Abstract
The molecular nature of a severe multisystemic disorder with a recurrent nonimmune hydrops fetalis was identified as deficiency of GDP-Man:GlcNAc2-PP-dolichol mannosyltransferase, the human orthologue of the yeast ALG1 gene (MIM 605907). The disease belongs to the group of congenital disorders of glycosylation (CDG) and is designated as subtype CDG-Ik. In patient-derived serum, the total amount of the glycoprotein transferrin was reduced. Moreover, a partial loss of N-glycan chains was observed, a characteristic feature of CDG type I forms. Metabolic labeling with [6-3H]glucosamine revealed an accumulation of GlcNAc2-PP-dolichol and GlcNAc1-PP-dolichol in skin fibroblasts of the patient. Incubation of fibroblast extracts with [14C]GlcNAc2-PP-dolichol and GDP-mannose indicated a severely reduced activity of the β1,4-mannosyltransferase, elongating GlcNAc2-PP-dolichol to Man1GlcNAc2-PP-dolichol at the cytosolic side of the endoplasmic reticulum. Genetic analysis of the patient’s hALG1 gene identified a homozygous mutation leading to the exchange of a serine residue to leucine at position 258 in the hALG1 protein. The disease-causing nature of the hALG1 mutation for the glycosylation defect was verified by a retroviral complementation approach in patient-derived primary fibroblasts and was confirmed by the expression of wild-type and mutant hALG1 in the Saccharomyces cerevisiae alg1-1 strain.
Introduction
Glycosylation of proteins is one of the most widespread forms of co- and posttranslational modification that has been found in eukaryotes, as well as in prokaryotes. In eukaryotes, glycoproteins are located inside cells predominantly in membranes of the secretory and endocytic pathway. Moreover, they are abundant in extracellular fluids and matrices. Glycan moieties attached to glycoproteins were shown to affect their folding and their transport, as well as their biological activity and stability (Spiro et al. 2002). The process of protein glycosylation is essential for viability and normal development and requires >100 glycosyltransferases, glycosidases, and transport proteins for sugar nucleotides (Helenius and Aebi 2001; Trombetta 2003). So far, 14 different molecular causes for inherited deficiencies in this complex metabolic pathway have been described in humans; these are termed “congenital disorders of glycosylation” (CDG) (Jaeken 2003; Thiel et al. 2003; Wu et al. 2003). CDG comprise a group of multisystemic diseases with mostly severe psychomotor and mental retardation. Biochemically, the disorders are characterized by defective glycosylation of proteins caused by mutations in genes required for the biosynthesis of N-linked oligosaccharides. CDG are subdivided into two groups. CDG type I (CDG-I) comprise defects that affect biosynthesis of dolichol-linked oligosaccharides in the cytosol or the endoplasmic reticulum (ER), as well as defects involving the transfer of oligosaccharides onto nascent glycoproteins. CDG type II (CDG-II) encompass all defects of further trimming and elongation of N-linked oligosaccharides in the ER and the Golgi (Aebi et al. 1999).
Here we describe a molecular defect in glycoprotein biosynthesis that results in a new type of CDG-I in humans (termed “CDG-Ik”). The defective biosynthesis is due to the faulty transfer of mannosyl residues from GDP-Man to GlcNAc2-PP-dolichol, which is catalyzed by the enzyme hALG1 at the cytosolic side of the ER.
Methods
Patient
The patient described here is the second child (P.B.) in the original case report (de Koning et al. 1998). Ultrasound analysis at the 30th wk of pregnancy revealed fetal hydrops and hepatosplenomegaly. Pregnancy was interrupted at the 35th wk because of bradycardia. The severely hydropic boy showed multiple dysmorphic features with a large fontanelle, hypertelorism, micrognathia, hypogonadism, contractures, areflexia, cardiomyopathy, and multifocal epileptic activity. The patient died at 2 wk of age. Isoelectric focusing (IEF) of serum transferrin revealed a typical CDG-I pattern with the appearance of disialo- and asialotransferrin. Normal activity of phosphomannomutase excluded CDG-Ia.
IEF and SDS/PAGE of Serum Transferrin
IEF and SDS/PAGE of serum transferrin were performed as described elsewhere (Niehues et al. 1998).
Cell Lines and Cell Culture
Fibroblasts from the patient and the controls were maintained at 37°C under 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM [Gibco BRL]), which contained 10% fetal calf serum (FCS [PAN Biotech]). The ecotropic packaging cell line FNX-Eco (ATCC) and the amphotropic packaging cell line RetroPack PT67 (Clontech) were cultured in DMEM containing 10% FCS—which was heat-inactivated at 56°C for 30 min—at 37°C under 5% CO2, unless otherwise stated.
Analysis of Dolichol- and Protein-Derived Oligosaccharides
Fibroblasts derived from the controls and the patient were grown and metabolically labeled with [2-3H]mannose for 30 min at 37°C. Dolichol- and protein-linked oligosaccharides were extracted, released, and analyzed by high-performance liquid chromatography (HPLC), as described elsewhere (Körner et al. 1998).
TLC Analysis of Short Dolichol-Linked Oligosaccharides
Prior to the experiment (60 h), 3.6 × 106 control- or patient-derived fibroblasts were plated onto 100-mm cell-culture dishes. Cells were labeled for 60 min in the presence of 1 mCi [6-3H]glucosamine (29.0 Ci/mmol [Amersham Biosciences]) in DMEM containing 2% dialyzed FCS, 0.5 mM glucose, and 0.5 mM mannose. Fibroblasts were scrapped in methanol, and the short dolichol-linked oligosaccharides were extracted and separated by thin-layer chromatography (TLC), as described elsewhere (Körner et al. 1998).
