside ZIF-8 to use it as a Faradaic probe and demonstrate its application in ammonia sensing.10 This hybrid Faradaic probe, driven by the chronoamperometric principle, successfully detected ammonia gas at very low levels (400 ppb) while demonstrating high sensitivity and specificity. In subsequent work, the researchers synthesized a non-Faradaic probe by incorporating RTIL inside the ZIF-8 pore and using the hybrid material for isopentane sensing.70 The authors employed [BMIM]BF4@ZIF-8 to sense isopentane levels associated with lung cancer, ranging from 600 ppb to 12 ppm. The findings show an increase in the cathodic current corresponding to higher isopentane concentrations, indicating that the diffusion process influences the electrochemical microenvironment. This results in a greater number of species diffusing across the interface and an enhanced level of interaction. The study displays the operational efficacy and practicality of an internet of things (IoT)-centered microelectronic prototype.
In another work, Banga et al. synthesized ZIF-8/graphene oxide nanocomposite for sensing carene vapors in the case of lower respiratory tract related infections.71 ZIF-8 provides a high surface area and porosity, facilitating the adsorption and diffusion of carene molecules. Graphene oxide (GO) enhances electrical conductivity and mechanical stability, improving the sensor’s overall performance. An in situ synthesis approach was taken for the synthesis of ZIF-8/GO hybrid composite. The ZIF-8/GO-modified sensor platform shows high sensitivity and specificity toward carene, achieving detection limits as low as 100 parts per billion due to the large surface area and porosity enabling effective adsorption and diffusion of carene molecules.
Moreover, influenza is a prevalent infection that spreads easily and requires early screening. Isoprene levels can serve as an indicator of oxidative stress resulting from respiratory inflammation. To address this, a AuNP@ZeNose platform, where gold nanoparticles (AuNPs) are encapsulated within the cavities of ZIF-8, is designed for screening isoprene levels as a biomarker for influenza, enabling noninvasive flu detection through breath analysis.72 Thus, electrochemical sensing integrated with breath analysis enables real-time disease monitoring, offering enhanced precision and improved patient outcomes through advanced VOC detection.
Implementation of Biosensors Utilizing Artificial Intelligence for Breathomics
AI and ML analyze electrochemical sensing data using neural networks and regression to detect patterns and make precise disease-related decisions. They enable task automation without human intervention, thereby increasing efficiency, saving analysis time, and reducing associated costs.73,74 AI/ML can analyze large volumes of data quickly, providing insights into data patterns and trends and aiding in process optimization. Moreover, they can process data with high accuracy and consistency, minimizing errors.73,74 Researchers can utilize AI/ML to explore vast data sets and perform complex simulations and analyses, aiding understanding of underlying phenomena or data trends, depicted as a scheme in Figure 5
Figure 5.
Comprehensive scheme of the combination of electrochemistry, machine learning, and artificial intelligence.
Multidimensional data collected from hospitals, laboratories, and wearable devices can be leveraged to build predictive and prognostic models using statistical and deep learning techniques. AI/ML techniques have been employed for proteomic and metabolic profiling of diseases like COVID-19 and various cancer types.73 Statistical and mathematical models are often used to extract chemical information and design experiments in analytical chemistry, which can be extended to the extraction of quantitative and qualitative information from electrochemical sensors. These AI/ML techniques can be implemented to enhance the sensing performance in various steps:
-
(1)
Preprocessing of electrochemical data, including correcting baseline, reducing noise, and normalizing collected signals
-
(2)
Electrochemical features like steady-state current, open circuit potential, impedance, and peak characteristics from VOC interactions with sensor surfaces are analyzed and fed to the model to extract critical sensor data.
-
(3)
Choosing appropriate AI/ML models based on the type, volume, and complexity of data for accurate prediction. Examples include support vector machines (SVM), random forest, gradient-boosting networks, Naïve-Bayes, decision trees, and neural networks.
-
(4)
Training the models with extracted data set features, optimizing model parameters rigorously to improve prediction efficacy and minimize errors
-
(5)
Validating the performance of the trained model by presenting it with new real-time data, enabling adaptation to new VOCs by learning from existing training data sets. Here performance metrics such as accuracy, area under the curve (AUC), and F1-score are measured.
