Abstract
In Drosophila, asymmetric division occurs during proliferation of neural precursors of the central and peripheral nervous system (PNS), where a membrane-associated protein, Numb, is asymmetrically localized during cell division and is segregated to one of the two daughter cells (the pIIb cell) after mitosis. numb has been shown genetically to function as an antagonist of Notch signaling and also as a negative regulator of the membrane localization of Sanpodo, a four-pass transmembrane protein required for Notch signaling during asymmetric cell division in the CNS. Previously, we identified lethal giant larvae (lgl) as a gene required for numb-mediated inhibition of Notch in the adult PNS. In this study we show that Sanpodo is expressed in asymmetrically dividing precursor cells of the PNS and that Sanpodo internalization in the pIIb cell is dependent cytoskeletally associated Lgl. Lgl specifically regulates internalization of Sanpodo, likely through endocytosis, but is not required for the endocytosis Delta, which is a required step in the Notch-mediated cell fate decision during asymmetric cell division. Conversely, the E3 ubiquitin ligase neuralized is required for both Delta endocytosis and the internalization of Sanpodo. This study identifies a hitherto unreported role for Lgl as a regulator of Sanpodo during asymmetric cell division in the adult PNS.
INTRODUCTION
Asymmetric cell division is a conserved mechanism for generating cell fate diversity during development in a wide range of species (Roegiers and Jan, 2004). In Drosophila, asymmetric division occurs during proliferation of neural precursors of the central and peripheral nervous system (CNS and PNS). In the adult PNS, a sensory organ precursor (pI) cell undergoes four rounds of mitosis to give rise to five cells: two external cells, the socket and hair cell, which are visible on the cuticle surface, and three internal cells, the neuron, sheath, and small glial cell. The small glial cell undergoes apoptosis, whereas the remaining four cells assemble a functional external mechanosensory organ (or ES organ; Lai and Orgogozo, 2004). Notch, which is required to specify a subset of these cell fates, is a transmembrane receptor that binds its extracellular ligand, Delta, and undergoes a series of proteolytic cleavages resulting in the translocation of the Notch intracellular domain into the nucleus, where it interacts with Suppressor of Hairless to turn on transcription of target genes (Schweisguth, 2004).
The pI cell undergoes an asymmetric cell division by segregating a membrane-associated protein, Numb, to an anterior crescent, which is partitioned into the anterior pIIb daughter cell after cytokinesis (Rhyu et al., 1994). numb has been shown genetically to function as an antagonist of Notch signaling, thereby establishing a difference between the two daughter cells. The pIIb cell, in which Notch signaling is inhibited, will give rise to the internal cells of the lineage, whereas the pIIa cell, the other pI cell daughter, undergoes high levels of Notch signaling and will give rise to the external cells of the lineage. Studies have shown that several other genes besides numb and Notch are required for correct specification of pIIa and pIIb cell fates. Loss of function mutants of the endocytic protein, and member of the AP-2 complex, α-adaptin (ada) causes similar cell fate defects to loss of numb function. Numb protein interacts directly with α-Adaptin, and α-Adaptin localizes asymmetrically in dividing pI cells in a Numb-dependent manner (Berdnik et al., 2002). In a previous study, we showed that the cortical tumor suppressor lethal giant larvae (lgl) is also required for the inhibition of Notch signaling in the pIIa/pIIb cell fate decision (Justice et al., 2003). Loss of lgl results in similar cell fate changes as loss of numb or α-adaptin. These studies raise the possibility that Numb may regulate Notch signaling directly via endocytosis and degradation of the Notch receptor involving lgl and α-adaptin; however, to date, there is no in vivo evidence to support this model.
Notch signaling in the pIIa cell requires, in addition to Notch and Delta, the activity of neuralized and sanpodo. Loss of function of any of these genes causes cell fate transformations leading to formation of ectopic neurons. Neuralized, like Numb, is asymmetrically localized and is segregated to the pIIb cell (Le Borgne and Schweisguth, 2003). Drosophila Neuralized is a RING-finger E3 ubiquitin ligase that monoubiquitinates Delta, leading to Delta endocytosis (Lai et al., 2001; Pavlopoulos et al., 2001; Yeh et al., 2001). neuralized-dependent Delta internalization in the pIIb cell (the signal sending cell) is required for cleavage of the Notch receptor, and therefore high levels of Notch signaling in the pIIa cell (the signal receiving cell; Le Borgne and Schweisguth, 2003). The result of segregating both Numb and Neuralized in the same cell is that Notch is inhibited by Numb cell-autonomously in the pIIb cell, whereas Neuralized activity in the pIIb cell activates Notch non-cell-autonomously in the pIIa cell. Thus two seemingly independent systems ensure proper cell fate specification between the pIIa and pIIb cells (reviewed in Roegiers and Jan, 2004). In the embryonic CNS and PNS, sanpodo, a gene encoding a four-pass transmembrane protein (O'Connor-Giles and Skeath, 2003), is required for Notch signaling during asymmetric cell division (Dye et al., 1998; Skeath and Doe, 1998), but the mechanism of Sanpodo's function in Notch signaling is not known. Plasma membrane localization of Sanpodo is negatively regulated by numb (O'Connor-Giles and Skeath, 2003); however, the role of sanpodo in adult PNS development has yet to be investigated.
In this study we address five questions: (1) Is sanpodo required for Notch signaling during asymmetric cell divisions in adult PNS, and if so what is the distribution and subcellular localization of Sanpodo? (2) Is the membrane localization of Sanpodo regulated by numb and lgl? (3) Is cortical localization of lgl, which is regulated by atypical Protein Kinase C (aPKC) phosphorylation, required for lgl regulation of Sanpodo? (4) Does endocytosis play a role in regulating Sanpodo subcellular localization? And (5), Is lgl required for the neuralized-mediated endocytosis of Delta, and, conversely, is neuralized required for regulating membrane localization of Sanpodo? We find that sanpodo is required for Notch-dependent cell fates in the adult PNS. We further show that the punctate distribution of Sanpodo in the pIIb cell, likely due to dynamin-dependent endocytosis, requires not only lgl downstream of aPKC but also numb and neuralized. In contrast, Delta internalization depends on neuralized but not lgl. Our study extends our previous analysis of the role of the tumor suppressor Lgl in Numb-mediated inhibition of Notch during asymmetric cell division in the PNS by showing that Lgl regulates Sanpodo. In addition we show that Neuralized, in addition to its role as a regulator of Delta endocytosis, is also required for Sanpodo internalization.
