Abstract
A large French family including members affected by nonspecific X-linked mental retardation, with or without autism or pervasive developmental disorder in affected male patients, has been found to have a 2–base-pair deletion in the Neuroligin 4 gene (NLGN4) located at Xp22.33. This mutation leads to a premature stop codon in the middle of the sequence of the normal protein and is thought to suppress the transmembrane domain and sequences important for the dimerization of neuroligins that are required for proper cell-cell interaction through binding to β-neurexins. As the neuroligins are mostly enriched at excitatory synapses, these results suggest that a defect in synaptogenesis may lead to deficits in cognitive development and communication processes. The fact that the deletion was present in both autistic and nonautistic mentally retarded males suggests that the NLGN4 gene is not only involved in autism, as previously described, but also in mental retardation, indicating that some types of autistic disorder and mental retardation may have common genetic origins.
X-linked mental retardation (XLMR) is a highly heterogeneous condition, including >140 distinct disorders and affecting ∼1.6/1,000 males, with a carrier frequency of 2.4/1,000 females (Herbst and Miller 1980; Stevenson and Schwartz 2002). Two groups have been defined: nonspecific forms (MRX), in which mental retardation (MR) is the only clinical manifestation, and syndromic forms (MRXS), in which MR is associated with recognizable physical signs such as skeletal abnormalities or dysmorphic facial features (Chelly and Mandel 2001). Both MRX and MRXS genes have been located at various regions of the X chromosome (Chiurazzi et al. 2001). To date, it is estimated that at least 30 genes are involved in MRX, but only 14 have been isolated.
Autism is a neurodevelopmental disorder beginning before the age of 3 years and characterized by severe social and communication impairments, repetitive and ritualistic behaviors, and a restricted pattern of interests (American Psychiatric Association 1994). The prevalence of autism is estimated at ∼10/10,000 when broader definitions are used (Fombonne 2003). Many studies, including correlation in twins and association of autism with Mendelian diseases, have supported a genetic etiology for this condition. Nevertheless, autism appears to be clinically and genetically heterogeneous, suggesting that multiple loci are involved (Risch et al. 1999). The male/female sex ratio for autism is estimated at 4:1 (Fombonne 2003), and several genome screens have shown that at least two loci on the X chromosome are associated with a predisposition to autism—that is, Xp22.3 and Xq13-21 (Philippe et al. 1999; Auranen et al. 2002). The report of a de novo chromosomal deletion at Xp22.3 in three unrelated autistic females (Thomas et al. 1999) and a nonsense mutation recently found in the Neuroligin 4 (NLGN4) gene in a family with two non–mentally retarded brothers with autism and Asperger syndrome (MIM 300427) respectively (Jamain et al. 2003), suggest that this gene could be involved in autistic disorders.
Wide intra- and extrafamilial phenotype heterogeneity exists in MRX conditions. Mild-to-severe MR and association with psychiatric disorders such as autism is frequently reported. MR is present in ∼70% of individuals with autism, and in half of these individuals intellectual quotient (IQ) is evaluated as <50 (Fombonne 2002). Among 25 French families including 135 male patients with MRX135, we found 7 families with at least one mentally retarded male with autistic disorder, according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV [American Psychiatric Association 1994]). Fragile X syndrome (FRAXA) was excluded, and diagnosis of isolated MR or MR associated with autistic symptoms was made after examination of the patients (Gomot et al., unpublished data).
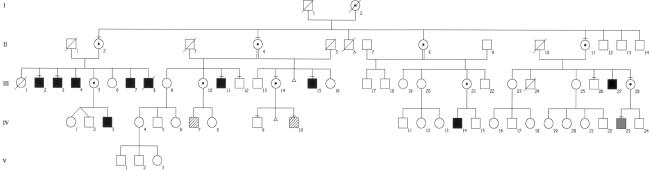
We present here the molecular and clinical data of one of these families (family T118) in which we found a null mutation in the NLGN4 gene in autistic and nonautistic mentally retarded males. The pedigree of the family is shown in figure 1. In this family, 13 males have been identified as having autism, MR, or pervasive developmental disorder; for eight subjects, this diagnosis was based on direct medical examination or on their medical records (table 1). Interviews of the nonaffected family members confirmed that the other subjects were institutionalized and that one of them was killed in a road accident (individual III-8).