-
(6)
Constantly monitoring the model’s accuracy and tuning its parameters to enhance its performance
-
(7)
Integration of the designed models with relevant hardware and software systems for deployment in real-time breath VOC detection
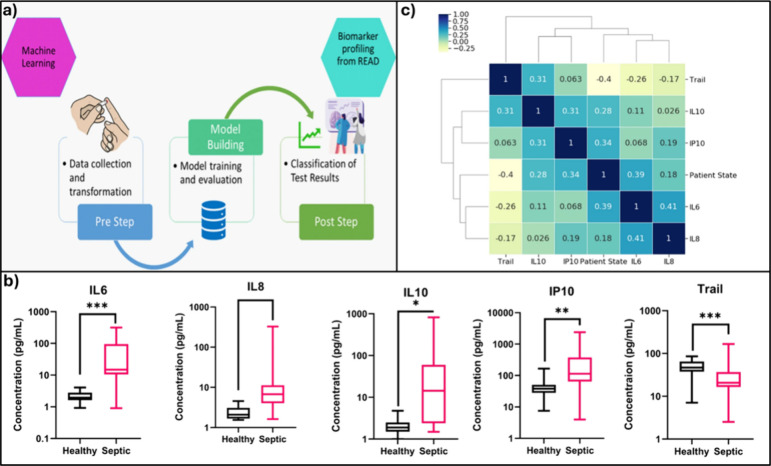
Classification algorithms, including random forests, support vector machines (SVM), and neural networks, are utilized to classify breath samples into healthy or diseased categories based on the presence or absence of target VOCs.75 Regression techniques such as linear regression, polynomial regression, lasso regression, and ridge regression are employed to model the relationship between the concentration of breath VOCs and the electrochemical output signal, allowing for forecasting of breath biomarker concentrations. Techniques such as principal component analysis (PCA)75 and t-distributed stochastic neighbor embedding (t-SNE) are used to reduce the complexity of the data while retaining important features. Feature extraction algorithms, such as time and frequency domain analysis and wavelet transform, are utilized to identify characteristic features associated with the target VOCs. Ensemble learning techniques like AdaBoost, gradient boosting, or bagging are employed to enhance prediction capabilities by combining the strengths of multiple models compared to a single model, particularly beneficial for noisy data sets. These methods improve the model’s robustness and stability and reduce bias and variance, resulting in more accurate predictions. A study by Sardesai et al. demonstrated the successful use of machine learning, with Naïve Bayes and decision tree algorithms to analyze sensor data for sepsis prognosis (Figure 6).
Figure 6.
(a) Machine learning model building process flow. (b) Comparing the healthy control vs septic patient samples for IL-6, IL-8, IL-10, IP-10, and Trail. (c) Correlation matrix with heatmap and cluster map showing relation between the study biomarkers and patient state. reprinted with permission from ref (76). Copyright Nature, 2021, under a Creative Commons license agreement (https://creativecommons.org/licenses/by/4.0/).
Data from a POC sensor platform for multiplexed cytokine detection have been integrated with AI models to support patient stratification and personalized treatment via study protein and patient state correlation. The selected models, Naïve Bayes and decision tree algorithms have shown 96.64% and 94.64% accuracy respectively highlighting the potential for patient stratification via biomarker profiling.76 Sankhala et al. have shown the design of a decision tree regression model to predict real-time sweat glucose levels from wearable sensor data using passively expressed eccrine sweat. The model has successfully integrated raw impedance signals with factors such as temperature and relative humidity to track and report sweat glucose trends.77 Deep learning methods such as convolutional neural networks (CNNs) and recurrent neural networks (RNNs) are popular due to their ability to automatically extract hierarchical features from raw data without the need for manual feature extraction, enabling the capture of complex data patterns. For instance, Kang et al. have incorporated CNN to overcome the challenge of low selectivity in semiconductor metal oxide (SMO) gas sensors. Sensor data have been used in matrix form as the input for the learning algorithm to conduct pattern recognition of the sensor responses.78 With the selected deep learning network, the study has been able to achieve real-time selective gas detection with an accuracy of 98%.
Among these different AI and ML techniques that are utilized, a major distinction lies in supervised and unsupervised learning. Supervised learning techniques, which are widely used, rely on labeled data sets to train models. On the contrary, unsupervised learning techniques complement by analyzing unlabeled data to reveal hidden patterns. This approach enables more comprehensive analysis of large data sets, especially in the space of breathomics with help in overcoming challenges such as data variability.79 Therefore, this synergy between advanced sensing technologies and intelligent data processing sets the stage for the next generation of diagnostic tools for chronic disease management.
