Abstract
Objectives:
Economic models assessing vaccinations commonly assume that inflation-adjusted vaccine costs are constant over time. This study assessed this assumption using historical vaccine cost data.
Methods:
Private sector and CDC contracted vaccine cost data (2001–2023) were collected from the CDC Vaccine Price List and converted to US$2023 to adjust for inflation. Trends in inflation-adjusted costs were described, and the annual percent changes in costs were calculated for each vaccine.
Results:
Changes in cost varied by vaccine. The average inflation-adjusted private sector costs increased by 0.9 % (IQR: −0.4 %—2.6 %) and 1.4 % (IQR: −0.4 %—3.2 %) annually among pediatric and adult vaccines, respectively, while CDC contracted costs increased by 0.9 % (IQR: 0.2 %—1.2 %) and 1.0 % (IQR: −0.8 %—2.1 %) annually among pediatric and adult vaccines, respectively.
Conclusions:
Inflation-adjusted vaccine costs increased on average since 2001, highlighting limitations of constant cost assumptions. These findings can inform cost-effectiveness analyses of multi-year vaccination programs and policies.
Keywords: United States, Vaccine cost, Cost-effectiveness analysis methods
1. Introduction
Routine vaccinations in the United States (US) generate substantial benefits by preventing an estimated 40,000 premature deaths with a net economic savings greater than $68.8 billion, among the cohort born in 2009 [1]. Since 1964, the Advisory Committee on Immunization Practices (ACIP) makes recommendations to the Centers for Disease Control and Prevention on the routine use of vaccines and related immunization pharmacological products in the US [2]. Part of ACIP deliberations on the recommended use of an immunization product considers the benefits and costs of vaccination strategies, estimated with cost-effectiveness models [3].
Identifying the most appropriate value for a cost per vaccine dose input in an economic model requires careful consideration of the objectives and perspective of the economic model [4]. In the case of a pre-market vaccine, a price or price range for the vaccine is often provided by the manufacturer. For simplicity and because future trends in the cost of the vaccine are unknown, models typically assume the cost per dose of the vaccine is constant in inflation-adjusted terms throughout the time horizon of the model.
Health care costs and pricing in the US are complex [5] and are generally not transparent [6]. At least three kinds of real-world costs may be relevant for economic decision-making in health care, including: (1) the list price or wholesale price of a product or service, (2) the contracted price, which is the negotiated price of a product, service, or group of either products or services between government agencies, health care systems or providers and the product manufacturers, and (3) the reimbursement for health care services paid by private insurance or public payers, such as Medicare and Medicaid.
The purpose of this study was to investigate the inflation-adjusted price trends of ACIP recommended vaccines using the manufacturers’ listed price and CDC contracted price as reported on the CDC Vaccine Price List (Price List). The CDC contracted price on the Price List documents the costs paid by federal vaccine contracts. The proportion of vaccines administered under the CDC pricing varies by vaccine. For example, the CDC administered Vaccines for Children (VFC) federal program provides no cost vaccines for eligible children. In 2014, an estimated 50 % of all children in the U.S. were VFC eligible [7]. The trends of the two reported vaccine prices can be used to assess the reasonableness of an assumption of constant future costs per dose of vaccines and can inform best practices for cost-effectiveness analyses of vaccines, whether done pre-market or post-market introduction.
2. Methods
2.1. Data collection
Data were collected from the Price List website [8]. The terms “price” and “cost” could be considered interchangeable in some contexts. The Price List labels the data as “cost per dose” so the term “cost” is used for consistency throughout this article. The Price List includes two types of cost. One is the private sector (private) cost, which is the cost per dose reported annually by vaccine manufacturers to the CDC. The other cost type is the CDC cost, which is the contract cost established for the purchase of vaccines by CDC on behalf of federally supported immunization programs (i.e., state health departments, select city and territory immunization programs). The CDC costs reported on the Price List get updated periodically as federal vaccine contracts are initiated, updated, or renewed. Therefore, during any given year, multiple costs per vaccine can be reported on the Price List. For the purposes of this study, a weighted annual cost was calculated. For example, the CDC cost of PCV13 was $150.83 on January 1, 2022, and was updated to $158.18 on April 1, 2022, so the weighted annual CDC cost of $156.37 was used ($150.83*(90/365) + $158.18*(275/365)).