MATERIALS AND METHODS
Fly Stocks
Experiments were conducted at 22°C unless otherwise noted. The MARCM experiments were conducted using y,w, ubx-FLP; ada[ear5] FRT40A/CyO (kindly provided by J. Knoblich, IMP), y,w, ubx-FLP; FRT82b Sb spdoc55 e/TM6,y+, y,w, ubx-FLP; nb2 FRT40A/CyO, y,w, ubx-FLP; l(2)gl4 FRT40A/CyO, y,w, ubx-FLP; y+ ck FRT40A/CyO and w; FRT82b neur1F65/TM6C (kindly provided by E. Lai, Berkeley), UAS-protein kinase CΔN (kindly provided by J. Knoblich, IMP) crossed to y,w ubx-FLP; Gal80 FRT40A; neurGal4, Kgv, UAS-mCD8-GFP/TM6,Ubx, y+ or yw ubx-FLP;scaGal4, UAS-Pon-GFP, UAS-Tau-GFP; FRT82b Gal80 (as described in Roegiers et al., 2001; Justice et al., 2003). Other stocks used include w, for immunocytochemistry of Sanpodo and Numb. shibirets1 (shits1) were harvested as white pupa and aged for 22–24 h at 22°C, and the pupae were then placed in one of three conditions: immersed in a water bath at 37°C, immersed in a water bath at 32°C, or left at room temperature 22°C for 1 h. All samples were rapidly dissected in phosphate-buffered saline (PBS) equilibrated to the incubation temperature (37, 32, or 22°C), and fixed.
Immunocytochemistry, Endocytosis Assay, and Imaging
Staining and imaging of isolated pupal nota and adult thorax was performed according to Justice et al. (2003). Unless otherwise noted all images are single XY planes. The endocytosis assay was performed according to Le Borgne and Schweisguth (2003). Antibodies used were rabbit anti-Sanpodo (1/1000) and rat anti-Sanpodo (1/100; both courtesy of J. Skeath, Washington University), rat anti-Lgl (courtesy of C. Q. Doe, University of Oregon), guinea pig anti-Numb (1/500), guinea pig anti-Asense (1/5000), rat anti-CD8 (Caltag Laboratories, Burlingame, CA, 1/100), rabbit anti-GFP, mouse anti-Dl (C-594, DSHB, 1/3000), guinea pig anti-Dl (courtesy of M. Muskavitch, 1/3000).
RESULTS
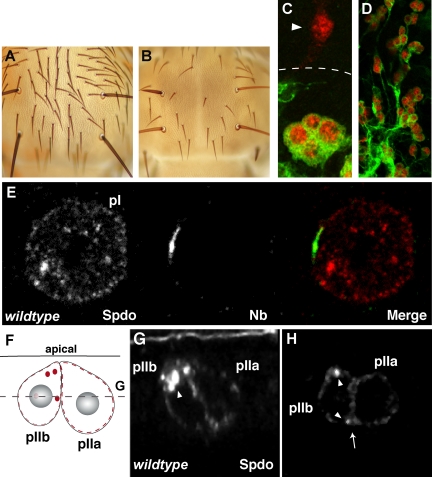
To ask if sanpodo is required for differentiation of the adult external sensory organ (Figure 1, A–C), we generated mitotic clones on the pupal thorax of the sanpodoC55 allele on a chromosome marked with the bristle marker Sb. Although we do detect some Sb/Sb bristles, there are significant regions of bristle loss in flies where sanpodo clones are induced (n = 17 flies, Figure 1B). Because of the balding phenotype, which could indicate a transformation of pIIa cells into pIIb cells, we could not rely on Sb as a marker indicating boundaries of the clone, so we generated mitotic clones of sanpodo mutant tissue using the MARCM system (Lee and Luo, 2001) in order to label mutant cells with GFP and asked whether these cells expressed markers for neurons, progeny of the pIIb cell. In pupae fixed and labeled shortly after completion of the asymmetric cell divisions (24 h APF), we observe clusters of either four or five GFP-positive sanpodo mutant cells. A majority of these clusters (n = 10/13) showed expression of the neuronal marker ELAV in at least three cells (Figure 1C), in contrast to regions outside the clone where only single ELAV-positive cells are detected (n = 26/26). In pupae fixed and labeled several hours after completion of the asymmetric cell division (28 h APF) we find large clusters of GFP-positive ELAV-positive cells exhibiting a neuronal morphology (n = 5 pupae, Figure 1D), suggesting that most sanpodo mutant cells differentiate into neurons. Taken together these data indicate that sanpodo is required for correct specification of the pIIa cell, which gives rise to the external cells of the ES lineage and that loss of sanpodo causes a transformation of the pIIa cell to a pIIb cell, which causes a loss of sensory bristles and overproduction of neurons.
Figure 1.