Conclusion
Utilizing the analysis of volatile organic compounds (VOC) in exhaled breath, breathomics presents promising advancements in disease diagnostics and management. The noninvasive approach in detecting biomarkers associated with CKD, COPD, and lung cancer improves patient outcomes and healthcare costs. Electrochemical sensing systems help decode these biomarkers by converting them to electrical signals. Furthermore, integrating AI into this equation helps to enhance the diagnostic accuracy of breathomics. It helps bridge the gap between raw data and actionable insights. The noninvasive nature of breathomics and analytical power of AI, combined with the sensitivity and specificity of electrochemical sensing, hold great potential in disease diagnosis and monitoring. In addition to chronic diseases addressed in this Review, with more research, similar relationships between changes in biological pathways upon disease onset and the resulting breath profiles can be built for other diseases as well. Ultimately, this knowledge can be incorporated to build sensing platforms targeting specific biomarkers, expanding this technology beyond chronic kidney disease, chronic pulmonary disease, and lung cancer.
The authors declare the following competing financial interest(s): S.P. and S.M. have a significant interest in EnLiSense LLC, a company that may have a commercial interest in the results of this research and technology. The potential individual conflict of interest has been reviewed and managed by The University of Texas at Dallas and played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
- Gambert S. R. The Burden of Chronic Disease. Clinical Geriatrics 2006, 5. 10.1016/j.mayocpiqo.2023.08.005. [DOI] [Google Scholar]
- Jeong S.; Park M.-J.; Song W.; Kim H.-S. Current Immunoassay Methods and Their Applications to Clinically Used Biomarkers of Breast Cancer. Clin Biochem 2020, 78, 43–57. 10.1016/j.clinbiochem.2020.01.009. [DOI] [PubMed] [Google Scholar]
- Banerjee S. Empowering Clinical Diagnostics with Mass Spectrometry. ACS Omega 2020, 5 (5), 2041–2048. 10.1021/acsomega.9b03764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemizu D.; Mori T.; Akiyama S.; Higaki S.; Watanabe H.; Sakurai T.; Niida S.; Ozaki K. Identification of Potential Blood Biomarkers for Early Diagnosis of Alzheimer’s Disease through RNA Sequencing Analysis. Alzheimers Res. Ther 2020, 12 (1), 87. 10.1186/s13195-020-00654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoubnasabjafari M.; Mogaddam M. R. A.; Rahimpour E.; Soleymani J.; Saei A. A.; Jouyban A. Breathomics: Review of Sample Collection and Analysis, Data Modeling and Clinical Applications. Crit Rev. Anal Chem. 2022, 52 (7), 1461–1487. 10.1080/10408347.2021.1889961. [DOI] [PubMed] [Google Scholar]
- Chen T.; Liu T.; Li T.; Zhao H.; Chen Q. Exhaled Breath Analysis in Disease Detection. Clin. Chim. Acta 2021, 515, 61–72. 10.1016/j.cca.2020.12.036. [DOI] [PubMed] [Google Scholar]
- Rosenblum S.; Pal A.; Reidy K.. Renal Development in the Fetus and Premature Infant. Seminars in Fetal and Neonatal Medicine; W.B. Saunders: 2017; pp 58–66 10.1016/j.siny.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Giacoman S. Biomarkers in Chronic Kidney Disease, from Kidney Function to Kidney Damage. World J. Nephrol 2015, 4 (1), 57. 10.5527/wjn.v4.i1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M. J.; Li Y. J.; Wu C. C.; Lee Y. C.; Zan H. W.; Meng H. F.; Hsieh M. H.; Lai C. S.; Tian Y. C. Breath Ammonia Is a Useful Biomarker Predicting Kidney Function in Chronic Kidney Disease Patients. Biomedicines 2020, 8 (11), 468. 10.3390/biomedicines8110468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga I.; Paul A.; Muthukumar S.; Prasad S. ZENose (ZIF-Based Electrochemical Nose) Platform for Noninvasive Ammonia Detection. ACS Appl. Mater. Interfaces 2021, 13 (14), 16155–16165. 10.1021/acsami.1c02283. [DOI] [PubMed] [Google Scholar]