This study collected data on pediatric and adult vaccines from the Price List and online archived lists on August 1, 2023. The vaccines’ historical costs were collected for as far back as either the earliest archived list (April 6, 2001), or the date when the vaccine was first introduced to the list, whichever date was later. Products added to the list after December 31, 2021, were not included in the analysis given they had less than 1 year of data at the time of data collection, where longer term trends in cost could not be assessed. These criteria excluded Dengvaxia, MenQuadfi™ (adults only), Prevnar 20™, Priorix, and Vaxneuvance™, along with all vaccines and immunization products for COVID-19 and RSV from the analysis. Influenza vaccines were also not included in this analysis. Cost information between influenza and noninfluenza products are less comparable due to differences in recommended ages across products, and the influenza vaccination recommendations are annual, not once-per-lifetime or multi-year booster recommendations.
All annual costs were adjusted for inflation using the health care component of the Personal Consumption Expenditures (PCE) index [9] and are reported in US$2023 [10]. For the inflation adjustment, an average of the PCE index values from quarter 1, 2, and 3 of 2023 was used as the US$2023 PCE index value. Results reported as “costs” in the rest of the article are inflation-adjusted costs. In a sensitivity analysis, a comparison of the main results was conducted using the medical care component of the Consumer Price Index (CPI) [11], as an alternative to the PCE index.
2.2. Analysis
The main analysis calculated changes in costs observed between the earliest year a vaccine’s cost was available and the cost as of August 1, 2023. Average annual changes in costs over time were reported as (1) the total change in the cost per dose divided by the number of years each vaccine was on the list, and (2) as an annual percent change in the cost per dose (see supplement for additional details). The two measures were reported for individual vaccines and summarized using descriptive statistics, stratified by age range (pediatric or adult), vaccine group (e.g., hepatitis B, pneumococcal, rotavirus), and time of introduction (before 2010, or 2010 and later). The study spans from 2001 to 2023, so the year 2010 was used as a threshold for separating the sample into two groups to assess if vaccines introduced earlier have different trends than vaccines introduced later in the observation period. We reported the initial analysis year cost, most recent year (2023) cost, and changes in cost for each vaccine. All analyses were conducted using R statistical software (version 4.1.2; The R Foundation) [12] using tidyverse [13]. All aggregated data and code are available at https://github.com/cdcgov/VaccinePriceList.
3. Results
An increase was observed for many vaccines for both private (32 of 50, or 64 %, Table 1, Fig. 1) and CDC costs (36 of 51, or 71 %, Table 2). Negative changes in cost per year was observed for the remaining vaccines. Adult vaccines had larger average annual percent increase in private cost (1.4 %) than pediatric vaccines (0.9 %) (Table 3). Among pediatric vaccines, the HPV vaccine Gardasil®9 had the largest change in costs per year in private cost ($9.25, 4.1 % increase per year) and in the CDC cost ($7.89, 4.3 % increase per year). Among adult vaccines, Gardasil®9 had the largest change in cost per year in private cost ($9.03, 4.0 % increase per year) and the pneumococcal vaccine Pneumovax®23 had the largest change in cost per year in CDC cost ($4.66, 10.0 % increase per year). Vaccines with the largest declines in costs include the Hib vaccine Hiberix® ($1.21, 6.0 % decrease per year), and the meningococcal vaccine Bexsero® ($2.59, 1.9 % decrease per year, adult only) which declined the most in cost per year in private and CDC cost, respectively.
Table 1.
Annual absolute and percentage changes in inflation-adjusted private costs (US$2023) of vaccines in the United States, by vaccine. 2001–2023 CDC Vaccine Price List. a.