Figure 1.
Pedigree of family T118. The diagram shows the 13 males who were identified as having nonspecific MR (blackened squares), nonspecific MR with autism (diagonally striped squares), or pervasive developmental disorder (gray square). For eight subjects, diagnoses were made by direct examination or from medical records, and the five other males were institutionalized. A minus sign (−) above a symbol indicates that a blood sample was obtained for analysis.
Table 1.
Summary of Clinical Findings[Note]
| Finding in Subject | ||||||||||||||
| Male |
||||||||||||||
| Affected |
||||||||||||||
| Characteristics | III-2a | III-3 | III-4a | III-7a | IV-3a | III-11 | IV-7a | IV-10 | III-15 | IV-14a | III-27a | IV-23 | Unaffected,IV-9 | Female,III-14 |
| Age at examination (years) | NA | 44 | NA | NA | NA | 42 | 11 | 8 | 30 | NA | NA | 16 | 13 | 34 |
| Dysmorphic features | No | Plagiocephaly | ND | ND | ND | No | ND | Plagiocephaly | No | ND | ND | No | No | No |
| Intelligence | ND | Mild MR | ND | ND | ND | Subnormal | ND | Mild MR | Subnormal | ND | ND | Subnormal | Normal | Normal |
| Autism | ND | No | ND | ND | ND | No | Yes | Yes | No | No | No | No | ||
| IQ: | ||||||||||||||
| Total | ND | 63 | ND | ND | ND | 77 | ND | 62 | 72 | ND | ND | 74 | 142 | 96 |
| Verbal | ND | 70 | ND | ND | ND | 82 | ND | 37 | 79 | ND | ND | 76 | 127 | 90 |
| Performance | ND | 60 | ND | ND | ND | 75 | ND | 88 | 63 | ND | ND | 75 | 141 | 105 |
Note.— NA = not available; ND = not determined.
Institutionalized patient.
Patient IV-10 was aged 8 years 1 mo at the time of the clinical evaluation by a child psychiatrist and by a psychologist. He met diagnostic criteria for autistic disorder (DSM-IV [American Psychiatric Association 1994]). Diagnostic evaluation also included the Autism Diagnostic Interview—Revised (ADI [Lord et al. 1994]). His ADI scores were 13 in the social domain (cutoff = 8), 13 in the language (verbal) domain (cutoff = 8), and 4 in the repetitive/restrictive behaviors domain (cutoff = 3). Developmental abnormalities were identified before 36 mo of age. Developmental ages for the Vineland Adaptative Behavior Scale (Sparrow et al. 1985) were 3 years 2 mo, for communication, 3 years 10 mo, for daily living skills, 2 years 9 mo, for socialization, and 4 years 11 mo, for motor skills. The intellectual assessment was made using the age-appropriate Wechsler Preschool and Primary Scale of Intelligence—Revised (WPPSI-R [Wechsler 1989]), providing verbal and performance IQs of 37 and 88, respectively, and an overall IQ of 62, which led to an associated diagnosis of mild MR. Physical examination revealed normal growth parameters with no discernible dysmorphic features, except for posterior plagiocephaly. Electroencephalogram (EEG) and morphometric MRI results showed no abnormalities. Late auditory evoked potentials with mismatch-negativity paradigm showed a typical profile for children with autism (Gomot et al. 2002).
His mother (III-14), a 34-year-old obligate carrier, was tested with the Wechsler Adult Intelligence Scale—III (WAIS-III [Wechsler 1997]) and had a verbal IQ of 90, a performance IQ of 105, and an overall IQ of 96. Her subscore in nonverbal analogic treatment indicated retardation, with a developmental age of 10 years.