- Saidi T.; Zaim O.; Moufid M.; El Bari N.; Ionescu R.; Bouchikhi B. Exhaled Breath Analysis Using Electronic Nose and Gas Chromatography-Mass Spectrometry for Non-Invasive Diagnosis of Chronic Kidney Disease, Diabetes Mellitus and Healthy Subjects. Sens Actuators B Chem. 2018, 257, 178–188. 10.1016/j.snb.2017.10.178. [DOI] [Google Scholar]
- Johns D. P.; Walters J. A. E.; Haydn Walters E. Diagnosis and Early Detection of COPD Using Spirometry. Journal of Thoracic Disease 2014, 1557–1569. 10.3978/j.issn.2072-1439.2014.08.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne S.; King G. G. Advanced Imaging in COPD: Insights into Pulmonary Pathophysiology. Journal of Thoracic Disease 2014, 1570–1585. 10.3978/j.issn.2072-1439.2014.11.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola M.; Segreti A.; Capuano R.; Bergamini A.; Martinelli E.; Calzetta L.; Rogliani P.; Ciaprini C.; Ora J.; Paolesse R.; Di Natale C.; D’Amico A. Analysis of Exhaled Breath Fingerprints and Volatile Organic Compounds in COPD. COPD Research and Practice 2015, 1 (1), 7. 10.1186/s40749-015-0010-1. [DOI] [Google Scholar]
- Gaugg M. T.; Nussbaumer-Ochsner Y.; Bregy L.; Engler A.; Stebler N.; Gaisl T.; Bruderer T.; Nowak N.; Sinues P.; Zenobi R.; Kohler M. Real-Time Breath Analysis Reveals Specific Metabolic Signatures of COPD Exacerbations. Chest 2019, 156 (2), 269–276. 10.1016/j.chest.2018.12.023. [DOI] [PubMed] [Google Scholar]
- Bregy L.; Nussbaumer-Ochsner Y.; Martinez-Lozano Sinues P.; García-Gómez D.; Suter Y.; Gaisl T.; Stebler N.; Gaugg M. T.; Kohler M.; Zenobi R. Real-Time Mass Spectrometric Identification of Metabolites Characteristic of Chronic Obstructive Pulmonary Disease in Exhaled Breath. Clinical Mass Spectrometry 2018, 7, 29–35. 10.1016/j.clinms.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty P.; Pande B.; Acharya R.; Bhaskar L. V. K. S.; Verma H. K. Unravelling the Triad of Lung Cancer, Drug Resistance, and Metabolic Pathways. Diseases 2024, 12, 93. 10.3390/diseases12050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi-Agmon I.; Abud-Hawa M.; Liran O.; Gai-Mor N.; Ilouze M.; Onn A.; Bar J.; Shlomi D.; Haick H.; Peled N. Exhaled Breath Analysis for Monitoring Response to Treatment in Advanced Lung Cancer. Journal of Thoracic Oncology 2016, 11 (6), 827–837. 10.1016/j.jtho.2016.02.017. [DOI] [PubMed] [Google Scholar]
- Chang J.-E.; Lee D.-S.; Ban S.-W.; Oh J.; Jung M. Y.; Kim S.-H.; Park S.; Persaud K.; Jheon S. Analysis of Volatile Organic Compounds in Exhaled Breath for Lung Cancer Diagnosis Using a Sensor System. Sens Actuators B Chem. 2018, 255, 800–807. 10.1016/j.snb.2017.08.057. [DOI] [Google Scholar]
- Capuano R.; Santonico M.; Pennazza G.; Ghezzi S.; Martinelli E.; Roscioni C.; Lucantoni G.; Galluccio G.; Paolesse R.; Di Natale C.; D’Amico A. The Lung Cancer Breath Signature: A Comparative Analysis of Exhaled Breath and Air Sampled from inside the Lungs. Sci. Rep 2015, 5, 16491. 10.1038/srep16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V.; Taha B. A.; Lucky N.; Rustagi S.; Khosla A.; Papakonstantinou P.; Bhalla N. Nose-on-Chip Nanobiosensors for Early Detection of Lung Cancer Breath Biomarkers. ACS Sensors 2024, 9, 4469. 10.1021/acssensors.4c01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga I.; Paul A.; France K.; Micklich B.; Cardwell B.; Micklich C.; Prasad S. E.Co. Tech-Electrochemical Handheld Breathalyzer COVID Sensing Technology. Sci. Rep 2022, 12 (1), 4370. 10.1038/s41598-022-08321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatoon Z.; Fouad H.; Alothman O. Y.; Hashem M.; Ansari Z. A.; Ansari S. A. Doped SnO2 Nanomaterials for E-Nose Based Electrochemical Sensing of Biomarkers of Lung Cancer. ACS Omega 2020, 5 (42), 27645–27654. 10.1021/acsomega.0c04231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga I.; Paul A.; Churcher N. K. M.; Kumar R. M.; Muthukumar S.; Prasad S. Passive Breathomics for Ultrasensitive Characterization of Acute and Chronic Respiratory Diseases Using Electrochemical Transduction Mechanism. TrAC Trends in Analytical Chemistry 2024, 170, 117455. 10.1016/j.trac.2023.117455. [DOI] [Google Scholar]