Enzyme Assays
To determine ALG1-encoded mannosyltransferase activity, GlcNAc2-PP-Dol was used as the glycosyl acceptor. Analysis of subsequent mannose elongation steps was performed with Man1GlcNAc2-PP-Dol as the acceptor. The reactions contained the following, in a final volume of 0.06 ml: [14C]GlcNAc2-PP-Dol (4,000 cpm) or Man1[14C]GlcNAc2-PP-Dol (4,000 cpm), 0.13% Nonidet P40, 10 mM MgCl2, 0.9 mM DTT, 0.14 mM Na-EDTA, 19 mM Tris-HCl (pH 7.2), 1 mM GDP-Man, and solubilized enzyme (equivalent to 0.05 mg membrane protein). Incubations were performed at 37°C for 12 min. The reaction was stopped by the addition of chloroform:methanol to give a ratio of chloroform:methanol:water of 2:1:1 (by volume) and was processed further by phase separation (Sharma et al. 1982) by use of an upper phase of chloroform:methanol:water of 1:32:48 (by volume) and by collecting both lower phase and inter phase. The solubilized extract was obtained from a particulate fibroblast fraction, prepared as described elsewhere (Knauer and Lehle 1994; Thiel et al. 2002), except that membranes were suspended in 20 mM Tris (pH 7.2), 10 mM MgCl2, and 1 mM DTT. Solubilization was performed at a protein concentration of 7 mg/ml and 1% Nonidet NP40. Preparation of [14C]GlcNAc2-PP-dolichol and Man1[14C]GlcNAc2-PP-dolichol glycosyl acceptors was performed as described elsewhere (Thiel et al. 2003).
Mutation Analysis
Total RNA was extracted from fibroblasts and leukocytes from the controls, the patient, and the patient's parents by use of the RNAeasy kit (Qiagen). First-strand cDNA was synthesized from 0.5 μg of total RNA with Omniscript reverse transcriptase (Qiagen) and the primer R1 (5′-CAAAGCTTCCCGGGTCACAG-3′). In the first round of PCR, the cDNA was amplified using the primers F1 (5′-GATCCCAATACAGTAACAGCTTT-3′) and R1 by use of the HotStar Taq polymerase kit (Qiagen), with a preincubation at 95°C for 15 min followed by 28 cycles with 1 min at 94°C, 0.5 min at 55°C, and 3 min at 72°C. Further amplification was performed with the nested primers F2 (5′-GGGAACCGCGTCCACTTCTC-3′) and R2 (5′-CACTGGGAGGTGCTGCTCG-3′). Reverse transcriptase PCR products were run on 1% agarose gels. The 1,593-bp fragment was prepared with the QIAquick PCR purification kit (Qiagen) and subcloned into the pGEM-T-Easy vector (Promega). Sequence analyses of the PCR products and the plasmids were performed by dye-determined cycle sequencing with the primers pUC M13 forward and pUC M13 reverse (Stratagene), F2, R2, R3 (5′-CTCAGGAGTTTCTGGTCCGGT-3′), and R4 (5′-GTGGAGCCGGAGGTCGGTC-3′) on an Applied Biosystems model 373A automated sequencer.
Genomic DNA was prepared from control and patient fibroblasts, as well as from blood leukocytes of the parents, by use of standard procedures (Maniatis 1989). PCR was performed with the primers gen-F1 (5′-GATGTGGCTGGGCACCCCA-3′) and gen-R1 (5′-CTGGCAGGGGTGAGGAGAAC-3′), as described above. Nested PCR was performed using the primers gen-F2 (5′-GGGAGCCTGCAGGCCTCG-3′) and gen-R2 (5′-AGGTGCCCGTCACACCAACC-3′), resulting in a 301-bp fragment. The PCR products were analyzed on a 1% agarose gel and prepared as described above. Sequence analyses were performed using primers gen-F3 (5′-GGAGATGCCTCTCCTGGGTC-3′).
Site-Directed Mutagenesis
A 1.6-kb fragment of a wild-type ALG1-cDNA representing the coding sequence (nt 107–nt 1486) was amplified by PCR, by use of primers F2 and R2. The resulting fragment was purified and cloned into the pGEM-T-Easy vector (pGEM-T-Easy–wild-type). The mutation C773T was inserted into the cDNA by use of the QuickChange site-directed mutagenesis kit (Stratagene), according to the manufacturer’s instructions, with the primers Mut773-A (5′-GGAGCGGTTGGCCTTCACG-3′) and Mut773-B (5′-CGTGAAGGCCAACCGCTCC-3′) to obtain plasmid pGEM-T-Easy-Pat. Wild-type and patient ALG1 cDNA was subcloned into the MoMuLV-derived vector pLNCX2 (Clontech).
Retroviral Complementation
Ecotropic FNX-Eco cells (5 × 105) were seeded onto dishes (60-mm diameter) 1 d before transfection. Transient transfection by FuGENE6 reagent was performed according to the manufacturer’s protocol (Roche), with 1 μg of LNCX2 vector (mock), LNCX2 wild type, and LNCX2 patient. Further procedures were performed as described elsewhere (Thiel et al. 2002). The supernatant with the amphotropic retroviral particles was used to transfect patient and control fibroblasts. After infection of the fibroblasts, the medium was replaced by DMEM, containing 10% heat-inactivated FCS with geneticin (335 μg/ml [Gibco BRL]). Selection was performed for 10 d.