| Private costs (US$2023) | Annual change (%)c | |||||
|---|---|---|---|---|---|---|
| Vaccine group | Brandname/ Tradename | Initial year on CDC Price Lista | Cost, initial year | Cost, 2023b | Annual change in cost | |
| Pediatric | ||||||
| DTaP/Tdap/Td | Adacel® | 2005 | 51.78 | 52.24 | 0.03 | 0.05 |
| Boostrix® | 2005 | 51.06 | 45.97 | −0.28 | −0.58 | |
| Daptacel® | 2003 | 32.16 | 35.61 | 0.17 | 0.51 | |
| Infanrix® | 2001 | 30.53 | 27.94 | −0.12 | −0.40 | |
| TDVAX™ | 2019 | 27.69 | 27.78 | 0.02 | 0.09 | |
| Tenivac® | 2012 | 25.57 | 39.58 | 1.27 | 4.05 | |
| DTaP/Hepatitis B/IPV | Pediarix® | 2003 | 97.21 | 92.41 | −0.24 | −0.25 |
| DTaP/IPV | Kinrix® | 2008 | 63.36 | 59.20 | −0.28 | −0.45 |
| Pentacel® | 2008 | 96.24 | 110.86 | 0.98 | 0.95 | |
| Quadracel™ | 2017 | 59.03 | 60.89 | 0.31 | 0.52 | |
| DTaP/IPV/HIB/Hepatitis B | Vaxelis™ | 2021 | 138.69 | 146.01 | 3.66 | 2.61 |
| Hepatitis A | Havrix® | 2001 | 49.06 | 36.83 | −0.56 | −1.29 |
| Vaqta® | 2001 | 41.90 | 36.59 | −0.24 | −0.61 | |
| Hepatitis A/B | Twinrix® | 2005 | 113.21 | 121.01 | 0.43 | 0.37 |
| Hepatitis B | Engerix B® | 2001 | 39.93 | 27.27 | −0.58 | −1.72 |
| Recombivax HB® | 2001 | 34.17 | 26.28 | −0.36 | −1.19 | |
| HiB | ActHIB® | 2001 | 25.16 | 18.88 | −0.29 | −1.30 |
| Hiberix® | 2009 | 29.34 | 12.41 | −1.21 | −5.96 | |
| PedvaxHIB® | 2001 | 30.35 | 28.80 | −0.07 | −0.24 | |
| HPV | Gardasil®9 | 2015 | 194.74 | 268.71 | 9.25 | 4.11 |
| Meningococcal | Bexsero® | 2015 | 188.50 | 210.47 | 2.75 | 1.39 |
| MenQuadfi™ | 2021 | 147.74 | 155.48 | 3.87 | 2.59 | |
| Menveo® | 2010 | 129.38 | 148.12 | 1.44 | 1.05 | |
| Trumenba® | 2015 | 135.73 | 178.34 | 5.33 | 3.47 | |
| MMR | M-M-R®II | 2001 | 46.60 | 89.67 | 1.96 | 3.02 |
| MMRV | ProQuad® | 2005 | 170.34 | 265.18 | 5.27 | 2.49 |
| Pneumococcal | Pneumovax®23 | 2012 | 74.90 | 117.08 | 3.83 | 4.14 |
| Prevnar 13™ | 2010 | 136.30 | 226.43 | 6.93 | 3.98 | |
| Polio | IPOL® | 2001 | 25.44 | 40.48 | 0.68 | 2.13 |
| Rotavirus | Rotarix® | 2008 | 135.29 | 134.39 | −0.06 | −0.04 |
| RotaTeq® | 2006 | 88.91 | 93.13 | 0.25 | 0.27 | |
| Varicella | Varivax® | 2001 | 77.10 | 159.93 | 3.76 | 3.37 |
| Adult | ||||||
| DTaP/Tdap/Td | Adacel® | 2009 | 48.10 | 52.24 | 0.30 | 0.59 |
| Boostrix® | 2009 | 48.25 | 45.97 | −0.16 | −0.35 | |
| TDVAX™ | 2019 | 27.69 | 27.78 | 0.02 | 0.09 | |
| Tenivac | 2012 | 25.57 | 39.58 | 1.27 | 4.05 | |
| Hepatitis A | Havrix® | 2001 | 98.10 | 79.45 | −0.85 | −0.95 |
| Vaqta® | 2001 | 85.32 | 76.63 | −0.40 | −0.49 | |
| Hepatitis A/B | Twinrix® | 2003 | 121.81 | 121.01 | −0.04 | −0.03 |
| Hepatitis B | Engerix-B® | 2001 | 85.36 | 66.63 | −0.85 | −1.12 |
| Heplisav-B™ | 2018 | 129.82 | 137.39 | 1.52 | 1.14 | |
| Recombivax HB® | 2001 | 87.77 | 64.84 | −1.04 | −1.37 | |
| HPV | Gardasil®9 | 2015 | 196.48 | 268.71 | 9.03 | 3.99 |
| Meningococcal | Bexsero® | 2015 | 188.50 | 210.47 | 2.75 | 1.39 |
| Menveo® | 2011 | 131.07 | 148.12 | 1.42 | 1.02 | |
| Trumenba® | 2015 | 135.51 | 178.34 | 5.35 | 3.49 | |
| MMR | M-M-R®II | 2009 | 62.08 | 89.67 | 1.97 | 2.66 |
| Pneumococcal | Pneumovax®23 | 2012 | 69.67 | 117.08 | 4.31 | 4.83 |
| Varicella | Varivax® | 2009 | 103.54 | 159.93 | 4.03 | 3.15 |
| Zoster (Shingles) | Shingrix® | 2018 | 157.01 | 182.40 | 5.08 | 3.04 |
Note(s): DTaP = diphtheria and tetanus toxoids and acellular pertussis vaccine; Hib = Haemophilus influenzae type b; HPV = human papillomavirus; IPV = inactivated polio vaccine; MMR = measles, mumps, and rubella; MMRV = measles, mumps, rubella, and varicella; Td = tetanus and reduced diphtheria toxoid; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine. All costs are reported in US$2023.