Sanpodo is required for Notch-mediated fates in the adult PNS. (A) A wild-type adult thorax. (B) Adult thorax containing a large sanpodoC55 Sb mutant clone; patches of the notum are bald, lacking external sensory structures. (C) Z-projection of MARCM clones of sanpodoC55 Sb, in a 24-h (C) and a 28-h (D) APF (after pupae formation) pupa. (C) Outside the clone (approximate clone border marked by dashed line), external sensory organs contain one neuron (arrowhead), which expresses ELAV (red), whereas in sanpodo mutant clusters (marked by GFP, green) more than two cells expresses ELAV. (D) Sanpodo mutant cells (green) form clusters of ELAV-positive (red) neurons. (E) A mitotic pI cell labeled with Sanpodo (red) and Numb (green) antibodies. Images taken from pupae collected at 22 h APF. Sanpodo is uniformly distributed along the cell membrane and is also present in large puncta in the cytoplasm. Sanpodo does not strongly colocalize with Numb at the anterior cortex. (F) A schematic representation of a pIIa and pIIb cell cluster the gray dashed line indicates the depth of the XY plane shown in H, with Sanpodo represented in red. An XZ plane (G) and an XY plane (H) through the same wild-type pIIa/pIIb cell cluster. Sanpodo is present in strongly labeled apical cytoplasmic puncta (white arrowheads) in the pIIb cell and is detectable at the cell membrane (white arrow) in the pIIa cell.
We next performed immunohistochemistry on pupal nota during and after pI mitosis to determine Sanpodo's distribution and subcellular localization (Figure 1, E–H). Sanpodo is present only in cells undergoing asymmetric cell division (neural precursor cells), whereas Sanpodo is undetectable in cells of the surrounding epithelium (unpublished data). In mitotic pI cells, we observe Sanpodo protein in cytoplasmic puncta as well as along the cell membrane, but Sanpodo is not significantly enriched in the region of the membrane where Numb accumulates during asymmetric cell division (Figure 1E). After completion of the pI cell division, Sanpodo accumulates in large (0.719 ± 0.2 μm, n = 31 puncta in 9 pIIb cells) primarily apical (within 1.5 μm of the apical cell surface), cytoplasmic puncta in the pIIb cell (Figure 1, F–H). In the pIIa cell, Sanpodo puncta are present, but are smaller (0.45 ± 0.11 μm), less abundant (5 puncta in 9 pIIa sisters of pIIb cells quantified above), and are not restricted to the apical domain of the cell. Sanpodo protein is also detected at the cell surface in pIIa cells (Figure 1, G and H).
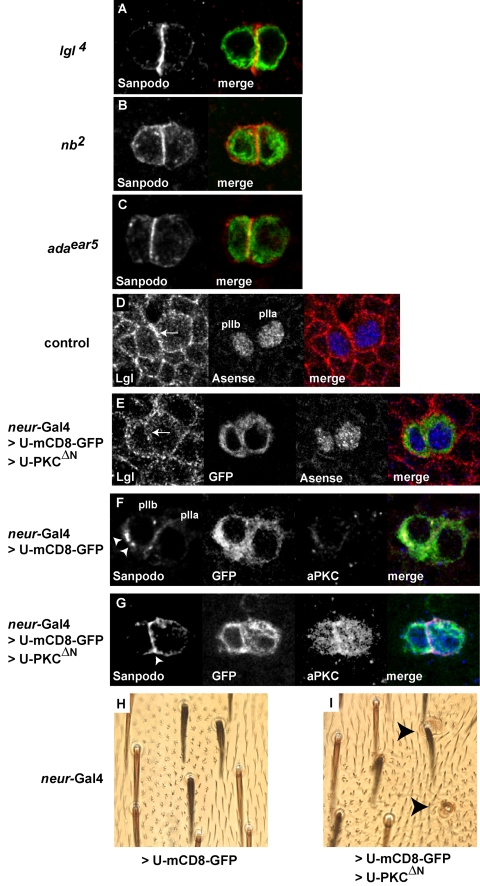
Previously, we reported that lgl is required for inhibiting Notch signaling in the pIIb-pIIa cell fate decision, leading to transformation of pIIb cells into pIIa cells in lgl mutants (Justice et al., 2003). We asked whether the cell fate transformations observed in lgl mutants is accompanied with misregulation of Sanpodo localization. We generated lgl4 MARCM clones. In regions outside the mutant clone, wild-type pIIa and pIIb cell pairs are clearly labeled with anti-Sanpodo antibody (for wild type see Figure 1, F and G). In the pIIb cell, Sanpodo is present in the cytoplasm in large puncta, whereas in the pIIa cell, Sanpodo protein was primarily at the cell surface. In contrast, pIIa and pIIb cells in lgl4 mutant clones show strong accumulation of Sanpodo at the cell membrane (Figure 2A, n = 31 pairs). Correspondingly, cytoplasmic Sanpodo puncta are reduced or absent. We observe a similar accumulation of Sanpodo at the membrane in both nb2 and adaear5 mutants (Figure 2, B and C). From these results we conclude that lgl, numb, and α-adaptin are required for accumulation of cytoplasmic puncta of Sanpodo protein in the pIIb cell after asymmetric cell division.
Figure 2.