Yeast Genetics
The cDNAs of the human alg1 mannosyltransferases from the controls and the patient, cloned into the pLNCX2 vector (Clontech), were isolated as NotI 1.4-kbp fragments and ligated into the NotI site of the yeast shuttle vector pNEV-N, under the control of the PMA1 promotor, to give pNEV-hALG1-Wt and pNEV-ALG1-Pat, respectively. Plasmids were transformed into the alg1-1 yeast strain (MATα ura3-52) by use of standard techniques (Gietz and Schiestl 1991). Yeast cells were grown in YNBD medium (0.67% yeast nitrogen dropout ura, 2% glucose).
Growth Complementation of alg1 Yeast Cells
Transformants were grown in selective liquid medium overnight at 25°C, and 3 μl of serial 10-fold dilutions were spotted on agar plates, starting at 104 cells, and were incubated at 25°C or 36°C for 4 d.
In Vivo Labeling of Dolichol-Linked Oligosaccharides in Yeast
Yeast cells were grown at 25°C to midlogarithmic phase in selective medium and were shifted to 36°C for 40 min. Subsequent labeling with [2-3H]mannose at 36°C, extraction, and analysis of dolichol-linked oligosaccharides were performed as described elsewhere (Knauer and Lehle 1999).
Metabolic Labeling of Carboxypeptidase Y (CPY)
Yeast cells were grown at 25°C in selective medium to midlogarithmic phase. Cells were harvested, resuspended in fresh medium, adapted to 25°C or 36°C for 30 min, and labeled with 75 μCi [35S] methionine/cysteine (Pro-mix [Amersham Biosciences]) for 45 min. Preparation of cell lysates, immunoprecipitation of CPY, and analysis by SDS-PAGE were performed as described elsewhere (Knauer and Lehle 1999).
Results
Partial Lack of Entire N-Linked Oligosaccharide Side Chains in Serum Transferrin
IEF of serum transferrin is the standard diagnostic procedure for CDG. Previous data for the index patient revealed a partial loss of sialic acids leading to an increase of di- and asialotransferrin at the expense of tetrasialotransferrin, indicating a case of CDG-I. To exclude a diagnosis of CDG-Ia, which is the most frequent type of CDG-I, the activity of phosphomannomutase 2, the enzyme deficient in CDG-Ia, was determined and was found to be normal (de Koning et al. 1998).
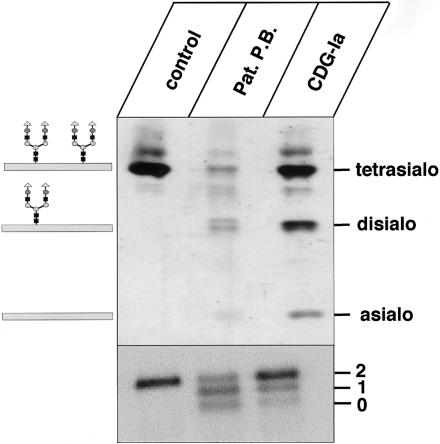
Our analysis of the transferrin IEF confirmed the data published for patient P.B. (de Koning et al. 1998) and demonstrated, moreover, that the total amount of transferrin was reduced in the serum of the patient, compared with serum of a control person and a CDG-Ia patient (fig. 1, upper panel). The mass of the protein was determined by SDS-PAGE (fig. 1, lower panel). The presence of faster-migrating transferrin forms indicated the loss of one or both of the two N-linked oligosaccharide side chains, which are normally present in transferrin. Deficiency of phosphomannose isomerase, which leads to a comparable IEF pattern of transferrin in CDG-Ib, was excluded, since enzyme activity determined in patient fibroblasts was normal (data not shown).
Figure 1.
IEF pattern and SDS-PAGE of serum transferrin. Sera from a control, patient P.B., and a patient with CDG-Ia were analyzed by IEF (upper panel) and SDS-PAGE, followed by western blotting (lower panel) and immunodetection of transferrin. “Tetrasialo,” “disialo,” and “asialo” on the upper panel indicate transferrin forms with four, two, or no sialic acid residues. The numerals “2,” “1,” and “0” in the lower panel indicate transferrin forms with two, one, or zero oligosaccharide chains.
Accumulation of Shortened Dolichol-Linked Oligosaccharides in Patient-Derived Fibroblasts
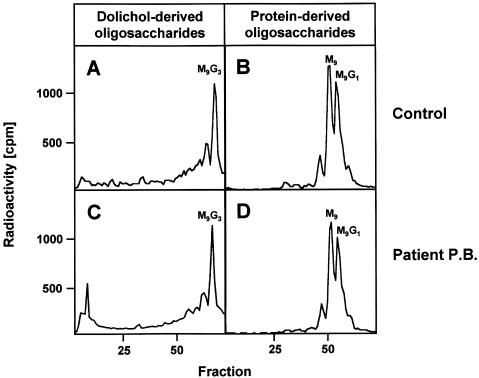
First, to investigate the molecular mechanism of the glycosylation defect, we attempted to study the transfer of GlcNAc2Man9Glc3 moieties from dolichol onto newly synthesized glycoproteins, which is catalyzed by the oligosaccharyltransferase complex. Control- and patient-derived fibroblasts were metabolically labeled with [2-3H]mannose, followed by extraction of total glycoproteins. N-glycan chains from equal amounts of glycoproteins were released by peptide:N-glycosidase F (PNGase F) treatment and subsequently were analyzed by HPLC (fig. 2B and 2D). The oligosaccharides released from newly synthesized glycoproteins of controls and the patient eluted mainly at positions corresponding to GlcNAc2Man9Glc1 and GlcNAc2Man9 standards, respectively. In four independent experiments, no significant difference in the amount of labeled oligosaccharides was observed between the patient and the controls. These results indicate that, at least under the experimental conditions, sufficient dolichol-linked oligosaccharides in patient fibroblasts are transferred onto newly synthesized glycoproteins with a normal activity of oligosaccharyltransferase.