The archived CDC Vaccine Price List ranges from 2001 to present. Available at https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html.
Most recent price for all products was from the CDC Vaccine Price List dated August 1, 2023.
Reported values in this column are percentages, not proportions. For example, the Adacel® pediatric vaccine inflation-adjusted CDC cost decreased by 1.27 % per year over the observation period.
Fig. 1. Time trends of inflation-adjusted cost per dose (US$2023) among pediatric and adult vaccines for two vaccine cost types, private sector cost and CDC cost, from the CDC Vaccine Price List.
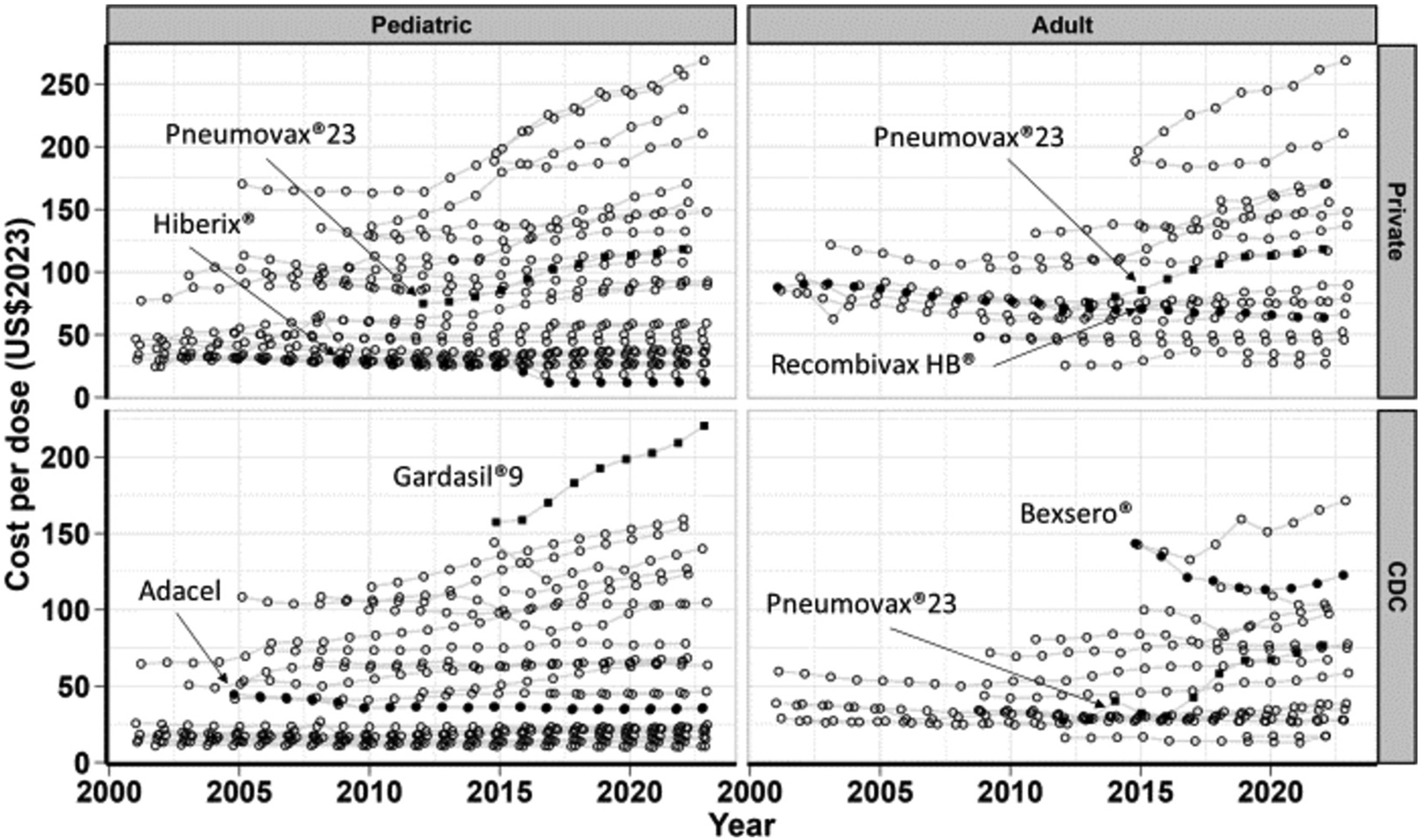
In each panel, the data points formatted as solid black circles indicate the vaccines with the lowest average annual percent change. Similarly, the data points formatted as solid black squares indicate the vaccines with the highest average annual percent change in each panel. See supplement for table of data.