Regulation of Sanpodo localization by lgl, numb, and α-adaptin. (A–C) Sanpodo protein labeled with anti-Sanpodo antibody (red) and mutant clone tissue is labeled with mCD8-GFP (green) in MARCM clones. A two-cell cluster of lgl4 (A), nb2 (B), α-adaptinear5 (adaear5; C), in mutant cells (green); Sanpodo protein (red) is strongly enriched at the membrane in both cells and cytoplasmic puncta of Sanpodo are reduced or absent (see Figure 1, G and H, for Sanpodo protein in wild-type pIIa and pIIb cells). (A–G) Images taken from pupae collected at 22 h APF. (D and E) Lgl protein labeled with anti-Lgl antibody (red), and clone tissue is labeled with mCD8-GFP (green) in MARCM clones, and pIIa and pIIb cells are labeled with Asense (blue). Outside the mutant clone (D), Lgl protein localizes to the cortical region of both pIIa and pIIb cell (white arrow). (E) A two-cell cluster (green) within a clone overexpressing both mCD8-GFP and aPKCΔN, Lgl protein (red) is distributed throughout in the cytoplasm, but is largely absent from the cortical region (white arrow). (F and G) Sanpodo protein labeled with anti-Sanpodo antibody (red), and clone tissue is labeled with mCD8-GFP (green) and overexpressed PKCΔN (blue) in MARCM clones. (F) A pIIa and pIIb cell cluster (green) in clone tissue overexpressing GFP, Sanpodo (red) is present in strongly labeled cytoplasmic puncta (arrowheads) in the pIIb cell and is present at the cell membrane and in the cytoplasm in the pIIa cell, and low levels of endogenous PKCΔN (blue) are detected. (G) A two cell cluster (green) within a clone overexpressing both mCD8-GFP and PKCΔN, Sanpodo protein is strongly enriched at the membrane in both cells, specifically at the cell-cell border (arrowhead); little Sanpodo protein is present in the cytoplasm, and the overexpressed PKCΔN (blue) is detected in both cells. (H and I) Adult external sense organs overexpressing mCD8-GFP (Neur-Gal4>U-mCD8-GFP) or overexpressing both mCD8-GFP and activated PKCΔN (Neur-Gal4>U-mCD8-GFP>U-PKCΔN) in MARCM clones. Adult ES organs overexpressing the transgenes are marked by the presence of dark (y+) bristles and are surrounded by epidermal hairs exhibiting the ck phenotype (multiple epidermal hairs). (H) Overexpression of mCD8-GFP alone results in ES organs that develop into single bristle cell, single socket cell; which is characteristic of wild-type ES organs (see light colored y–bristles outside the ck marked clone). (I) Overexpression of both mCD8-GFP and PKCΔN results in organs developing supernumerary socket cells (black arrowheads) within the y+ ck marked clone.
We next labeled pIIb/pIIa cell pairs with an anti-Lgl antibody to determine if Lgl protein is localized to the cell cortex. In wild-type pupae, we found that Lgl protein is localized to the cortical region of pIIb/pIIa cell pairs labeled with Asense (Figure 2D). We reasoned that the cortical localization of Lgl in pIIa and pIIb cells could be important for regulating Sanpodo. Recent studies in both flies and vertebrates (Betschinger et al., 2003; Plant et al., 2003) have shown that Lgl is a substrate for atypical protein kinase C (aPKC) and that phosphorylated Lgl is unable to associate with the cytoskeleton. We expressed an activated version of aPKC, aPKCΔN, in pI cells and their progeny using neur-Gal4 in order to disrupt cortical localization of Lgl. Expression of aPKCΔN in embryonic and larval neural precursors lead to larval lethality, which prevented us from analyzing the pupal phenotype, so we generated MARCM clones expressing aPKCΔN in pIIb/pIIa cells (using neur-Gal4). Overexpression of aPKCΔN results in decrease in Lgl protein detected at the cell cortex in eight of eight pIIa/pIIb (100%) cell pairs, compared with controls expressing mCD8-GFP alone (2/7 or 29% pIIb/pIIa cell pairs had low levels of cortical Lgl; Figure 2E). The loss of Lgl protein is particularly evident at the juxtamembrane region of these two cells when overexpressing aPKCΔN. We also observed that aPKCΔN overexpression leads to an increased accumulation of Sanpodo protein at the membrane and a reduction of Sanpodo puncta in the cytoplasm (n = 10, Figure 2G), compared with controls expressing only GFP (n = 12, Figure 2F). We then asked whether aPKCΔN overexpression leads to cell fate transformations similar to what we observe in lgl mutants. We generated mitotic clones that express either mCD8-GFP or mCD8-GFP and aPKCΔN under control of neur-Gal4. When mCD8-GFP alone is expressed, a single hair and socket arise, indicating no duplication of external structures (0%, n = 52 organs, Figure 2H). We find clones overexpressing both mCD8-GFP and aPKCΔN show an increased frequency of ectopic external cells (43% of ES organs with at least 3 external cells, n = 87 organs, Figure 2I), a phenotype similar to the lgl loss of function mutant phenotype (Justice et al., 2003). In summary, overexpression of aPKCΔN disrupts cortical localization of Lgl, causes increase in membrane Sanpodo and cell fate transformations consistent with the lgl loss of function phenotype. We interpret these data to suggest that cytoskeletal association of Lgl is required for regulation of Sanpodo internalization and that misregulation of Sanpodo may cause the cell fate transformations we observe.
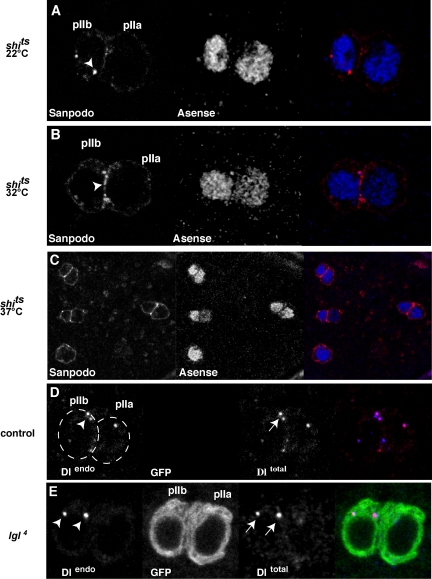
We next asked whether blocking endocytosis causes changes in Sanpodo distribution in pIIb cells. We reasoned that a defect in endocytosis in lgl mutants could lead to accumulation of Sanpodo protein at the membrane. We used the temperature-sensitive allele of shibire (Drosophila dynamin), shits1, to block endocytosis during and shortly after asymmetric cell division and labeled cells with the Sanpodo antibody. We observed the distribution of Sanpodo protein in pIIb/pIIa cell pairs in three different conditions: at the permissive temperature (22°C), at 32°C and at 37°C. At 22°C, shits1 mutants displayed wild-type distribution of Sanpodo protein in both pIIa and pIIb cells (Figure 3A, n = 42 pIIa/pIIb cell pairs). At 32°C Sanpodo accumulates at membrane and in small puncta at the membrane (Figure 3B, n = 27 pIIa/pIIb cell pairs), whereas at 37°C, Sanpodo protein is distributed primarily on the membrane (Figure 3C), similar to the phenotype we observe in the lgl mutant pIIa/pIIb cell pairs, suggesting that Lgl may regulate Sanpodo internalization by endocytosis.