Figure 2.
Analysis of protein- and dolichol-derived oligosaccharides in CDG-Ik. Fibroblasts of a control (B) and the patient (D) were metabolically labeled with [2-3H]mannose for 30 min, [2-3H]glycans were released from equal amounts of the glycoprotein fraction by PNGase F digestion and were size fractionated by HPLC. M9 and M9G1 refer to the positions of GlcNAc2Man9 and GlcNAc2Man9Glc1 standards, respectively. Control- (A) and patient-derived (C) fibroblasts (in equal amounts) were metabolically labeled with [2-3H]mannose for 30 min. The [2-3H]oligosaccharides were released from the dolichol-PP moiety by mild acid hydrolysis and were size fractionated by HPLC. M9G3 refers to the position of a GlcNAc2Man9Glc3 standard.
Next, we investigated the size of dolichol-linked oligosaccharides by metabolic labeling of fibroblasts from a control and the patient with [2-3H]mannose for 30 min, followed by extraction with chloroform:methanol:water (10:10:3), release of the glycan moieties by mild acid hydrolysis, and subsequent size fractionation of the oligosaccharides by HPLC. The majority of dolichol-linked oligosaccharides from the control and the patient eluted at the position of a GlcNAc2Man9Glc3 standard (fig. 2A and 2C), indicating the formation of full-length dolichol-linked oligosaccharides. Radioactivity eluting in fractions 1–6 (e.g., fig. 2C) represents [2-3H]mannose that is occasionally carried over into the chloroform:methanol:water (10:10:3) fraction during extraction of dolichol-linked oligosaccharides.
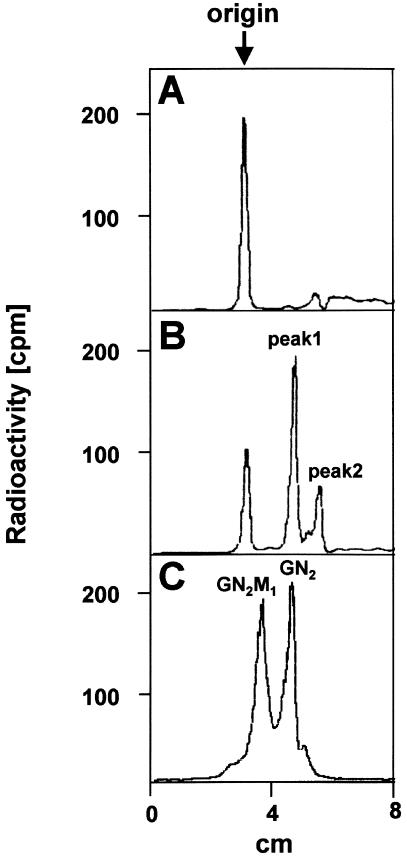
For CDG-Ii [MIM 607906], it was shown (Thiel et al. 2003) that a defect in one of the early steps of dolichol-linked oligosaccharide biosynthesis leads to an accumulation of short dolichol-linked oligosaccharides that escape the extraction procedure described above. Moreover, defects in the initiating steps of dolichol-linked oligosaccharide biosynthesis, such as the addition of the two glucosamine residues and the first mannose residue, are missed by metabolic labeling with [2-3H]mannose. Therefore, we labeled fibroblasts from a control and the patient for 60 min in the presence of [6-3H]glucosamine, extracted the cells with chloroform:methanol (3:2), and analyzed the extract by TLC (fig. 3). Besides nonmigrating radioactivity at the origin in the control (fig. 3A) and the patient (fig. 3B) extracts, we observed two additional peaks in the case of the patient (fig. 3B; peak 1 and peak 2). Peak 1 migrated with GlcNAc2-PP-dolichol from a standard mixture containing GlcNAc2-PP-dolichol and Man1GlcNAc2-PP-dolichol (fig. 3C). Peak 2 is supposed to be dolichol-PP-GlcNAc1, which migrates faster due to its more hydrophobic nature.
Figure 3.
TLC analysis of [6-3H]glucosamine-labeled short dolichol-linked oligosaccharides. Fibroblasts from a control (A) and from patient P.B. (B) were metabolically labeled for 60 min with [6-3H]glucosamine. After extraction of the short lipid-linked oligosaccharide fraction with chloroform:methanol (3:2), further analysis was performed by TLC on silica gel 60 plates with chloroform:methanol:water (65:25:4) as solvent. The position of the origin and the positions of a [14C]GlcNAc2-PP-dolichol standard and a Man1[14C]GlcNAc2-PP-dolichol standard are indicated as GN2 and GN2M1, respectively (C).