Table 2.
Annual absolute and percentage changes in inflation-adjusted CDC costs (US$2023) of vaccines in the United States, by vaccine. 2001–2023 CDC Vaccine Price List.a.
| CDC costs (US$2023) | Annual change (%)c | |||||
|---|---|---|---|---|---|---|
| Vaccine group | Brandname/ Tradename | Initial year on CDC Price Lista | Cost, initial year | Cost, 2023b | Annual change in cost | |
| Pediatric | ||||||
| DTaP/Tdap/Td | Adacel® | 2005 | 44.54 | 35.36 | −0.51 | −1.27 |
| Boostrix® | 2005 | 41.64 | 35.76 | −0.33 | −0.84 | |
| Daptacel® | 2003 | 19.76 | 20.54 | 0.04 | 0.19 | |
| Infanrix® | 2001 | 17.46 | 20.91 | 0.16 | 0.82 | |
| TDVAX™ | 2019 | 17.66 | 18.22 | 0.14 | 0.79 | |
| Tenivac® | 2012 | 20.53 | 23.22 | 0.24 | 1.13 | |
| DTaP/Hepatitis B/IPV | Pediarix® | 2003 | 50.76 | 63.82 | 0.65 | 1.15 |
| DTaP/IPV | Kinrix® | 2008 | 42.57 | 46.54 | 0.26 | 0.60 |
| Pentacel® | 2008 | 66.13 | 67.64 | 0.10 | 0.15 | |
| Quadracel™ | 2017 | 45.21 | 45.72 | 0.09 | 0.19 | |
| DTaP/IPV/HIB/Hepatitis B | Vaxelis™ | 2021 | 95.67 | 97.12 | 0.72 | 0.75 |
| Hepatitis A | Havrix® | 2001 | 18.40 | 22.84 | 0.20 | 0.99 |
| Vaqta® | 2001 | 18.40 | 23.14 | 0.22 | 1.05 | |
| Hepatitis A/B | Twinrix® | 2005 | 53.46 | 69.67 | 0.90 | 1.48 |
| Hepatitis B | Engerix B® | 2001 | 14.85 | 16.74 | 0.09 | 0.55 |
| Recombivax HB® | 2001 | 14.85 | 13.84 | −0.05 | −0.32 | |
| HiB | ActHIB® | 2001 | 9.49 | 10.68 | 0.05 | 0.54 |
| Hiberix® | 2009 | 11.13 | 10.60 | −0.04 | −0.35 | |
| PedvaxHIB® | 2001 | 13.37 | 15.46 | 0.09 | 0.66 | |
| HPV | Gardasil®9 | 2015 | 157.43 | 220.53 | 7.89 | 4.30 |
| Meningococcal | Bexsero® | 2015 | 144.17 | 140.05 | −0.52 | −0.36 |
| MenQuadfi™ | 2021 | 104.51 | 107.07 | 1.28 | 1.22 | |
| Menveo® | 2010 | 99.95 | 104.84 | 0.38 | 0.37 | |
| Trumenba® | 2015 | 112.28 | 129.53 | 2.16 | 1.80 | |
| MMR | M-M-R®II | 2001 | 25.63 | 24.64 | −0.04 | −0.18 |
| MMRV | ProQuad® | 2005 | 108.41 | 162.23 | 2.99 | 2.26 |
| Pneumococcal | Pneumovax®23 | 2012 | 45.93 | 65.80 | 1.81 | 3.32 |
| Prevnar 13™ | 2010 | 114.99 | 158.18 | 3.32 | 2.48 | |
| Polio | IPOL® | 2001 | 13.61 | 15.77 | 0.10 | 0.67 |
| Rotavirus | Rotarix® | 2008 | 108.56 | 104.70 | −0.26 | −0.24 |
| RotaTeq® | 2006 | 73.10 | 78.67 | 0.33 | 0.43 | |
| Varicella | Varivax® | 2001 | 64.58 | 130.01 | 2.97 | 3.23 |
| Adult | ||||||
| DTaP/Tdap/Td | Adacel® | 2009 | 34.25 | 27.99 | −0.45 | −1.43 |
| Boostrix® | 2009 | 34.03 | 27.66 | −0.45 | −1.47 | |
| TDVAX™ | 2019 | 16.91 | 17.85 | 0.24 | 1.37 | |
| Tenivac | 2012 | 16.19 | 21.75 | 0.51 | 2.72 | |
| Hepatitis A | Havrix® | 2001 | 27.75 | 38.57 | 0.49 | 1.51 |
| Vaqta® | 2001 | 28.96 | 39.25 | 0.47 | 1.39 | |
| Hepatitis A/B | Twinrix® | 2001 | 59.67 | 71.96 | 0.56 | 0.86 |
| Hepatitis B | Engerix-B® | 2001 | 38.36 | 34.87 | −0.16 | −0.43 |
| Heplisav-B™ | 2018 | 78.23 | 74.94 | −0.66 | −0.85 | |
| Recombivax HB® | 2001 | 38.83 | 32.43 | −0.29 | −0.82 | |
| HPV | Gardasil®9 | 2015 | 142.64 | 171.48 | 3.61 | 2.33 |
| Meningococcal | Bexsero® | 2015 | 143.47 | 122.74 | −2.59 | −1.93 |
| Menveo® | 2011 | 80.64 | 77.54 | −0.26 | −0.33 | |
| Trumenba® | 2015 | 99.95 | 105.53 | 0.70 | 0.68 | |
| MMR | M-M-R®II | 2009 | 43.77 | 58.56 | 1.06 | 2.10 |
| Pneumococcal | Pneumovax®23 | 2012 | 27.58 | 78.82 | 4.66 | 10.02 |