- Jiang B.; Liu F.; Fu H.; Mao J. Advances in Imaging Techniques to Assess Kidney Fibrosis. Renal Failure 2023, 45, 2171887. 10.1080/0886022X.2023.2171887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksu F.; Capan N.; Aksu K.; Ofluoǧlu R.; Canbakan S.; Yavuz B.; Akin K. O. C-Reactive Protein Levels Are Raised in Stable Chronic Obstructive Pulmonary Disease Patients Independent of Smoking Behavior and Biomass Exposure. J. Thorac Dis 2013, 5 (4), 414–421. 10.3978/j.issn.2072-1439.2013.06.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M.; Parthasarathi A.; S K. C.; Biligere Siddaiah J.; Mahesh P. A. Fibrinogen: A Feasible Biomarker in Identifying the Severity and Acute Exacerbation of Chronic Obstructive Pulmonary Disease. Cureus 2021, 13, e16864. 10.7759/cureus.16864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S. Z.; He Q.; Su Y. S.; Wang P.; Xiang S. T.; Su W.; Mao C. W. Analysis of High-Resolution Computed Tomography Phenotypes and Pulmonary Function in Chronic Obstructive Pulmonary Disease. Journal of International Medical Research 2020, 48 (1), 300060519889459. 10.1177/0300060519889459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y. H.; Hsu C. L.; Li Y. Y.; Chang C. H.; Lai M. S. Bronchodilators Use in Patients with COPD. International Journal of COPD 2015, 10 (1), 1769–1779. 10.2147/COPD.S86198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mkorombindo T.; Dransfield M. T. Inhaled Corticosteroids in Chronic Obstructive Pulmonary Disease: Benefits and Risks. Clinics in Chest Medicine 2020, 475–484. 10.1016/j.ccm.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaida A.; Holz O.; Nell C.; Schuchardt S.; Lavae-Mokhtari B.; Kruse L.; Boas U.; Langejuergen J.; Allers M.; Zimmermann S.; Vogelmeier C.; Koczulla A. R.; Hohlfeld J. M. A Dual Center Study to Compare Breath Volatile Organic Compounds from Smokers and Non-Smokers with and without COPD. J. Breath Res. 2016, 10 (2), 026006. 10.1088/1752-7155/10/2/026006. [DOI] [PubMed] [Google Scholar]
- Wan J. C. M.; Massie C.; Garcia-Corbacho J.; Mouliere F.; Brenton J. D.; Caldas C.; Pacey S.; Baird R.; Rosenfeld N. Liquid Biopsies Come of Age: Towards Implementation of Circulating Tumour DNA. Nature Reviews Cancer 2017, 17, 223–238. 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Yuan Z.; Wang H.; Zhang H.; Duan G.; Zhang X. Role of Circulating Tumor Cells in Diagnosis of Lung Cancer: A Systematic Review and Meta-Analysis. Journal of International Medical Research 2021, 49 (3), 300060521994926. 10.1177/0300060521994926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.; Li J.; Qi X.; Qi J. Diagnostic Value of CYFRA 21–1 and Carcinoembryonic Antigen in Diagnosis of Operable Lung Cancer from Benign Lung Disease. J. Cancer Res. Ther 2018, 14 (9), S400–S404. 10.4103/0973-1482.174180. [DOI] [PubMed] [Google Scholar]