Deficiency of GDP-Mannose:GlcNAc2-PP-Dolichol β1,4-Mannosyltransferase (hAlg1) in Patient-Derived Fibroblasts
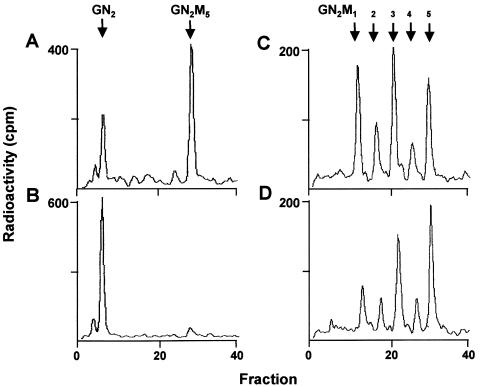
The analysis of dolichol-linked oligosaccharides revealed a predominant accumulation of GlcNAc2-PP-dolichol and a minor accumulation of GlcNAc1-PP-dolichol in fibroblasts of the patient. A comparable accumulation pattern of biosynthetic intermediates of dolichol-linked oligosaccharides has been described for the temperature-sensitive alg1-1 mutant from Saccharomyces cerevisiae, in which the GDP-mannose:GlcNAc2-PP-dolichol β1,4-mannosyltransferase is defective at the restrictive temperature (Huffacker and Robbins 1982). To determine whether elongation of GlcNAc2-PP-dolichol is impaired in the patient, an in vitro assay was established by incubating microsomal extracts from control and patient fibroblasts with GDP-mannose and [14C]GlcNAc2-PP-dolichol. An extension of the newly synthesized glycan up to Man5[14C]GlcNAc2-PP-dolichol was observed in presence of the microsomal extract from control fibroblasts but not from fibroblasts of patient P.B. (fig. 4A and 4B).
Figure 4.
In vitro determination of hALG1 and hALG2 activity. Microsomal extracts from fibroblasts of a control (A, C) and the patient (B, D) were incubated for 10 min with either [14C]GlcNAc2-PP-dolichol (A, B) or with Man1[14C]GlcNAc2-PP-dolichol (C, D), respectively, in the presence of GDP-mannose. Dolichol-linked oligosaccharides were extracted from the incubation mixture and were treated by mild acid hydrolysis, and the released oligosaccharides were separated by HPLC. The positions of a GlcNAc2-standard (GN2) and Man1–5GlcNAc2 (GN2M1–5)-standards are marked by arrows.
Next, we investigated the ability of control and patient cell extracts to elongate Man1GlcNAc2-PP-dolichol, which is catalyzed by the hALG2-encoded α1,3 mannosyltransferase. The reaction catalyzes the transfer of the second mannosyl residue in the dolichol-linked oligosaccharide assembly. Fibroblast extracts from a control and from patient P.B. were incubated in the presence of GDP-mannose and Man1[14C]GlcNAc2-PP-dolichol. We found that both cell extracts were able to elongate the oligosaccharide chain up to Man5 [14C]GlcNAc2-PP-dolichol (fig. 4C and 4D). These findings demonstrate that the transfer of the first mannosyl residue to GlcNAc2-PP-dolichol, which is catalyzed by hALG1, is impaired in case of the patient.
Detection of a Homozygous Missense Mutation in the hALG1 Gene
Sequencing of the hALG1 cDNA (accession number BAA90748 [NCBI Entrez Database]) revealed homozygosity for a C773T transition in the case of the patient (data not shown). The C773T transition causes the substitution of serine residue 258 with a leucine residue. The mutation was confirmed on the level of genomic DNA. Both parents were shown to be heterozygous for the C773T transition mutation on the cDNA, as well as on the genomic level (data not shown).
Retroviral Expression of Wild-Type hALG1 Complements for GDP-Man:GlcNAc2-PP-Dolichol Mannosyltransferase Deficiency in the Patient
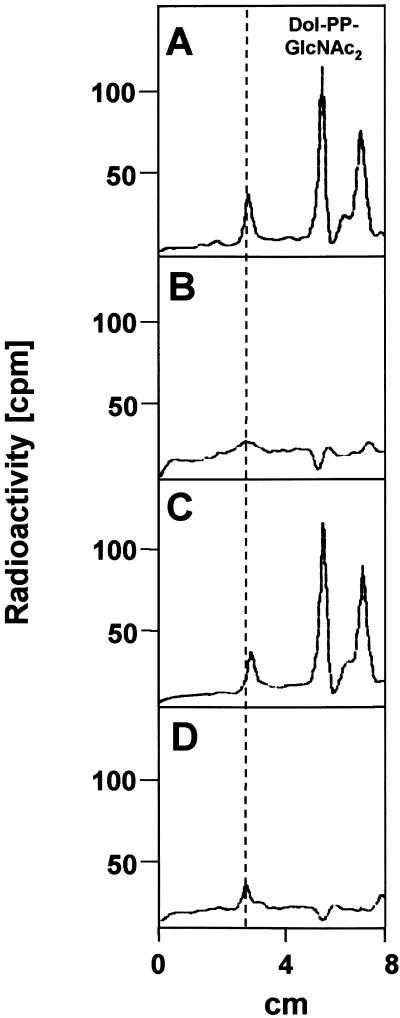
To confirm that the C773T transition mutation in the hALG1 gene is the disease-causing mutation in the patient, we expressed hALG1 wild-type cDNA (fig. 5B), as well as the hALG1 cDNA carrying the C773T mutation (fig. 5C) in patient-derived fibroblasts, by use of a retroviral expression system. The effect of the retroviral vector alone was investigated in patient fibroblasts, as well as control fibroblasts (fig. 5A and 5D). TLC analysis of extracts from [6-3H]glucosamine-labeled patient fibroblasts expressing the retroviral vector alone (fig. 5A) showed a major peak corresponding to a Dol-PP-GlcNAc2 standard and a second peak that is supposed to be Dol-PP-GlcNAc1, which is not present in control fibroblasts (fig. 5D). Retroviral transduction of the wild-type hALG1 cDNA, but not of the C773T mutant hALG1 cDNA, led to normalization of dolichol-linked oligosaccharide biosynthesis in the patient, demonstrating that the hALG1 deficiency causes the biochemical defect in the patient and that the C773T mutation causes inactivity of hALG1.