| Varicella | Varivax® | 2009 | 71.94 | 101.53 | 2.11 | 2.49 |
| Zoster (Shingles) | Shingrix® | 2018 | 114.61 | 112.77 | −0.37 | −0.32 |
Note(s): DTaP = diphtheria and tetanus toxoids and acellular pertussis vaccine; Hib = Haemophilus influenzae type b; HPV = human papillomavirus; IPV = inactivated polio vaccine; MMR = measles, mumps, and rubella; MMRV = measles, mumps, rubella, and varicella; Td = tetanus and reduced diphtheria toxoid; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine. All costs are reported in US$2023.
The archived CDC Vaccine Price List ranges from 2001 to present. Available at https://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/index.html.
Most recent price for all products was from the CDC Vaccine Price List dated August 1, 2023.
Reported values in this column are percentages, not proportions. For example, the Adacel® pediatric vaccine inflation-adjusted CDC cost decreased by 1.27 % per year over the observation period.
Table 3.
Summary statistics of annual percentage (%) change in vaccine costs, stratified by cost type (private sector cost and CDC cost), age group, vaccine group. and time period (i.e., before 2010 vs. 2010 and later), 2001–2023 CDC Vaccine Price List.
| Private | CDC | ||||
|---|---|---|---|---|---|
| Average | IQR | Average | IQR | N | |
| Age group | |||||
| All pediatric | 0.85 | −0.43; 2.60 | 0.86 | 0.17; 1.18 | 32 |
| All adult | 1.40 | −0.35; 3.15 | 0.99 | −0.82; 2.10 | 18 |
| Vaccine group | |||||
| DTaP-IPV | 0.34 | 0.03; 0.73 | 0.31 | 0.17; 0.39 | 3 |
| DTaP/Tdap/Td | 0.81 | −0.35; 0.59 | 0.20 | −1.27; 1.13 | 10 |
| Hep A | −0.84 | −1.12; −0.55 | 1.23 | 1.02; 1.45 | 4 |
| Hep A/B | 0.17 | −0.03; 0.37 | 1.17 | 0.86; 1.48 | 2 |
| Hep B | −0.85 | −1.37; −1.12 | −0.38 | −0.82; −0.32 | 5 |
| HiB | −2.50 | −3.63; −0.77 | 0.29 | 0.10; 0.60 | 3 |
| HPV | 4.05 | 3.99; 4.11 | 3.32 | 2.33; 4.30 | 2 |
| Meningococcal | 2.06 | 1.22; 3.03 | 0.21 | −0.34; 0.95 | 7 |
| MMR | 2.84 | 2.66; 3.02 | 0.96 | −0.18; 2.10 | 2 |
| Pneumococcal | 4.32 | 4.06; 4.49 | 5.27 | 2.9; 6.67 | 3 |
| Rotavirus | 0.11 | −0.04; 0.27 | 0.10 | −0.24; 0.43 | 2 |
| Varicella | 3.26 | 3.15; 3.37 | 2.86 | 2.49; 3.23 | 2 |
| Time period | |||||
| Before 2010 | 0.04 | −0.95; 0.59 | 0.53 | −0.32; 1.15 | 30 |
| 2010 and later | 2.55 | 1.09; 4.02 | 1.48 | −0.07; 2.41 | 20 |
Note(s): IQR = interquartile range (25th percentile; 75th percentile); DTaP = diphtheria and tetanus toxoids and acellular pertussis vaccine; Hib = Haemophilus influenzae type b; HPV = human papillomavirus; IPV = inactivated polio vaccine; MMR = measles, mumps, and rubella; Td = tetanus and reduced diphtheria toxoid; Tdap = tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine. Summary statistics combine values that appeared on the pediatric and the adult sections of the CDC vaccine price list. For example, one HPV vaccine (Gardasil®9) appeared on the pediatric and adult sections of the price list, and both of these prices were included in the summary results shown here. If a vaccine group had only one vaccine and the vaccine appeared on either the pediatric or adult sections of the price list but not both sections, then that vaccine was not included in the summary by groups section of this table. All costs are reported in US$2023.