Figure 3.
lgl specifically regulates the membrane localization Sanpodo, but not the endocytosis of Delta. shibire regulates the cytoplasmic localization of Sanpodo. (A–C) A two-cell cluster of ES lineage cells in a shits1 mutant pupae 22 h APF maintained at 22°C (A) and 32°C (B) or 37°C (C) labeled with anti-Sanpodo antibody (red) and pI, pIIb, and pIIa cell marker Asense (blue). At 22°C, Sanpodo protein is found in cytoplasmic puncta in the pIIb cell (white arrowhead). Inactivation of shibire (B and C) causes an accumulation of Sanpodo at the membrane. At 32°C (B), small cytoplasmic puncta and membrane-associated Sanpodo are observed (arrowhead), whereas at 37°C (C), Sanpodo is primarily at the membrane in both pI and pIIa/pIIb cell pairs as labeled by Asense (lower magnification view of one pI cell and three pIIa/pIIb cell pairs). (D–E) lgl is dispensable for the endocytosis of Delta into the pIIb cell. Endocytosis of Delta can be assayed by culturing nota in serum containing antibodies raised against the extracellular domain of Delta (Le Borgne and Schweisguth, 2003). Outside the mutant clone, control pIIa and pIIb cell clusters (D) internalize extracellular Delta (Dlendo, red) into the pIIb cell (white arrowhead). Similarly, Endocytosed Delta (arrowheads, red) is detected as large puncta that are labeled with anti-Delta antibodies (arrows, blue) in the pIIb cell in lgl4 (E) MARCM mutant ES clusters (green).
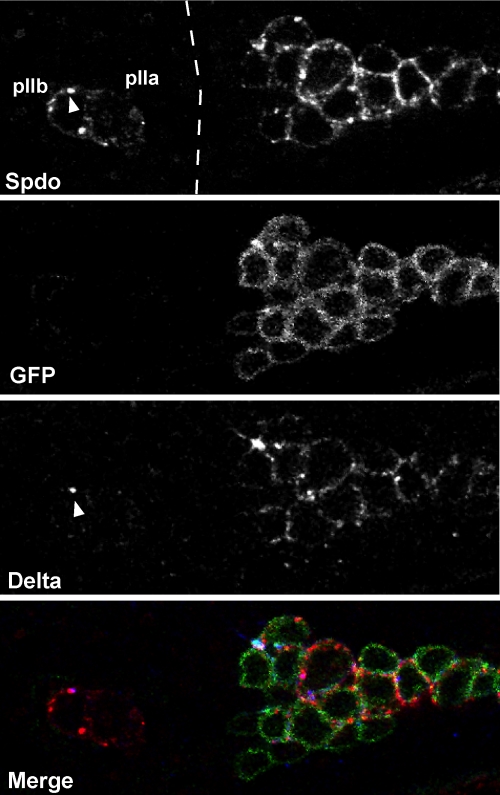
Endocytosis of both Notch, and Delta, plays an important role in regulating Notch signaling in both Drosophila and vertebrates (Schweisguth, 2004). We wanted to determine if lgl is required for endocytosis of other Notch pathway components or if the endocytic defect is specific for Sanpodo. In the pIIb/pIIa cells, Delta is endocytosed in the pIIb cell after ubiquitination of Delta by the ubiquitin ligase neuralized. We asked whether Delta endocytosis could occur in the absence of lgl. We applied the Delta internalization assay used in Le Borgne and Schweisguth (2003) to label the extracellular domain of Delta in living cells to determine whether Delta was internalized in lgl mutants. In both wild-type (n = 37 cells, Figure 3D) and lgl (100%, n = 14 cells, Figure 3E) mutants, we observe that Delta, as labeled with the antibody that recognizes the extracellular domain, was internalized in large puncta in the pIIb cell. We also examined Notch protein localization in wild-type and lgl mutant pIIa/pIIa cells. In wild-type epithelial, pIIa and pIIb cells the Notch extracellular domain (ECD) is detected in intracellular puncta (Le Borgne and Schweisguth, 2003), and we observed similar distribution of Notch ECD in lgl mutant cells (unpublished data), suggesting that loss of lgl function does not lead to Notch ECD accumulation at the membrane. From these data we conclude that cortical Lgl is required for the Numb-mediated regulation of Sanpodo in pIIb cells, perhaps via an endocytic mechanism, but that Lgl is dispensable for the formation of both Delta and Notch ECD intracellular puncta. neuralized is asymmetrically localized, along with Numb, to the anterior cortex of the pI cell and segregated to the pIIb cell at anaphase. Delta endocytosis is dependent on neur function, but independent of numb (Le Borgne and Schweisguth, 2003) and lgl. We next tested whether Sanpodo protein localization is dependent on neuralized function. We hypothesized that if Sanpodo regulation were totally independent of neuralized, we would observe a wild-type distribution of Sanpodo in clusters of pIIb/pIIa cells in neuralized mutant clones. We generated MARCM clones of a null allele of neuralized, neur1F65 (Lai and Rubin, 2001). Loss of neuralized function causes a lateral inhibition defect, resulting in specification of ectopic pI cells, which complicated the task of establishing the identity of the pIIb/pIIa cells with certainty. We therefore used live imaging of neuralized mutant cell clusters to visualize asymmetric cell divisions of pI cells using the GFP reporters, Pon-GFP and Tau-GFP (Roegiers et al., 2001; Justice et al., 2003). Using this method, we observed clusters of four to eight pI cells undergoing asymmetric cell division in a near synchronous manner, and no defects in coordination between Pon-GFP localization and mitotic spindle orientation (unpublished data). After completion of pI asymmetric cell division, we dissected, fixed, and labeled nota from pupae used in the live imaging experiment, thus ensuring that we were observing progeny of the pI cell. We labeled these nota with antibodies directed against Sanpodo and against Delta to determine the localization of these proteins in neur mutant clones. In clones of neur1F65 Sanpodo protein was detected in a large number of cells (Figure 4), consistent with the neurogenic phenotype of neuralized, which was in contrast to the Sanpodo distribution in discrete two cell pairs in the neighboring wild-type tissue (Figure 4). In neur1F65 clones show high levels of membrane localization of Sanpodo, making individual cell outlines clearly visible (n = 23 multicell clusters, Figure 4), in addition, Sanpodo was detected in cytoplasmic puncta in these cells, and we analyzed the size and distribution of these puncta compared with wild-type pIIa/pIIb cell pairs. In neur1F65 cells, smaller Sanpodo puncta (0.547 ± 0.125, n = 21 cells) were distributed throughout the cytoplasm, in contrast to the larger apical puncta seen in wild-type pIIb cells (Figure 1, G and H).