Patients III-3 and III-11 were 44 and 42 years of age, respectively, when they were examined (using the WAIS-III) by a psychiatrist with experience of working with children and adults with developmental disorders. They met DSM-IV criteria for MR without an associated psychiatric disorder. Patient III-3 had mild MR with a verbal IQ of 70, a performance IQ of 60, and an overall IQ of 63. Patient III-11 had subnormal intelligence with a verbal IQ of 82, a performance IQ of 75, and an overall IQ of 77. Patient III-11 had no dysmorphic features. He had a head trauma injury at the age of 4 years that resulted in coma for 6 d. Patient III-3 presented posterior plagiocephaly. His work environment was adapted for mentally handicapped adults. Patient III-4 declined psychiatric examination but was admitted to a psychiatric unit for aggressive behavior at the time of his brother’s clinical interview.
Clinical information on patient III-15, a 30-year-old man, was taken from an interview with his mother and from his medical records. He was born after three miscarriages. Delivery was normal. He experienced hypoxic-ischemic events in the first weeks of life, with epileptic seizures from 1 to 9 years of age. Psychomotor development was retarded, and he could sit alone at the age of 2 years. He was tested with the WAIS-III and had subnormal intelligence with a verbal IQ of 79, a performance IQ of 63, and an overall IQ of 72. He receives an allowance for mentally handicapped adults. He is friendly and sociable.
Clinical information on patient IV-23 was taken from medical records. At the age of 2.5 years, he was admitted to a children's psychiatric unit. He was identified as having pervasive developmental disorder, not otherwise specified (PDD-NOS). He was 16 years of age when he was assessed with the Weschler Adult Intelligence Scale—Revised (WAIS-R [Wechsler 1981]), and he had a verbal IQ of 76, a performance IQ of 75, and an overall IQ of 74. He also presented transient tic disorder and received neuroleptic treatment. The only available medical information concerning patient IV-7, an 11-year-old boy, is that his condition was diagnosed as autism in a children's psychiatric unit.
In family T118, two-point linkage analysis by use of 41 DNA markers spanning the X chromosome indicated a strong linkage of XLMR to Xp21.2-pter, with a maximum LOD score of 3.30 (θ=0) for DXS996 and DXS989. Several previously isolated MRX genes fall within the linkage interval, such as RSK2 (Merienne et al. 1999), IL1RAPL (Carrie et al. 1999), VCX (Fukami et al. 2000), and ARX (Bienvenu et al. 2002; Stromme et al. 2002). Furthermore, the report of de novo Xp deletions in three females with autism (Thomas et al. 1999) and the description of a susceptibility locus at DXS996 (Philippe et al. 1999) suggested that a gene involved in autistic disorder was located at Xpter. A gene encoding for a member of the neuroligin family, named “Neuroligin 4” (NLGN4 or KIAA1260), has recently been mapped to Xp22.33 (obtained from the Ensembl Genome Browser) and closely linked to DXS996 (Bolliger et al. 2001), and a mutation was found in two brothers with autism and Asperger syndrome, both without MR (Jamain et al. 2003).
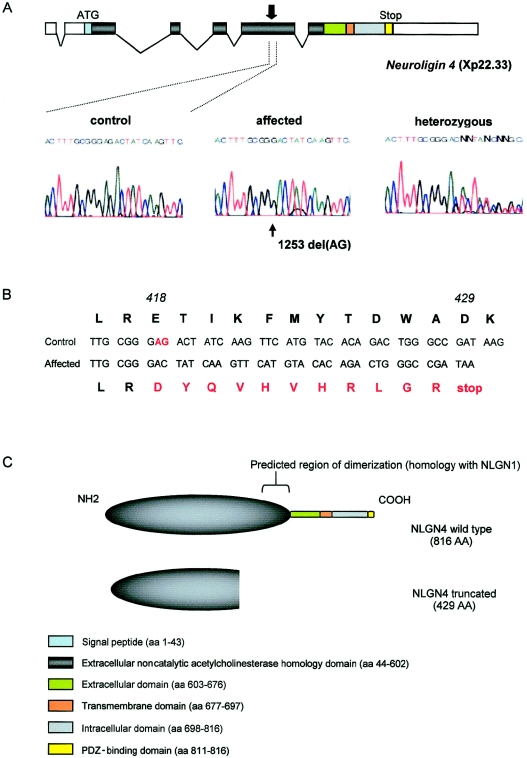
We sequenced this gene in the members of family T118 for whom genomic DNA was available (fig. 1). After PCR analysis of the coding exons, the products were directly sequenced. We detected a 2-bp deletion, nt1253del(AG) (GenBank accession numbers: NM_020742, AF376803), in the fifth exon of NLGN4 in all affected patients (fig. 2a). This deletion caused a frameshift in the coding sequence and was predicted to create a stop codon at position 429 (fig. 2b). The healthy males did not carry the deletion, and the obligate carrier females were heterozygous for the mutation. We did not observe this deletion in 200 healthy male controls, and analysis of the X-chromosome inactivation profile in the obligate carrier females was not informative. The resulting mutated protein is predicted to be truncated by ∼50% of its normal sequence (429 amino acids, instead of 816) and to lose the C-terminal transmembrane domain.