Vaccines added to the CDC Price List in 2010 or later had 2.6 % and 1.5 % annual increase in private and CDC costs, respectively. In contrast, vaccines added to the CDC Price List before 2010 had an average 0.04 % and 0.5 % annual increase in private and CDC costs, respectively. Additional sensitivity analyses did not impact findings and are included in the supplementary materials.
4. Discussion
In this study, many vaccines’ costs increased more than the PCE health care rate of inflation, i.e., faster than other health care services that are captured in the PCE index. The annual change in costs was greater for products that were added to the Price List in 2010 or later compared to products added earlier, while vaccines with the greatest increase in cost were not always the most expensive. These findings have important implications for cost-effectiveness analyses best practices, and public-health programs.
For cost-effectiveness analyses of vaccines in the US, our findings suggest that multi-year vaccination programs can underestimate the long-term costs of vaccination when assuming a constant vaccine cost per dose. Price increases for vaccines included in the pediatric vaccine schedule accounted for a 19.5 % increase in the overall vaccine purchase cost per child over a 18 year period (1996 to 2014) [14]. This is similar to observed increases in per capita excess medical cost for diabetes over a 10 year period, which was 22 % between 2007 and 2017, and 18 % between 2012 and 2022 [15,16]. Higher vaccine costs (that are constant through time) are typically assessed in the sensitivity analyses of cost-effectiveness models, but for newly introduced vaccines it may be reasonable to include a scenario with an increasing inflation-adjusted cost per dose in addition to an appropriate discount rate to the costs and benefits occurring in the future [17]. Further, our results suggest CEAs could be periodically updated to understand vaccines’ cost-effectiveness over their lifespans. If the cost of a vaccine increases faster than other health care costs, then previous assessments of cost-effectiveness of vaccines could be too favorable, relative to a more up-to-date assessments that incorporate recent changes in costs.
About one third of vaccines examined in this study exhibited decreasing costs, which occurs if a vaccine’s yearly cost increase is less than the rate of inflation. A previous study found a set of specific vaccines that were subject to a price cap did not experience increasing CDC costs per dose over time [18]. In general, costs per dose that are decreasing or stable over time would be less of a concern than increasing costs per dose for vaccination programs and decision makers, because decreasing costs per dose yields lower program expenditures and better economic value for public health systems. On the other hand, increasing costs per dose can be a major obstacle for effective public health programs, like the entitlement VFC or discretionary Section 317 immunization programs. If these programs receive funding that is constant through time while vaccine dose costs increase through time, then the amount of vaccine doses that could be purchased in future years and, by extension, the number of individuals that can be vaccinated would decrease. This decreased ability to provide routine or outbreak response vaccinations could place additional financial strain on immunization programs. Better anticipating future changes to vaccine cost can help vaccine programs and decision makers ensure adequate budget projections to meet program needs.
This study considered the manufacturer reported and the negotiated CDC cost per vaccine dose. We did not gather other contracted costs with the manufacturers, or payer costs based on health insurance claims data. Previous studies have reported the proportion of vaccine private insurance reimbursements exceeding the private List Price ranges from 95 % (pediatric HPV) to as little as 25 % (adult Td) of claims by vaccine [19,20]. This would be an important area for future research along with causal analysis focused on understanding the factors contributing to observed differences in changes in cost per dose by vaccine, age group, year of introduction, etc. Future studies could also examine factors that affect negotiated vaccine prices internationally, and the potential influence of ACIP cost-effectiveness considerations on vaccine prices in the U.S.
To summarize, inflation-adjusted costs of vaccines have increased, on average, and larger annual increases were among vaccines introduced in 2010 or later than vaccines introduced before 2010. These vaccine cost changes highlight the limitations of a constant vaccine cost assumption used in many economic models, especially for new vaccines. These findings can inform cost-effectiveness analyses of long-term vaccination programs and policy.
