Abstract
Intracellular persistence of the protozoan parasite, Trypanosoma cruzi, is an aggravating cause of Chagas' disease, involving that the protozoan infection specifically inhibits death receptor-mediated apoptosis of host cells. Here we demonstrate that the parasite dramatically up-regulates cellular FLICE inhibitory protein (c-FLIP), the only known mammalian inhibitor specific for death receptor signaling, in infected cells by an unusual, posttranscriptional stabilization of the short-lived protein. We also show that c-FLIP is accumulated in T. cruzi–infected mouse heart muscle cells in vivo. Stimulation of death receptor Fas in infected cells induces recruitment of c-FLIP to block the procaspase-8 activation at the most upstream caspase cascade. c-FLIP knock-down with a small interfering RNA significantly restores Fas-mediated apoptosis in infected cells. Taken together, our findings indicate that T. cruzi posttranscriptionally up-regulates and exploits host c-FLIP for the inhibition of death-inducing signal, a mechanism that may allow parasites to persist in host cells.
INTRODUCTION
Apoptosis is used as a defense strategy for the elimination of virus-, bacteria-, and parasite-infected cells by the immune system, as well as in cell selection during the development and maintenance of tissue homeostasis (Vaux et al., 1994). Pathogen-infected apoptotic cells are recognized and phagocytosed by macrophages, and the pathogen is eliminated, together with host cells, in an immunologically silent manner (Ren and Savill, 1998). In contrast, pathogens have been found to antagonize apoptotic death of invaded host cells, prolonging their survival and allowing them more time to replicate (Moss et al., 1999). Typically, viruses inhibit host cell apoptosis through the expression of antiapoptotic genes, such as viral FLIP (v-FLIP) in herpesvirus, p35 in baculovirus, E1B 19K in adenovirus, and CrmA in cow pox virus (Benedict et al., 2002). Little is known, however, regarding the molecule(s) through which intracellular parasites interfere with host cell apoptosis (Heussler et al., 2001; James and Green, 2004).
Trypanosoma cruzi, the protozoan parasite that causes Chagas' disease in Latin America (Morel and Lazdins, 2003), occurs as two forms in mammalian hosts. The nondividing trypomastigote form circulates in the bloodstream and invades a wide variety of nucleated cells, preferably heart muscle cells. Once in the host cell cytoplasm, the parasite transforms into the amastigote form. The intracellular parasite multiplies by binary fission, kills the host cell, and returns to the circulation as trypomastigotes that propagate the infection (Brener, 1973). CD8+ T lymphocytes are involved in T. cruzi infection, killing infected cells by triggering their death through the interaction of Fas ligand with its receptor and of tumor necrosis factor (TNF)-α with its receptor, TNFR (Tarleton et al., 1992; Rottenberg et al., 1993; Locksley et al., 2001). T. cruzi, however, can persist for many years in the mammalian host as intracellular amastigotes, suggesting that the parasite antagonizes apoptotic death of the invaded host cells. Chagas' disease is characterized by two distinct phases (Brener, 1973). The acute phase, which lasts 2–4 mo, involves a number of parasites detected in the blood stream as well as in host tissues, followed by a lifelong chronic phase in up to 30% of the patients. In chronic phase of Chagas' disease, T. cruzi persists in human with a nearly undetectable parasite load, and then the ultimate cause of the disease has been still hotly debated. However, currently accumulating evidence indicates that inefficient immune response to the parasites results in increased parasite load and increased incidence of chronic phase of Chagas' disease and that intracellular persistence of the parasites is an aggravating cause of even chronic phase of Chagas' disease (Tarleton and Zhang, 1999; Higuchi Mde et al., 2003). Therefore, how T. cruzi persists in host cell is one of the most important studies to understand the pathogenicity.