Figure 5.
Retroviral transduction of patient fibroblasts with wild-type hALG1 cDNA leads to complementation of the hALG1 deficiency. Dolichol-PP-[3H]GlcNAc2 and dolichol-PP-[3H]GlcNAc1 were extracted with chloroform:methanol (3:2) from [6-3H]glucosamine-labeled control fibroblasts expressing the retroviral vector alone (D) and from patient fibroblasts, which were either transduced with the retroviral vector alone (A), the wild-type cDNA (B), or the C773T hALG1 cDNA (C). Further analysis was performed by TLC. The elution position of a [14C]GlcNAc2-PP-dolichol standard and the origin (dotted line) are indicated.
Wild-Type hALG1 Complements for the Defect in an S. Cerevisiae alg1 Mutant Strain
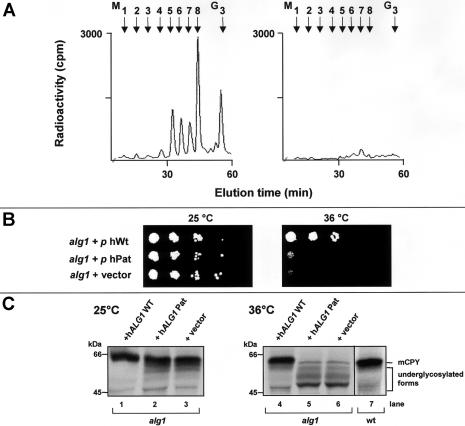
To confirm the complementation results in patient fibroblasts, we introduced cDNAs encoding wild-type hALG1 or the hALG1 C773T transition mutation into the temperature-sensitive alg1-1 yeast strain. We found that only transformation with the wild type but not with the mutant hALG1 cDNA led to normalization of dolichol-linked oligosaccharide biosynthesis in alg1-1 cells (fig. 6A), when analyzed by metabolic labeling with [2-3H]mannose.
Figure 6.
Defects in dolichol-linked oligosaccharide biosynthesis (A), growth (B), and CPY glycosylation (C) in the alg1-1 yeast mutant are complemented by expression of wild-type hALG1. A, Biosynthesis of dolichol-linked oligosaccharides was investigated in an alg1-1 strain transformed with the wild-type hALG1 cDNA (left panel) or the hALG1 cDNA encoding the C773T mutation (right panel). Yeast cells were metabolically labeled with [2-3H]mannose for 30 min, [2-3H] oligosaccharides were released from the dolichol moiety by mild acid hydrolysis and further analyzed by HPLC. M1–M8 and G3 refer to Man1–8GlcNAc2 and Glc3Man9GlcNAc2 standards, respectively. B, Growth of yeast alg1-1 cells either transformed with wild-type hALG1 cDNA, the C773T hALG1 or the expression vector was investigated under permissive (25°C, left panel) and nonpermissive temperature (36°C, right panel). C, The glycosylation status of CPY is shown at the permissive temperature (25°C, left panel) and at the nonpermissive temperature (36°C, right panel). Yeast cells were metabolically labeled with 35S-methionine for 30 min, and CPY was immunoprecipitated and analyzed by SDS-PAGE. The position of the mature form of CPY in wild-type yeast cells (mCPY) and in the complemented alg1-1 cells are indicated on the right. Molecular weight standards are indicated on the left.
Complementation was also evident when the growth behavior in the alg1-1 strain was investigated (fig. 6B). At the permissive temperature of 25°C, alg1-1 cells that were transformed with either the vector alone, the hALG1 C773T mutant cDNA, or the hALG1 wild-type cDNA showed comparable growth (fig. 6, left panel). At the nonpermissive temperature of 36°C, correction of the growth phenotype occurred only by transformation with wild-type hALG1 cDNA, but not with the hALG1 C773T mutant (fig. 6, right panel).
Next, we analyzed the glycosylation of the vacuolar glycoprotein CPY (fig. 6C). At the permissive temperature, the glycosylation state of CPY was comparable between alg1-1 cells either transformed with the vector alone (lane 3), the hALG1 C773T cDNA (lane 2), or the hALG1 wild-type cDNA (lane 1). The glycosylation state of CPY is severely reduced in the alg1-1 strain at the nonpermissive temperature of 36°C because of a reduced transfer of truncated oligosaccharides onto the newly synthesized protein (fig. 6C, lane 6). Again, only transformation of the alg1-1 strain with the wild type (lane 4), but not with the C773T hALG1 cDNA (lane 5), led to normalization of CPY glycosylation, as occurring in yeast wild-type cells (lane 7). Taken together, these results confirm, first, that the C773T transition in the hALG1 gene is the disease-causing mutation in CDG-Ik and, second, that the isolated gene is indeed the human orthologue to the yeast ALG1 gene.