Supplementary Material
Acknowledgements
We would like the thank H. Rosenblum and D. Ekwueme for useful comments during the conceptualization and initial drafting stages.
Role of the funding source:
The authors received no external financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
RajReni Kaul: Writing – review & editing, Writing – original draft, Methodology. Andrew J. Leidner: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Harrell W. Chesson: Writing – review & editing, Methodology.
Data statement
All data used in this analysis is publicly available at Vaccines for Children (VFC): Archived Vaccine Price Lists | CDC. Code to recreate the analysis and figures along with the data is available at https://github.com/cdcgov/VaccinePriceList.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. The use of trade names is for identification only and does not imply endorsement by the Department of Health and Human Services or the CDC.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2024.126667.
Data availability
I have included a link to all data and code in the methods section.
References
- [1].Zhou F, et al. Economic evaluation of the routine childhood immunization program in the United States, 2009. Pediatrics 2014;133(4):577–85. [DOI] [PubMed] [Google Scholar]
- [2].Walton LR, Orenstein WA, Pickering LK. The history of the United States advisory committee on immunization practices (ACIP). Vaccine 2015;33(3):405–14. [DOI] [PubMed] [Google Scholar]
- [3].Leidner A, et al. Guidance for health economics studies presented to the Advisory Committee on Immunization Practices (ACIP), 2019 update. In: Centers for Disease Control and Prevention. National Center for Immunization and Respiratory Diseases: Atlanta GA; 2019. p. 1–21. [Google Scholar]
- [4].Hay JW, et al. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR drug cost task force report—part I. Value Health 2010;13(1):3–7. [DOI] [PubMed] [Google Scholar]
- [5].Frank RG. Prescription drug prices: why do some pay more than others do? Health Aff 2001;20(2):115–28. [DOI] [PubMed] [Google Scholar]
- [6].Reinhardt UE. Health care price transparency and economic theory. Jama 2014; 312(16):1642–3. [DOI] [PubMed] [Google Scholar]
- [7].Whitney CG, et al. Benefits from immunization during the vaccines for children program era—United States, 1994–2013. Morb Mortal Wkly Rep 2014;63(16):352. [PMC free article] [PubMed] [Google Scholar]
- [8].Centers for Disease Control and Prevention. CDC Vaccine Price List.. 2023. [cited 2023 August 1], Available from, https://www.cdc.gov/vaccines-for-children/php/awardees/current-cdc-vaccine-price-list.html.
- [9].US Bureau of Economic Analysis. Table 2.3.4. Price Indexes for Personal Consumption Expenditures by Major Type of Product. 2023. [cited 2023 October 26]; Available from, https://www.bea.gov/itable/national-gdp-and-personal-income.
- [10].Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res 2018;53(1):175–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bureau of Labor Statistics (BLS). Medical care in U.S. city average, all urban consumers, seasonally adjusted (Series ID: CUSR0000SAM). 2023. [cited. Available from: https://data.bls.gov/cgi-bin/srgate; 2024.
- [12].R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria. 2021. [Google Scholar]
- [13].Wickham H, et al. Welcome to the Tidyverse. Journal of open source software 2019;4(43):1686. [Google Scholar]
- [14].Chen W, Messonnier M, Zhou F. Trends in childhood vaccine purchase costs in the US public sector: 1996–2014. Vaccine 2016;34(39):4706–11. [DOI] [PubMed] [Google Scholar]
- [15].American Diabetes A Economic costs of Diabetes in the U.S. in 2017. Diabetes Care 2018;41(5):917–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Parker ED, et al. Economic costs of Diabetes in the U.S. in 2022. Diabetes Care 2024;47(1):26–43. [DOI] [PubMed] [Google Scholar]
- [17].Haddix AC, Teutsch SM, Corso PS. Prevention effectiveness: A guide to decision analysis and economic evaluation. Oxford University Press; 2002. [Google Scholar]
- [18].Chen W, Messonnier M, Zhou F. Factors associated with the pricing of childhood vaccines in the US public sector. Health Econ 2018;27(2):252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tsai Y, et al. Provider payments and the receipt of human papillomavirus vaccine among privately insured adolescents. Health Aff (Millwood) 2018;37(10):1587–95. [DOI] [PubMed] [Google Scholar]
- [20].Tsai Y, Zhou F, Lindley MC. Insurance reimbursements for routinely recommended adult vaccines in the private sector. Am J Prev Med 2019;57(2):180–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
I have included a link to all data and code in the methods section.