- Dall’Olio F. G.; Abbati F.; Facchinetti F.; Massucci M.; Melotti B.; Squadrilli A.; Buti S.; Formica F.; Tiseo M.; Ardizzoni A. CEA and CYFRA 21–1 as Prognostic Biomarker and as a Tool for Treatment Monitoring in Advanced NSCLC Treated with Immune Checkpoint Inhibitors. Ther Adv. Med. Oncol 2020, 12, 1758835920952994. 10.1177/1758835920952994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooreldeen R.; Bach H. Current and Future Development in Lung Cancer Diagnosis. International Journal of Molecular Sciences 2021, 22, 8661. 10.3390/ijms22168661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cainap C.; Balacescu O.; Cainap S. S.; Pop L. A. Next Generation Sequencing Technology in Lung Cancer Diagnosis. Biology 2021, 10, 864. 10.3390/biology10090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon E. B.; Rizvi N. A.; Hui R.; Leighl N.; Balmanoukian A. S.; Eder J. P.; Patnaik A.; Aggarwal C.; Gubens M.; Horn L.; Carcereny E.; Ahn M.-J.; Felip E.; Lee J.-S.; Hellmann M. D.; Hamid O.; Goldman J. W.; Soria J.-C.; Dolled-Filhart M.; Rutledge R. Z.; Zhang J.; Lunceford J. K.; Rangwala R.; Lubiniecki G. M.; Roach C.; Emancipator K.; Gandhi L. Pembrolizumab for the Treatment of Non-Small-Cell Lung Cancer. New England Journal of Medicine 2015, 372 (21), 2018–2028. 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- Johnson M.; Garassino M. C.; Mok T.; Mitsudomi T. Treatment Strategies and Outcomes for Patients with EGFR-Mutant Non-Small Cell Lung Cancer Resistant to EGFR Tyrosine Kinase Inhibitors: Focus on Novel Therapies. Lung Cancer 2022, 170, 41–51. 10.1016/j.lungcan.2022.05.011. [DOI] [PubMed] [Google Scholar]
- Wang P.; Huang Q.; Meng S.; Mu T.; Liu Z.; He M.; Li Q.; Zhao S.; Wang S.; Qiu M. Identification of Lung Cancer Breath Biomarkers Based on Perioperative Breathomics Testing: A Prospective Observational Study. eClinicalMedicine 2022, 47, 101384. 10.1016/j.eclinm.2022.101384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S. S.; Abdul S. S.; Shannon Chiu H. Y.; Roy R. B.; Huang P. Y.; Malwade S.; Nursetyo A. A.; Jack Li Y. C. Artificial-Intelligence-Based Prediction of Clinical Events among Hemodialysis Patients Using Non-Contact Sensor Data. Sensors (Switzerland) 2018, 18 (9), 2833. 10.3390/s18092833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Granero M. A.; Sanchez-Morillo D.; Leon-Jimenez A. An Artificial Intelligence Approach to Early Predict Symptom-Based Exacerbations of COPD. Biotechnology & Biotechnological Equipment 2018, 32 (3), 778–784. 10.1080/13102818.2018.1437568. [DOI] [Google Scholar]
- Binson V. A.; Subramoniam M. Artificial Intelligence Based Breath Analysis System for the Diagnosis of Lung Cancer. Journal of Physics: Conference Series 2021, 1950, 012065. 10.1088/1742-6596/1950/1/012065. [DOI] [Google Scholar]
- Kalra N.; Verma P.; Verma S. Advancements in AI Based Healthcare Techniques with FOCUS ON Diagnostic Techniques. Computers in Biology and Medicine 2024, 179, 108917. 10.1016/j.compbiomed.2024.108917. [DOI] [PubMed] [Google Scholar]
- Lazanas A. C.; Prodromidis M. I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Measurement Science Au 2023, 3, 162–193. 10.1021/acsmeasuresciau.2c00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upasham S.; Banga I. K.; Jagannath B.; Paul A.; Lin K. C.; Muthukumar S.; Prasad S. Electrochemical Impedimetric Biosensors, Featuring the Use of Room Temperature Ionic Liquids (RTILs): Special Focus on Non-Faradaic Sensing. Biosens Bioelectron 2021, 177, 112940. 10.1016/j.bios.2020.112940. [DOI] [PubMed] [Google Scholar]
- Dorledo de Faria R. A.; Dias Heneine L. G.; Matencio T.; Messaddeq Y. Faradaic and Non-Faradaic Electrochemical Impedance Spectroscopy as Transduction Techniques for Sensing Applications. Int. J. Biosens Bioelectron 2019, 5 (1), 29. 10.15406/ijbsbe.2019.05.00148. [DOI] [Google Scholar]
- Brenden C. K.; Iyer H.; Zhang Y.; Kim S.; Shi W.; Vlasov Y. A. Enhancement of Faradaic Current in an Electrochemical Cell Integrated into Silicon Microfluidic Channels. Sens Actuators B Chem. 2023, 385, 133733. 10.1016/j.snb.2023.133733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong M. E.; Richards J. R.; Torres M.; Beck C. M.; La Belle J. T. Faradaic Electrochemical Impedance Spectroscopy for Enhanced Analyte Detection in Diagnostics. Biosens Bioelectron 2021, 177, 112949. 10.1016/j.bios.2020.112949. [DOI] [PubMed] [Google Scholar]