Discussion
In a patient suffering from multiple dysmorphic features, hypertelorism, micrognathia, hepatosplenomegaly, hypogonadism, contractures, areflexia, cardiomyopathy, and multifocal epileptic activity (de Koning et al. 1998), we determined a new molecular defect in the biosynthesis of N-linked glycans. The defect in this new disorder, termed “CDG-Ik,” affecting an early step of dolichol-linked oligosaccharide biosynthesis catalyzed by the ALG1-encoded mannosyltransferase gene, was independently identified in two other patients with CDG (T. Marquardt, personal communication).
Dolichol-linked oligosaccharide biosynthesis comprises a series of reactions by which, in a stepwise, ordered manner, two GlcNAc and five mannose residues are added from nucleotide sugar donors to dolichol-phosphate by glycosyltransferases on the cytosolic side of the ER. After the translocation of Man5GlcNAc2-PP-dolichol into the lumen of the ER, the oligosaccharide moiety is further elongated to Glc3Man9GlcNAc2-PP-dolichol, with Dol-P-Man and Dol-P-Glc as donor substrates (Snider and Rogers 1984). The molecular defect of the CDG-Ik patient affects the ALG1-encoded mannosyltransferase, catalyzing the transfer of the first β1,4-linked mannose residue onto the dolichol-bound chitobiose residue at the outer leaflet of the ER, causing the accumulation of GlcNAc2-PP-dolichol. Accumulation of GlcNAc1-PP-dolichol, the biosynthetic precursor of GlcNAc2-PP-dolichol, was also observed. This is probably caused by feedback inhibition of the preceding reaction resulting from the accumulation of GlcNAc2-PP-dolichol, which interferes with a so-far-unknown enzyme catalyzing the elongation of GlcNAc1-PP-dolichol. A comparable accumulation of GlcNAc2-PP-dolichol and GlcNAc1-PP-dolichol for the temperature-sensitive S. cerevisiae alg1-1 strain has been described elsewhere. In yeast, deficiency of ALG1 leads to underglycosylation of glycoproteins and cell death (Huffacker and Robbins 1982). The cDNA for the human orthologue of the yeast ALG1 encodes a transmembrane protein with 464 amino acids containing several regions highly conserved between yeast and humans (Takahashi et al. 2000). In the case of the patient with CDG-Ik, we identified homozygosity for a C773T transition mutation in hALG1 at the mRNA and genomic DNA levels, whereas the patient's parents were heterozygous carriers of the mutation. The mutation leads to replacement of a serine by a leucine residue at position 258 in the hALG1 protein. To prove the disease-causing nature of the C773T mutation in the hALG1 gene, we expressed the wild-type and the mutant hALG1 cDNA in patient-derived fibroblasts and in an S. cerevisiae alg1-1 strain, respectively. In both cases, only the wild-type cDNA complemented for the ALG1 deficiency, but not the C773T mutation.
The serine residue at position 258 in the hAlg1 protein is conserved in the mouse and is replaced by a threonine residue in Drosophila melanogaster, as well as in S. cerevisiae, indicating the importance of the hydroxyl group. Computer-assisted protein structure analysis (TMpred) did not predict phosphorylation or O-glycosylation of serine 258. The hALG1 protein is predicted to have four transmembrane domains. Serine 258 is part of a loop, 222 amino acids in length, located in the lumen of the ER. This would indicate that the mutation does not directly affect either the binding sites for the substrates or the catalytic sites that are supposed to reside in the cytosolic domain. Instead, the Ser258Leu mutation causes a conformational change of the hAlg1 protein in the patient.
The comparable HPLC size patterns of dolichol-linked oligosaccharides in the chloroform:methanol:water (10:10:3) fraction in control and patient fibroblasts metabolically labeled with [2-3H]mannose points to the leaky nature of the hALG1 deficiency in the patient. Manifestation of the defect may be restricted to tissues, such as liver or pancreas, with high rates of glycoprotein biosynthesis.
The biosynthesis of dolichol-linked oligosaccharides is routinely investigated by metabolic labeling of cells with [2-3H]mannose and analysis of the glycan moieties in the fraction extracted with chloroform:methanol:water (10:10:3). Similar to the metabolic defect in patients with CDG-Ii, in which the Alg2-mediated elongation of the dolichol-linked Man1GlcNAc2 trisaccharide is deficient, the metabolic defect in patients with CDG-Ik would have escaped detection if just the routine protocol for dolichol-linked oligosaccharide analysis had been applied. Early biosynthetic intermediates of dolichol-linked oligosaccharides are seldom (and poorly) labeled with [2-3H]mannose. Moreover, they are extracted with chloroform:methanol (3:2) preceding extraction with chloroform:methanol:water (10:10:3). Therefore, we recommend that, in the growing number of patients with a CDG-I IEF pattern for transferrin of unknown origin, the fibroblasts should be metabolically labeled with [6-3H]glucosamine and the chloroform:methanol (3:2) extract should be analyzed for dolichol-linked sugar residues.