Figure 2.
Identification of the NLGN4 mutation in family T118. A, Schematic representation of the organization of the NLGN4 gene and representative NLGN4 sequence of normal, affected, and heterozygous subjects from family T118. PCR products of each coding exon (from 100 ng of DNA) were amplified (primers and PCR conditions available on request), purified through Sephadex G-50 columns, and analyzed by agarose-gel electrophoresis. The products were then sequenced using the ABI PRISM BigDye Terminator Sequencing Kit (PE Applied Biosystems) on an ABI PRISM 377 automated sequencer. The arrows mark the position of the NLGN4 mutation (cDNA nucleotide 1253). B, Sequence alignments between the normal and the predicted mutated NLGN4 proteins. The position of the mutation and the consequence on the amino acid sequence are indicated in red. C, Schematic representation of the NLGN4 protein with the predicted constitutive domains by homology with the other neuroligins. The region of dimerization is positioned at the base of the AchE domain and would be absent in the truncated NLGN4.
We also screened for mutations of this gene in eight families collected by the European XLMR consortium with MRX overlapping Xp22.33 regions and DXS996 (including family MRX49 [Claes et al. 1997] and seven small families with two affected brothers), but we failed to detect any variations in the coding sequence. The other families with MRX and autism were not screened for the NLGN4 gene because their linkage area did not fall in Xp22.33.
Jamain et al. (2003) recently reported a nonsense mutation in NLGN4 that led to a predicted truncated protein of 396 amino acids in a Swedish family with two affected brothers who exhibited autism or Asperger syndrome, both without MR. Our study showed the involvement of the same gene in XLMR with or without autism. Altogether, these results suggest that mutations in NLGN4 are involved in a wide spectrum of phenotypes, ranging from mild isolated MR without communication deficits (family T118) to Asperger syndrome with normal or supranormal intelligence (Jamain et al. 2003). The phenotypic heterogeneity of this X-linked condition suggests that the expression of the mutation is modulated by genetic or nongenetic factors.
The NLGN4 gene belongs to the neuroligin family made up of neuronal cell-surface proteins located in the synaptic structures. They are composed of a large extracellular noncatalytic acetylcholinesterase homology domain, which is required for presynaptic neurexin binding, a transmembrane domain, and a short cytoplasmic tail with a postsynaptic density 95-disc large zona-occludens-1 (PDZ) binding domain (fig. 2). They are particularly abundant in the postsynaptic membrane of glutamatergic synapses, suggesting specific targeting to excitatory synapses (Song et al. 1999).
Experiments in vitro have shown that the intercellular neuroligin-neurexin adhesion complex is able to trigger formation of functional presynaptic elements and leads to axon specialization (Scheiffele et al. 2000). As the neuroligin C-terminus binds the postsynaptic proteins PSD-95 and S-SCAM that link neuroligins to NMDA receptors and downstream signal-transducing proteins, the neuroligin-neurexin interaction may also mediate postsynaptic differentiation (Rao et al. 2000).
A previous study has shown that oligomerization of neuroligins is required for their activity in synaptogenesis. Dean et al. (2003) demonstrated that two alpha helices located at the base of the AchE-homologous domain of NLGN1 were critical for interaction between other neuroligins, with inactivating amino acid mutations that affect multimerization and reduce the synapse-promoting activity of the protein.