On binding of the trimeric Fas ligands or agonistic antibodies, Fas receptors recruit adaptor molecules (FADD) and procaspase-8 to form the death-inducing signaling complex (DISC). In this complex, procaspase-8 is activated to caspase-8, triggering the proteolytic cascade of effecter caspases leading to cell death (Medema et al., 1997). We previously demonstrated that induction of Fas- and TNFR-mediated apoptosis was more strongly inhibited in T. cruzi–infected cells than in uninfected cells and that caspase-8 activity could not be measured upon Fas stimulation of T. cruzi–infected cells (Nakajima-Shimada et al., 2000). We also showed that x-ray, H2O2, cholchicine, and etoposide, respectively, induced an essentially same degree of apoptosis between T. cruzi–infected and uninfected cells. These findings indicate that parasite infection inhibits one of the earliest steps of death receptor-mediated apoptosis.
Here we report that T. cruzi uses the host's cellular FLICE inhibitory protein (c-FLIP), the only known inhibitor specific for death receptor–mediated apoptosis in mammals (Thome and Tschopp, 2001), for the inhibition of Fas-mediated apoptosis by posttranscriptional up-regulation. This finding indicates that T. cruzi modulates and exploits a host molecule to counteract death receptor signaling, a finding consistent with the view that parasites hijack the host cell, placing themselves in the driver's seat (Beverley, 1996).
MATERIALS AND METHODS
Cells and Parasites
HT1080 cells, a human fibrosarcoma cell line obtained from Japan Health Sciences Foundation (Tokyo, Japan), were cultured in DMEM (Nissui Pharmaceutical, Tokyo, Japan) supplemented with 10% fetal bovine serum. HT1080 cells (2 × 105) were infected with 4 × 105 T. cruzi trypomastigotes (Tulahuen strain) and cultured for 4 d. A cell population with an infection rate greater than 80% was considered as infected cells. The infection complex of HT1080 cells and T. cruzi was maintained as described (Nakajima-Shimada et al., 2000).
Antibodies
Rabbit anti-c-FLIP polyclonal antibodies specific for amino acid residues 2–17 was obtained from Upstate Biotechnology (Lake Placid, NY). Mouse anti-c-FLIP monoclonal antibody (mAb; Dave-2) was from Alexis Biochemicals (San Diego, CA). Mouse anti-FADD mAb (clone 1) was purchased from BD Biosciences Clontech (Palo Alto, CA). Rabbit anti-caspase-8 polyclonal antibody (GD-13) was from Sigma-Aldrich (St. Louis, MO) and mouse anti-caspase-8 mAb (12F5) from Alexis Biochemicals. Mouse anti-p53 mAb (antibody-6) was from Oncogene (San Diego, CA). Mouse anti-Fas mAb (APO1–3) was purchased from Wako (Osaka, Japan). Rabbit anti-actin polyclonal antibody was from Sigma-Aldrich.
Western Blotting
Cell lysates were prepared in lysis buffer (20 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, and 1 mM phenylmethylsulfonyl fluoride [PMSF]), and the protein concentration was determined by the Bradford assay. Proteins were resolved by SDS-PAGE and transferred to Immobilon Transfer Membranes (Millipore, Bedford, MA) by electroblotting. Immunoblot analyses were performed with the indicated antibodies. Bound primary antibodies were visualized with alkaline phosphatase–conjugated specific antibodies and with CSPD (Roche, Mannheim, Germany).
Northern Blotting
Total RNA was isolated using TRIzol (Life Technologies, Tokyo, Japan) according to the manufacturer's instruction. Total RNA was size-fractionated, and blotting was performed using the VacuGene XL protocol (Amersham Biosciences, Piscataway, NJ). The c-FLIP probe was DIG-labeled using PCR Probe Synthesis Kit (Roche) in the presence of 5′-GAGTTGGAGAAACTAAAT-3′ and 5′-ACACTCTGGGAGCCTCCT-3′, the forward and reverse primers, respectively.