Acknowledgments
The authors thank Professor Gert Matthijs, Catholic University Leuven, Belgium, for supplying us with patient fibroblasts. The authors acknowledge the support of the European Commission (contract QLG1-CT2000-00047 [Euroglycan]), the Deutsche Forschungsgemeinschaft, and the Fonds der Chemischen Industrie.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- National Center for Biotechnology Information (NCBI) Entrez Database, http://www.ncbi.nlm.nih.gov/Entrez/ (for hALG1 cDNA [accession number BAA90748])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for ALG1 and CDG-Ii)
References
- Aebi M, Helenius A, Schenk B, Barone R, Fiumara A, Berger EG, Hennet T, et al (1999) Carbohydrate-deficient glycoprotein syndromes become congenital disorders of glycosylation: an updated nomenclature for CDG. First International Workshop on CDGS. Glycoconj J 16:669–671 10.1023/A:1017249723165 [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH (1991) Applications of high efficiency lithium acetate transformation of intact yeast cells using single stranded nucleic acids as carrier. Yeast 7:253–263 [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi A (2001) Intracellular functions of N-linked glycans. Science 291:2364–2369 10.1126/science.291.5512.2364 [DOI] [PubMed] [Google Scholar]
- Huffaker T, Robbins P (1982) Temperature-sensitive yeast mutants deficient in asparagine-linked glycosylation. J Biol Chem 257:3203–3210 [PubMed] [Google Scholar]
- Jaeken J (2003) Congenital disorders of glycosylation (CDG): it’s all in it! J Inherit Metab Dis 26:99–118 10.1023/A:1024431131208 [DOI] [PubMed] [Google Scholar]
- Knauer R, Lehle L (1994) The N-oligosaccharyltransferase complex from yeast. FEBS Letters 344:83–86 10.1016/0014-5793(94)00356-4 [DOI] [PubMed] [Google Scholar]
- Knauer R, Lehle L (1999) The oligosaccharyltransferase complex from Saccharomyces cerevisiae: isolation of the OST6 gene, its synthetic interaction with OST3, and analysis of the native complex. J Biol Chem 274:17249–17256 10.1074/jbc.274.24.17249 [DOI] [PubMed] [Google Scholar]
- Körner C, Lehle L, von Figura K (1998) Abnormal synthesis of mannose-1-phosphate derived carbohydrates in carbohydrate deficient glycoprotein syndrome type I fibroblasts with phosphomannomutase deficiency. Glycobiology 8:165–171 10.1093/glycob/8.2.165 [DOI] [PubMed] [Google Scholar]
- de Koning TJ, Toet M, Dorland L, de Vries LS, van den Berg IET, Duran M, Poll-The BT (1998) Recurrent nonimmune hydrops fetalis associated with carbohydrate-deficient glycoprotein syndrome. J Inher Metab Dis 21:681–682 10.1023/A:1005496920435 [DOI] [PubMed] [Google Scholar]
- Maniatis NT, Fritsch EF, Sambrook J (1989) Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- Niehues R, Hasilik M, Alton G, Körner C, Schiebe-Sukumar M, Koch, HG, Zimmer KP, Wu R, Harms E, Reiter K, von Figura K, Freeze HH, Harms HK, Marquardt T (1998) Carbohydrate-deficient glycoprotein syndrome type 1b: phosphomannose isomerase deficiency and mannose therapy. J Clin Invest 101:1414–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma CB, Lehle L, Tanner W (1982) Solubilization and characterization of the initial enzymes of the dolichol pathway from yeast. Eur J Biochem 126:319–325 [DOI] [PubMed] [Google Scholar]
- Snider MD, Rogers OC (1984) Transmembrane movement of oligosaccharide-lipids during glycoprotein synthesis. Cell 36:753–761 [DOI] [PubMed] [Google Scholar]
- Spiro R (2002) Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 12:43R–56R 10.1093/glycob/12.4.43R [DOI] [PubMed] [Google Scholar]
- Takahashi T, Honda R, Nishikawa Y (2000) Cloning of the human cDNA which can complement the defect of the yeast mannosyltransferase I-deficient mutant alg1. Glycobiology 10:321–327 10.1093/glycob/10.3.321 [DOI] [PubMed] [Google Scholar]
- Thiel C, Schwarz M, Hasilik M, Grieben U, Hanefeld F, Lehle L, von Figura K, Körner C (2002) Congenital disorder of glycosylation-Ig is caused by deficiency of dolichyl-P-Man:Man7 GlcNAc2-PP-dolichyl mannosyltransferase. Biochem J 367:195–201 10.1042/BJ20020794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel C, Schwarz M, Peng J, Grzmil M, Hasilik M, Braulke T, Kohlschütter A, von Figura K, Lehle L, Körner C (2003) A new type of congenital disorders of glycosylation (CDG-Ii) provides new insights into the early steps of dolichol-linked oligosaccharide biosynthesis. J Biol Chem 278:22498–22505 10.1074/jbc.M302850200 [DOI] [PubMed] [Google Scholar]
- Trombetta S (2003) The contribution of N-glycans and their processing in the endoplasmic reticulum to glycoprotein biosynthesis. Glycobiology 13:77R–91R 10.1093/glycob/cwg075 [DOI] [PubMed] [Google Scholar]
- Wu R, Rush J, Karaoglu D, Krasnewich D, Lubinsky M, Waechter C, Gilmore R, Freeze H (2003) Deficiency of UDP-GlcNAc:dolichol-phosphate N-acetylglucosamine-1 phosphate transferase (DPAGT1) causes a novel congenital disorder of glycosylation type Ij. Hum Mutat 22:144–150 10.1002/humu.10239 [DOI] [PubMed] [Google Scholar]