Figure 4.
neuralized regulates Sanpodo protein localization. Sanpodo protein (red) and Delta protein (blue) localization in neur1F65 mutant clones (green). (A–C) A region of the pupal thorax containing both wild-type and neur1F65 mutant tissue, to the left, a wild-type pIIa/pIIb cell pair shows strong accumulation of Sanpodo (red) and Delta (blue) in cytoplasmic puncta that can colocalize (arrowhead), in the pIIb cell. At the right, the GFP+ neur1F65 mutant tissue (green) exhibits a large cluster of cells that have high levels of Sanpodo (red) primarily at the membrane, as well as in cytoplasmic puncta, and accumulation of Delta (blue) at the cell surface.
Interestingly, Sanpodo membrane localization correlates with the accumulation of Delta protein at the membrane in neur1F65 mutant clones (Figure 4 and Le Borgne and Schweisguth, 2003). These data are consistent with the idea that neuralized functions to negatively regulate the accumulation of Delta protein at the membrane. In summary, these data indicate that although lgl is required for accumulation of Sanpodo puncta, but not Delta puncta, in the pIIb cell, neuralized function is required for both Delta endocytosis and contributes to the regulation of Sanpodo in pIIa/pIIb cells.
DISCUSSION
In this study we have established that Sanpodo is required for the specification of the pIIa cell in the developing adult PNS and that Sanpodo internalization in the pIIb cell is dependent on cytoskeletally associated Lgl, in addition to numb and α-adaptin. Lgl specifically regulates internalization of Sanpodo, likely through endocytosis, but is not required for the endocytosis of Delta, which is a necessary step in Notch-mediated cell fate decision during asymmetric cell division. Conversely, the E3 ubiquitin ligase neuralized is required for both Delta endocytosis and the internalization of Sanpodo.
Our analysis of Sanpodo function in the adult PNS suggests that, like in the embryo, Sanpodo is expressed only in asymmetrically dividing precursor cells and is required for cell fates dependant on high levels of Notch signaling (Dye et al., 1998; Skeath and Doe, 1998), perhaps through the direct interaction between Sanpodo and the full-length Notch receptor (O'Connor-Giles and Skeath, 2003). Sanpodo also interacts directly with Numb in vivo (O'Connor-Giles and Skeath, 2003), and in both the embryonic CNS and the adult PNS (our study), numb inhibits plasma membrane association of Sanpodo. Therefore, it appears that Sanpodo plays a similar role in asymmetrically dividing precursor cells in both the CNS and PNS in Drosophila.
Although there are many similarities between the mechanisms of asymmetric cell divisions in embryonic neuroblasts and adult sensory organ precursor cells, one difference involves the role of lgl. In neuroblasts, lgl is required along with another cortical tumor suppressor, dlg, to target Numb to a basal crescent during mitosis (Ohshiro et al., 2000; Peng et al., 2000), whereas in pI cells, only dlg is required for Numb crescent formation (Bellaiche et al., 2001). Our previous study showed that although lgl is dispensable for segregation of Numb to the pIIb cell after pI cell mitosis, lgl is required for the inhibition of Notch signaling in the pIIb cell (Justice et al., 2003). On the basis of our current study, we propose that Lgl functions with Numb to remove Sanpodo from the membrane, leading to down-regulation of the Notch signaling pathway in the pIIb cell.
Through what mechanism might Lgl regulate Sanpodo localization? Studies in Drosophila, yeast, and vertebrate cells have implicated Lgl as both a regulator of exocytosis, through its interaction with t-SNARES (Lehman et al., 1999; Arquier et al., 2001; Musch et al., 2002) and as cytoskeletal effector (Strand et al., 1994a, 1994b; Kagami et al., 1998). In our study, we do not detect any phenotypes suggesting gross defects in exocytosis; in fact, we see increased accumulation of the membrane protein Sanpodo at the plasma membrane in lgl mutants. Accumulation of Sanpodo at the plasma membrane in lgl mutants, resembling the phenotype of three endocytic proteins numb, α-adaptin, and shibire, suggested that lgl may have a broader role in vesicle traffic. Although here we observe a potential role for Lgl in endocytosis, it appears to be specific to Sanpodo, because endocytosis of Delta occurs normally in lgl mutants, suggesting that Lgl is not required for bulk endocytosis.
Increasingly, selective endocytosis is being implicated as an important regulator of signaling pathways (Dudu et al., 2004). Two recent studies demonstrate that liquid facets, an endocytic epsin (Overstreet et al., 2004; Wang and Struhl, 2004) participates in the neuralized-mediated Delta endocytosis, apparently by targeting mono-ubiquitinated Delta to a specific, activating, endocytic compartment (Wang and Struhl, 2004). The Notch receptor is also subjected to an ubiquitin-mediated endocytic step required for activation via the E3 ubiquitin ligase Deltex, which targets Notch to the late endosome (Hori et al., 2004). However, the roles of liquid facets and deltex have not been explored in asymmetrically dividing neural precursors. One possible function for Lgl could be to direct Sanpodo toward a specific endocytic compartment. Alternatively, Lgl may be involved indirectly, by targeting molecules required for Sanpodo endocytosis to the membrane region. This scenario would be more consistent with Lgl's role as an exocytic regulator.