Immunohistochemistry
Five-week-old female BALB/c mice (Japan SLC, Hamamatsu, Japan) were intraperitoneally infected with T. cruzi trypomastigotes (5000 parasites/mouse) recovered from the preceding infected mice. Fourteen days later, heart samples were collected and frozen in blocks of optimal cutting temperature compound (OTC, Sakura, Tokyo, Japan) in liquid nitrogen according to standard procedure. The OTC block was cut in 4-μm sections onto silane-coated slide glasses. The sections were incubated with 10% normal goat blocking serum and then with anti-c-FLIP mAb G-11 (Santa Cruz Biotechnology, Santa Cruz, CA) diluted to 1:100. After washing with phosphate-buffered saline (PBS), the sections were incubated with secondary antibody (horse anti-mouse-FITC, Vector Laboratories, Burlingame, CA). After washing with PBS, the specimens were counterstained with 10 μM Hoechst 33342 (Calbiochem, La Jolla, CA) to visualize DNA. The quantitation of the fluorescence intensity of T. cruzi–infected and uninfected cells, the latter locating near the infected cells, were manually assessed by Image-Proplus ver. 4.0 software (Media Cybernetics, Silver Spring, MD). Background fluorescence intensity of the thin section was subtracted from the sample cell fluorescence intensity. Statistical evaluation was performed by SigmaPlot software (Systat Software, Point Richmond, CA).
Apoptosis Induction and Cell Death Assay
Cells were incubated with anti-Fas CH11 mAb (0.5 μg/ml; MBL, Nagoya, Japan) to induce apoptosis through Fas. The cells were washed in PBS, fixed with methanol, stained with Hoechst 33342, and photographed under a fluorescence microscope. Condensed nuclei were scored as apoptotic.
Immunoprecipitation
Immunoprecipitation was performed using magnetic beads as described by the manufacturer (New England Biolabs, Beverly, MA). Cells were lysed with IP buffer (150 mM NaCl, 10 mM Tris, pH 7.4, 1 mM EDTA, 1 mM EGTA, pH 8.0, 0.2 mM sodium orthovanadate, 0.2 mM PMSF, 1% Triton X-100, and 0.5% Nonidet P-40). The crude cell extract was preincubated with Protein G Magnetic Beads (New England Biolabs), and the resulting supernatant was incubated with the indicated antibody and the beads. Magnetic beads were washed with IP buffer and suspended in SDS sample loading buffer. After incubation at 70°C for 5 min, each sample was subjected to SDS-PAGE and Western blotting as described above.
DISC analysis was performed using a standard method (Krueger et al., 2001). Briefly, cells (1 × 107) were trypsinized, collected by centrifugation, and then resuspended in 5 ml of DMEM. Fas receptor was stimulated with 2 μg/ml anti-Fas mAb (APO1–3) for 20 min. Cells were washed with ice-cold PBS and lysed in 1 ml of lysis buffer (30 mM Tris, pH 7.5, 150 mM NaCl, 1 mM PMSF, 1% Triton X-100, and 10% glycerol) for 30 min at 4°C. Protein A-Sepharose beads (Amersham Bioscience) was added to the lysate. As a negative control, APO1–3 and protein A-Sepharose beads were added to the lysate of unstimulated cells. Sample and negative control lysates were incubated at 4°C on a rotator for 3 h. The beads were washed four times in lysis buffer and suspended and boiled in SDS sample loading buffer, and then each sample was subjected to SDS-PAGE and Western blotting as described above.
siRNA Experiment
For depletion of c-FLIP, we used siRNA generated in vitro by RNase III from Escherichia coli (Yang et al., 2002). To produce the long double-stranded RNA (dsRNA), c-FLIP gene (nucleotides 89–698 downstream from the start codon) were amplified by PCR using the specific primers 5′-gcgtaatacgactcactatagggagaagatgtggttccacctaatg-3′ (forward) and 5′-gcgtaatacgactcactatagggagagcttctgattcctgaatgga-3′ (reverse), which also contain a T7 promoter and a leader sequence. Double-stranded (ds) RNA was generated using TurboScript T7 Transcription Kit (Gene Therapy Systems, San Diego, CA). The dsRNA was converted to siRNA using ShortCut RNase III (New England Biolabs). The siRNAs were precipitated in ethanol and then dissolved in nuclease-free water. The concentration of the siRNAs was determined spectrophotometrically and by ethidium bromide staining in 3% agarose gels. As a negative control, we used luciferase siRNA (Luciferase GL2, Fasmac, Kanagawa, Japan).