Although these experiments were performed with constructs from the Neuroligin 1 gene that shares 71.2% identity with NLGN4 in their amino acid sequences, we can expect that the mutated NLGN4 protein will lack structural elements indispensable for its specific activity. Indeed, the sequence homology between NLGN1 and NLGN4 and the position of the mutation in NLGN4 in family T118 indicates that these regions, which are important for oligomerization, were deleted, thus potentially leading to a loss of synaptogenic activity (fig. 2c).
In summary, we report a new gene involved in XLMR, which is also associated with autism and pervasive developmental disorder. Moreover, this gene has also been reported to be involved in autism and Asperger syndrome without MR, suggesting strong phenotypic heterogeneity. We propose that NLGN4 deficiency in the brain may lead to abnormal development of synaptic structures and may have dramatic effects on communication processes and cognitive development. The generation of animal models with null mutations of this gene would provide an approach to the functional consequences of such a deficiency.
Acknowledgments
We thank the patients, members of family T118, and the European XLMR consortium for their participation in this study. We also thank Catherine Cherpi-Antar and Brigitte Jauffrion for technical assistance. This work was supported by grants from Institut National de la Santé et de la Recherche Medicale (INSERM), Fondation Jerome Lejeune, Fondation de France, Fondation France Telecom, and by the 5th EU Framework (RTD Project-QLRT-2001-01810) and the GIS maladies rares (project A021817GS).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- Ensembl Genome Browser, http://www.ensembl.org
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for NLGN4 mRNA [accession numbers NM_020742 and AF376803])
- Kazusa DNA Research Institute, http://www.kazusa.or.jp/huge (for information about KIAA clones)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/
References
- American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th ed. (DSM-IV) American Psychiatric Association, Washington, DC [Google Scholar]
- Auranen M, Vanhala R, Varilo T, Ayers K, Kempas E, Ylisaukko-Oja T, Sinsheimer JS, Peltonen L, Jarvela I (2002) A genomewide screen for autism-spectrum disorders: evidence for a major susceptibility locus on chromosome 3q25–27. Am J Hum Genet 71:777–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu T, Poirier K, Friocourt G, Bahi N, Beaumont D, Fauchereau F, Ben Jeema L, Zemni R, Vinet MC, Francis F, Couvert P, Gomot M, Moraine C, van Bokhoven H, Kalscheuer V, Frints S, Gecz J, Ohzaki K, Chaabouni H, Fryns JP, Desportes V, Beldjord C, Chelly J (2002) ARX, a novel Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in X-linked mental retardation. Hum Mol Genet 11:981–991 10.1093/hmg/11.8.981 [DOI] [PubMed] [Google Scholar]
- Bolliger MF, Frei K, Winterhalter KH, Gloor SM (2001) Identification of a novel neuroligin in humans which binds to PSD-95 and has a widespread expression. Biochem J 356:581–588 10.1042/0264-6021:3560581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie A, Jun L, Bienvenu T, Vinet MC, McDonell N, Couvert P, Zemni R, Cardona A, Van Buggenhout G, Frints S, Hamel B, Moraine C, Ropers HH, Strom T, Howell GR, Whittaker A, Ross MT, Kahn A, Fryns JP, Beldjord C, Marynen P, Chelly J (1999) A new member of the IL-1 receptor family highly expressed in hippocampus and involved in X-linked mental retardation. Nat Genet 23:25–31 [DOI] [PubMed] [Google Scholar]
- Chelly J, Mandel JL (2001) Monogenic causes of X-linked mental retardation. Nat Rev Genet 2:669–680 10.1038/35088558 [DOI] [PubMed] [Google Scholar]
- Chiurazzi P, Hamel BC, Neri G (2001) XLMR genes: update 2000. Eur J Hum Genet 9:71–81 [DOI] [PubMed] [Google Scholar]