An alternative hypothesis may be that Lgl regulates Sanpodo localization through its interaction with the cytoskeleton. lgl functions as an inhibitor of nonmuscle myosin II function in both Drosophila and yeast (Strand et al., 1994a,1994b; Kagami et al., 1998; Barros et al., 2003). Our data suggests that cytoskeletal association of Lgl is required for regulating Sanpodo localization, because phosphorylation of Lgl by aPKC, which causes an autoinhibitory conformational change in Lgl that disrupts the association with the cytoskeleton (Betschinger et al., 2005), causes membrane accumulation of Sanpodo. It remains to be determined if Sanpodo endocytosis requires inhibition of myosin II activity.
Previously, numb and neuralized had been implicated in two complementary, and possibly independent, mechanisms to determine cell fate in PNS precursor cells. Numb functions to inhibit Notch autonomously by internalizing Sanpodo in the pIIb cell: although Neuralized acts on Delta in the pIIb cell to induce Notch signaling nonautonomously in the pIIa cell. Both neuralized-dependant uptake of Delta (Le Borgne and Schweisguth, 2003) and Sanpodo internalization (this study) require dynamin function, suggesting that these steps rely on endocytosis. Unexpectedly, we found that loss of neuralized function affects both Delta internalization (Le Borgne and Schweisguth, 2003) and Sanpodo internalization. Failure to internalize Delta into the pIIb cell causes a cell fate transformation of the pIIa cell into a pIIb cell in neuralized mutants, and this transformation occurs despite the accumulation of Sanpodo at the membrane, suggesting that accumulation of Sanpodo at the membrane is not sufficient to induce Notch signaling in the pIIb cell in the absence of neuralized. It is unclear whether membrane accumulation of Sanpodo in neuralized mutants is due to a direct interaction between Neuralized and Sanpodo, perhaps through ubiquitination of Sanpodo, or through an indirect mechanism. Regardless, our data show that regulation of Sanpodo membrane localization is not completely independent of neuralized function. In summary, our study suggests that Sanpodo is regulated by both neuralized and lgl, whereas Delta is regulated by neuralized independently of lgl. In addition, we show that lgl appears to contribute to the endocytosis of Sanpodo, which suggests a broader role for lgl in vesicle trafficking, which may have important implications for its role as a tumor suppressor (Bilder, 2004).
Could the regulation of Notch signaling by Sanpodo, Lgl, and Numb be conserved across species? Sequence analysis did not reveal any homologues of sanpodo beyond other insect species (O'Connor-Giles and Skeath, 2003). However, loss-of-function studies of the mouse homologues of Drosophila numb and lgl in the developing brain show strikingly similar phenotypes. Targeted numb/numblike (Li et al., 2003) knockouts in dorsal forebrain and Lgl1 (Klezovitch et al., 2004) knockouts cause profound disorganization of the layered regions of the cortex and striatum and formation of rosette-like accumulations of neurons. These phenotypes may indicate that Numb and Lgl function together to regulate Notch signaling in mouse neurogenesis as well as in Drosophila PNS development, but a functional homologue sanpodo has yet to be identified in the mouse.
Acknowledgments
We thank C. Doe, J. Knoblich, E. Lai, J. Skeath, the Bloomington Drosophila Stock Center, and the Developmental Studies Hybridoma Bank (DSHB) for providing fly stocks and reagents. We also thank members of the Jan lab for helpful discussions.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–03–0177) on May 18, 2005.
Abbreviations used: APF, after pupae formation; ES, external sensory; GFP, green fluorescent protein; Lgl, lethal giant larvae; MARCM, mosaic analysis with a repressible cell marker; Pon, partner of Numb; PNS, peripheral nervous system.
References
- Arquier, N., Perrin, L., Manfruelli, P., and Semeriva, M. (2001). The Drosophila tumor suppressor gene lethal(2)giant larvae is required for the emission of the Decapentaplegic signal. Development 128, 2209–2220. [DOI] [PubMed] [Google Scholar]
- Barros, C. S., Phelps, C. B., and Brand, A. H. (2003). Drosophila nonmuscle myosin II promotes the asymmetric segregation of cell fate determinants by cortical exclusion rather than active transport. Dev. Cell 5, 829–840. [DOI] [PubMed] [Google Scholar]
- Bellaiche, Y., Radovic, A., Woods, D. F., Hough, C. D., Parmentier, M. L., O'Kane, C. J., Bryant, P. J., and Schweisguth, F. (2001). The Partner of Inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell 106, 355–366. [DOI] [PubMed] [Google Scholar]
- Berdnik, D., Torok, T., Gonzalez-Gaitan, M., and Knoblich, J. A. (2002). The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev. Cell 3, 221–231. [DOI] [PubMed] [Google Scholar]
- Betschinger, J., Eisenhaber, F., and Knoblich, J. A. (2005). Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr. Biol. 15, 276–282. [DOI] [PubMed] [Google Scholar]
- Betschinger, J., Mechtler, K., and Knoblich, J. A. (2003). The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422, 326–330. [DOI] [PubMed] [Google Scholar]
- Bilder, D. (2004). Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 18, 1909–1925. [DOI] [PubMed] [Google Scholar]
- Dudu, V., Pantazis, P., and Gonzalez-Gaitan, M. (2004). Membrane traffic during embryonic development: epithelial formation, cell fate decisions and differentiation. Curr. Opin. Cell Biol. 16, 407–414. [DOI] [PubMed] [Google Scholar]
- Dye, C. A., Lee, J. K., Atkinson, R. C., Brewster, R., Han, P. L., and Bellen, H. J. (1998). The Drosophila sanpodo gene controls sibling cell fate and encodes a tropomodulin homolog, an actin/tropomyosin-associated protein. Development 125, 1845–1856. [DOI] [PubMed] [Google Scholar]