- Bigdeli I. K.; Yeganeh M.; Shoushtari M. T.; Zadeh M. K.. Electrochemical Impedance Spectroscopy (EIS) for Biosensing. In Nanosensors for Smart Manufacturing; Elsevier, 2021; pp 533–554 10.1016/B978-0-12-823358-0.00025-3. [DOI] [Google Scholar]
- Paul A.; Chiriacò M. S.; Primiceri E.; Srivastava D. N.; Maruccio G. Picomolar Detection of Retinol Binding Protein 4 for Early Management of Type II Diabetes. Biosens Bioelectron 2019, 128, 122–128. 10.1016/j.bios.2018.12.032. [DOI] [PubMed] [Google Scholar]
- Liu W.; Lewis S. E.; di Lorenzo M.; Squires A. M. Development of Redox-Active Lyotropic Lipid Cubic Phases for Biosensing Platforms. Langmuir 2024, 40 (1), 170–178. 10.1021/acs.langmuir.3c02307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magar H. S.; Hassan R. Y. A.; Mulchandani A. Electrochemical Impedance Spectroscopy (Eis): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. 10.3390/s21196578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.; Vyas G.; Paul P.; Srivastava D. N. Gold-Nanoparticle-Encapsulated Zif-8 for a Mediator-Free Enzymatic Glucose Sensor by Amperometry. ACS Appl. Nano Mater. 2018, 1 (7), 3600–3607. 10.1021/acsanm.8b00748. [DOI] [Google Scholar]
- Xu Q.; Pan Y.; Li W.; Yang Z.. Amperometric Sensors. In Fundamentals of Sensor Technology: Principles and Novel Designs; Elsevier, 2023; pp 123–145 10.1016/B978-0-323-88431-0.00007-7. [DOI] [Google Scholar]
- Rahman M. M.; Ahammad A. J. S.; Jin J. H.; Ahn S. J.; Lee J. J. A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors 2010, 10 (5), 4855–4886. 10.3390/s100504855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- Paul A.; Srivastava D. N. Amperometric Glucose Sensing at Nanomolar Level Using MOF-Encapsulated TiO2 Platform. ACS Omega 2018, 3 (11), 14634–14640. 10.1021/acsomega.8b01968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga I.; Paul A.; Poudyal D. C.; Muthukumar S.; Prasad S. Recent Advances in Gas Detection Methodologies with a Special Focus on Environmental Sensing and Health Monitoring Applications—A Critical Review. ACS Sens 2023, 8 (9), 3307–3319. 10.1021/acssensors.3c00959. [DOI] [PubMed] [Google Scholar]
- Silvester D. S. New Innovations in Ionic Liquid-Based Miniaturised Amperometric Gas Sensors. Curr. Opin Electrochem 2019, 15, 7–17. 10.1016/j.coelec.2019.03.001. [DOI] [Google Scholar]
- Galassetti P. R.; Novak B.; Nemet D.; Rose-Gottron C.; Cooper D. M.; Meinardi S.; Newcomb R.; Zaldivar F.; Blake D. R. Breath Ethanol and Acetone as Indicators of Serum Glucose Levels: An Initial Report. Diabetes Technol. Ther 2005, 7 (1), 115–123. 10.1089/dia.2005.7.115. [DOI] [PubMed] [Google Scholar]
- Belluzo M. S.; Ribone M. E.; Lagier C. M. Assembling Amperometric Biosensors for Clinical Diagnostics. Sensors 2008, 8, 1366–1399. 10.3390/s8031366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graef E. W.; Munje R. D.; Prasad S. A Robust Electrochemical CO2 Sensor Utilizing Room Temperature Ionic Liquids. IEEE Trans Nanotechnol 2017, 16 (5), 826–831. 10.1109/TNANO.2017.2672599. [DOI] [Google Scholar]
- Paul A.; Banga I. K.; Muthukumar S.; Prasad S. Engineering the ZIF-8 Pore for Electrochemical Sensor Applications—A Mini Review. ACS Omega 2022, 7 (31), 26993–27003. 10.1021/acsomega.2c00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Cheng S.; Liu H.; Hu S.; Zhang D.; Ning H. A Survey on Gas Sensing Technology. Sensors (Switzerland). 2012, 12, 9635–9665. 10.3390/s120709635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi D.; Wang H.; Huang W.; Hu L.; Tang Y.; Guo Z.; Ouyang Z.; Zhang H. Recent Advances in Two-Dimensional-Material-Based Sensing Technology toward Health and Environmental Monitoring Applications. Nanoscale 2020, 12 (6), 3535–3559. 10.1039/C9NR10178K. [DOI] [PubMed] [Google Scholar]