- Claes S, Vogels A, Holvoet M, Devriendt K, Rayemaekers P, Cassiman JJ, Fryns JP (1997) Regional localization of two genes for non-specific X-linked mental retardation to Xp22.3-p22.2 (MRX49) and Xp11.3-p11.21 (MRX50). Am J Med Genet 73:474–479 [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P (2003) Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci 6:708–716 10.1038/nn1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fombonne E (2002) Epidemiological trends in rates of autism. Mol Psychiatry Suppl 7:4–6 10.1038/sj.mp.4001162 [DOI] [PubMed] [Google Scholar]
- ——— (2003) Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord 33:365–382 10.1023/A:1025054610557 [DOI] [PubMed] [Google Scholar]
- Fukami M, Kirsch S, Schiller S, Richter A, Benes V, Franco B, Muroya K, Rao E, Merker S, Niesler S, Ballabio A, Ansorge W, Ogata T, Rappold GA (2000) A member of a gene family on Xp22.3, VCX-A, is deleted in patients with X-linked non-specific mental retardation. Am J Hum Genet 67:563–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomot M, Giard MH, Adrien JL, Barthelemy C, Bruneau N (2002) Hypersensivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology 39:577–584 10.1017/S0048577202394058 [DOI] [PubMed] [Google Scholar]
- Herbst DS, Miller JR (1980) Nonspecific X-linked mental retardation II: the frequency in British Columbia. Am J Med Genet 7:461–469 [DOI] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, Soderstrom H, Giros B, Leboyer M, Cillberg C, Bourgeron T (2003) Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Paris Autism Research International Sibpair Study. Nat Genet 34:27–29 10.1038/ng1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Lecouteur A (1994) Autism diagnostic interview—revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 24:659–685 [DOI] [PubMed] [Google Scholar]
- Merienne K, Jacquot S, Pannetier S, Zeniou M, Bankier A, Gecz J, Mandel JL, Mulley J, Sassone-Corsi P, Hanauer A (1999) A missense mutation in RPS6KA3 (RSK2) responsible for non-specific mental retardation. Nat Genet 22:13–14 10.1038/8719 [DOI] [PubMed] [Google Scholar]
- Philippe A, Martinez M, Guilloud-Bataille M, Gillberg C, Rastam M, Sponheim E, Coleman M, Zappella M, Aschauer H, van Maldergem L, Penet C, Feingold J, Brice A, Leboyer M, van Malldergerme L (1999) Genome-wide scan for autism susceptibility genes. Paris Autism International Sibpair Study. Hum Mol Genet 8:805–812 10.1093/hmg/8.5.805 [DOI] [PubMed] [Google Scholar]
- Rao A, Harms KJ, Craig AM (2000) Neuroligation: building synapses around the neurexin-neuroligin link. Nat Neurosci 3:747–749 10.1038/77636 [DOI] [PubMed] [Google Scholar]
- Risch N, Spiker D, Lotspeisch L, Nouri N, Hinds D, Hallmayer J, Kalaydjieva L, et al (1999) A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet 65:493–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T (2000) Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101:657-669 [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Südhof TC, Brose N (1999) Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci USA 96:1100–1105 10.1073/pnas.96.3.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV (1985) Vineland adaptative behavior scales: classroom edition. American Guidance Service, Circle Pines, MN [Google Scholar]
- Stevenson RE, Schwartz CE (2002) Clinical and molecular contributions to the understanding of X-linked mental retardation. Cytogenet Genome Res 99:265–275 10.1159/000071603 [DOI] [PubMed] [Google Scholar]
- Stromme P, Mangelsdorf ME, Shaw MA, Lower KM, Lewis SM, Bruyere H, Lutcherath V, Gedeon AK, Wallace RH, Scheffer IE, Turner G, Partington M, Frints SG, Fryns JP, Sutherland GR, Mulley JC, Gecz J (2002) Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet 30:441–445 10.1038/ng862 [DOI] [PubMed] [Google Scholar]
- Thomas NS, Sharp AJ, Browne CE, Skuse D, Hardie C, Dennis NR (1999) Xp deletions associated with autism in three females. Hum Genet 104:43–48 10.1007/s004390050908 [DOI] [PubMed] [Google Scholar]
- Wechsler D (1981) Wechsler adult intelligence scale—revised. Psychological Corp., NewYork [Google Scholar]
- ——— (1989) Manual for the Wechsler preschool and primary scale of intelligence—revised, Psychological Corporation, New York [Google Scholar]
- ——— (1997) Wechsler adult intelligence scale, 3rd ed. Psychological Corporation, New York [Google Scholar]