- Hori, K., Fostier, M., Ito, M., Fuwa, T. J., Go, M. J., Okano, H., Baron, M., and Matsuno, K. (2004). Drosophila Deltex mediates Suppressor of Hairless-independent and late-endosomal activation of Notch signaling. Development 131, 5527–5537. [DOI] [PubMed] [Google Scholar]
- Justice, N., Roegiers, F., Jan, L. Y., and Jan, Y. N. (2003). Lethal giant larvae acts together with numb in notch inhibition and cell fate specification in the Drosophila adult sensory organ precursor lineage. Curr. Biol. 13, 778–783. [DOI] [PubMed] [Google Scholar]
- Kagami, M., Toh-e, A., and Matsui, Y. (1998). Sro7p, a Saccharomyces cerevisiae counterpart of the tumor suppressor l(2)gl protein, is related to myosins in function. Genetics 149, 1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klezovitch, O., Fernandez, T. E., Tapscott, S. J., and Vasioukhin, V. (2004). Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 18, 559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, E. C., Deblandre, G. A., Kintner, C., and Rubin, G. M. (2001). Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev. Cell 1, 783–794. [DOI] [PubMed] [Google Scholar]
- Lai, E. C., and Orgogozo, V. (2004). A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev. Biol. 269, 1–17. [DOI] [PubMed] [Google Scholar]
- Lai, E. C., and Rubin, G. M. (2001). neuralized functions cell-autonomously to regulate a subset of notch-dependent processes during adult Drosophila development. Dev. Biol. 231, 217–233. [DOI] [PubMed] [Google Scholar]
- Le Borgne, R., and Schweisguth, F. (2003). Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell 5, 139–148. [DOI] [PubMed] [Google Scholar]
- Lee, T., and Luo, L. (2001). Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 24, 251–254. [DOI] [PubMed] [Google Scholar]
- Lehman, K., Rossi, G., Adamo, J. E., and Brennwald, P. (1999). Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol. 146, 125–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. S., Wang, D., Shen, Q., Schonemann, M. D., Gorski, J. A., Jones, K. R., Temple, S., Jan, L. Y., and Jan, Y. N. (2003). Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron 40, 1105–1118. [DOI] [PubMed] [Google Scholar]
- Musch, A., Cohen, D., Yeaman, C., Nelson, W. J., Rodriguez-Boulan, E., and Brennwald, P. J. (2002). Mammalian homolog of Drosophila tumor suppressor lethal (2) giant larvae interacts with basolateral exocytic machinery in Madin-Darby canine kidney cells. Mol. Biol. Cell 13, 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor-Giles, K. M., and Skeath, J. B. (2003). Numb inhibits membrane localization of Sanpodo, a four-pass transmembrane protein, to promote asymmetric divisions in Drosophila. Dev. Cell 5, 231–243. [DOI] [PubMed] [Google Scholar]
- Ohshiro, T., Yagami, T., Zhang, C., and Matsuzaki, F. (2000). Role of cortical tumour-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature 408, 593–596. [DOI] [PubMed] [Google Scholar]
- Overstreet, E., Fitch, E., and Fischer, J. A. (2004). Fat facets and Liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development 131, 5355–5366. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos, E., Pitsouli, C., Klueg, K. M., Muskavitch, M. A., Moschonas, N. K., and Delidakis, C. (2001). neuralized encodes a peripheral membrane protein involved in delta signaling and endocytosis. Dev. Cell 1, 807–816. [DOI] [PubMed] [Google Scholar]
- Peng, C. Y., Manning, L., Albertson, R., and Doe, C. Q. (2000). The tumour-suppressor genes lgl and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature 408, 596–600. [DOI] [PubMed] [Google Scholar]
- Plant, P. J., Fawcett, J. P., Lin, D. C., Holdorf, A. D., Binns, K., Kulkarni, S., and Pawson, T. (2003). A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat. Cell Biol. 5, 301–308. [DOI] [PubMed] [Google Scholar]
- Rhyu, M. S., Jan, L. Y., and Jan, Y. N. (1994). Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76, 477–491. [DOI] [PubMed] [Google Scholar]
- Roegiers, F., and Jan, Y. N. (2004). Asymmetric cell division. Curr. Opin. Cell Biol. 16, 195–205. [DOI] [PubMed] [Google Scholar]
- Roegiers, F., Younger-Shepherd, S., Jan, L. Y., and Jan, Y. N. (2001). Two types of asymmetric divisions in the Drosophila sensory organ precursor cell lineage. Nat. Cell Biol. 3, 58–67. [DOI] [PubMed] [Google Scholar]
- Schweisguth, F. (2004). Notch signaling activity. Curr. Biol. 14, R129–R138. [PubMed] [Google Scholar]
- Skeath, J. B., and Doe, C. Q. (1998). Sanpodo and Notch act in opposition to Numb to distinguish sibling neuron fates in the Drosophila CNS. Development 125, 1857–1865. [DOI] [PubMed] [Google Scholar]
- Strand, D., Jakobs, R., Merdes, G., Neumann, B., Kalmes, A., Heid, H. W., Husmann, I., and Mechler, B. M. (1994a). The Drosophila lethal(2)giant larvae tumor suppressor protein forms homo-oligomers and is associated with nonmuscle myosin II heavy chain. J. Cell Biol. 127, 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand, D., Raska, I., and Mechler, B. M. (1994b). The Drosophila lethal(2)giant larvae tumor suppressor protein is a component of the cytoskeleton. J. Cell Biol. 127, 1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., and Struhl, G. (2004). Drosophila Epsin mediates a select endocytic pathway that DSL ligands must enter to activate Notch. Development 131, 5367–5380. [DOI] [PubMed] [Google Scholar]
- Yeh, E., Dermer, M., Commisso, C., Zhou, L., McGlade, C. J., and Boulianne, G. L. (2001). Neuralized functions as an E3 ubiquitin ligase during Drosophila development. Curr. Biol. 11, 1675–1679. [DOI] [PubMed] [Google Scholar]