- Huang D.; Xin Q.; Ni Y.; Shuai Y.; Wang S.; Li Y.; Ye H.; Lin L.; Ding X.; Zhang Y. Synergistic Effects of Zeolite Imidazole Framework@graphene Oxide Composites in Humidified Mixed Matrix Membranes on CO 2 Separation. RSC Adv. 2018, 8 (11), 6099–6109. 10.1039/C7RA09794H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G.; Li S.; Guo Z.; Farha O. K.; Hauser B. G.; Qi X.; Wang Y.; Wang X.; Han S.; Liu X.; DuChene J. S.; Zhang H.; Zhang Q.; Chen X.; Ma J.; Loo S. C. J.; Wei W. D.; Yang Y.; Hupp J. T.; Huo F. Imparting Functionality to a Metal-Organic Framework Material by Controlled Nanoparticle Encapsulation. Nat. Chem. 2012, 4 (4), 310–316. 10.1038/nchem.1272. [DOI] [PubMed] [Google Scholar]
- Paul A.; Muthukumar S.; Prasad S. Review—Room-Temperature Ionic Liquids for Electrochemical Application with Special Focus on Gas Sensors. J. Electrochem. Soc. 2020, 167 (3), 037511. 10.1149/2.0112003JES. [DOI] [Google Scholar]
- Banga I.; Paul A.; Sardesai A. U.; Muthukumar S.; Prasad S. ZEUS (ZIF-Based Electrochemical Ultrasensitive Screening) Device for Isopentane Analytics with Focus on Lung Cancer Diagnosis. RSC Adv. 2021, 11 (33), 20519–20528. 10.1039/D1RA03093K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banga I.; Paul A.; Sardesai A. U.; Muthukumar S.; Prasad S. ZeNose/GO Hybrid Composite for Detection of Clinically Relevant VOCs in Lower Respiratory Tract (Case Study Using Carene). Mater. Lett. 2022, 307, 130975. 10.1016/j.matlet.2021.130975. [DOI] [Google Scholar]
- Banga I.; Paul A.; Sardesai A.; Muthukumar S.; Prasad S. AuNP@ZeNose (ZIF-Based Electrochemical Nose) for Detection of Flu Biomarker in Breath. Microchimica Acta 2022, 189 (6), 231. 10.1007/s00604-022-05334-1. [DOI] [PubMed] [Google Scholar]
- Xu Y.; Liu X.; Cao X.; Huang C.; Liu E.; Qian S.; Liu X.; Wu Y.; Dong F.; Qiu C. W.; Qiu J.; Hua K.; Su W.; Wu J.; Xu H.; Han Y.; Fu C.; Yin Z.; Liu M.; Roepman R.; Dietmann S.; Virta M.; Kengara F.; Zhang Z.; Zhang L.; Zhao T.; Dai J.; Yang J.; Lan L.; Luo M.; Liu Z.; An T.; Zhang B.; He X.; Cong S.; Liu X.; Zhang W.; Lewis J. P.; Tiedje J. M.; Wang Q.; An Z.; Wang F.; Zhang L.; Huang T.; Lu C.; Cai Z.; Wang F.; Zhang J. Artificial Intelligence: A Powerful Paradigm for Scientific Research. Innovation 2021, 2, 100179. 10.1016/j.xinn.2021.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F.; Yue Y.; Zhang Y.; Zhang Z.; Zhou H. S. Advancing Biosensors with Machine Learning. ACS Sens 2020, 5 (11), 3346–3364. 10.1021/acssensors.0c01424. [DOI] [PubMed] [Google Scholar]
- Chen M.; Cui D.; Haick H.; Tang N. Artificial Intelligence-Based Medical Sensors for Healthcare System. Advanced Sensor Research 2024, 3 (3), 2300009. 10.1002/adsr.202300009. [DOI] [Google Scholar]
- Sardesai A. U.; Tanak A. S.; Krishnan S.; Striegel D. A.; Schully K. L.; Clark D. V.; Muthukumar S.; Prasad S. An Approach to Rapidly Assess Sepsis through Multi-Biomarker Host Response Using Machine Learning Algorithm. Sci. Rep 2021, 11 (1), 16905. 10.1038/s41598-021-96081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankhala D.; Sardesai A. U.; Pali M.; Lin K. C.; Jagannath B.; Muthukumar S.; Prasad S. A Machine Learning-Based on-Demand Sweat Glucose Reporting Platform. Sci. Rep 2022, 12 (1), 2442. 10.1038/s41598-022-06434-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.; Cho I.; Park J.; Jeong J.; Lee K.; Lee B.; Del Orbe Henriquez D.; Yoon K.; Park I. High Accuracy Real-Time Multi-Gas Identification by a Batch-Uniform Gas Sensor Array and Deep Learning Algorithm. ACS Sens 2022, 7 (2), 430–440. 10.1021/acssensors.1c01204. [DOI] [PubMed] [Google Scholar]
- Rajpurkar P.; Chen E.; Banerjee O.; Topol E. J. AI in Health and Medicine. Nature Medicine 2022, 28, 31–38. 10.1038/s41591-021-01614-0. [DOI] [PubMed] [Google Scholar]