Transfection of siRNAs (200 ng/ml) into 40–50% confluent HT1080 cells cultured in wells of a 12-well plate was performed using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturer's instruction. After cultivation for 24–30 h, the cells were infected with 2 × 106 trypomastigotes as previously described (Nakajima-Shimada et al., 2000), incubated for 24 h further, and harvested for Western blotting or apoptosis induction followed by cell death assay.
RESULTS
T. cruzi Infection Up-regulates c-FLIPL Protein Expression
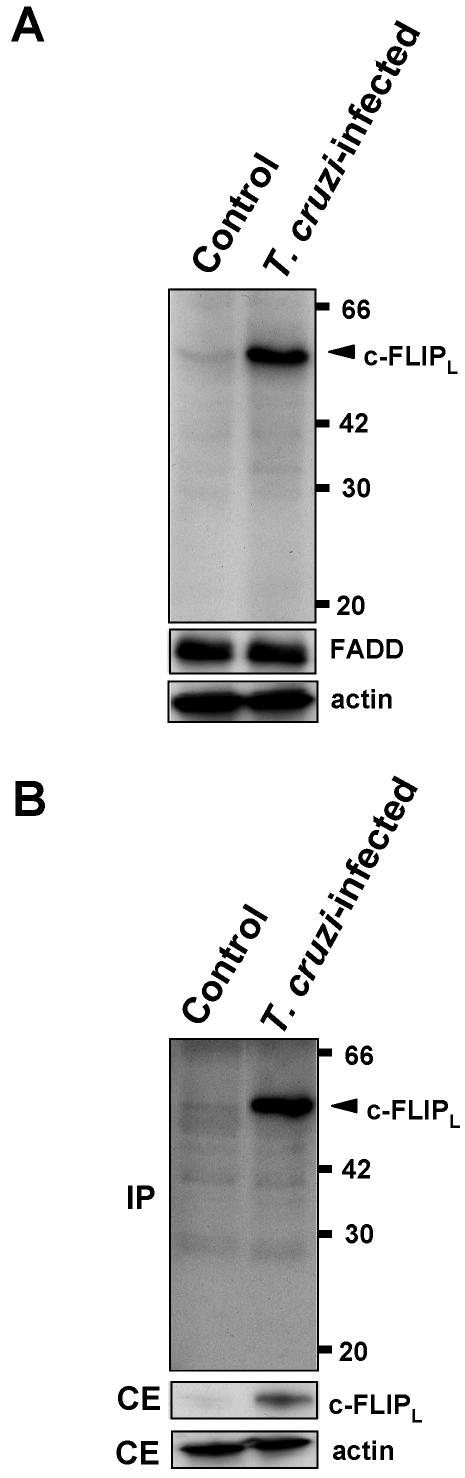
Because our previous study has demonstrated that caspase-8 activity could not be measured upon Fas stimulation of T. cruzi–infected cells (Nakajima-Shimada et al., 2000), expression of c-FLIP protein in T. cruzi–infected and uninfected cells was examined by Western blotting using an anti-c-FLIP antibody that recognizes both c-FLIP long (c-FLIPL) and c-FLIP short (c-FLIPS; Figure 1A). Both c-FLIPL and c-FLIPS, which are generated by alternative splicing, block procaspase-8 activation at the DISC and consequently inhibit death receptor–mediated apoptosis (Thome and Tschopp, 2001). Although we did not detect any 26-kDa c-FLIPS in either infected or control cells, the level of c-FLIPL (55 kDa) was dramatically up-regulated in infected cells when compared with uninfected cells. These two cell populations showed an essentially same level of FADD. We confirmed that, after immunoprecipitation by a c-FLIP-specific mAb (Dave-2, Alexis Biochemicals), Western blotting of the precipitated proteins using the above anti-c-FLIP antibody (see Figure 1A) also showed markedly up-regulated c-FLIPL protein level in T. cruzi–infected cells (Figure 1B).
Figure 1.
Expression of c-FLIP protein in T. cruzi–infected cells. (A) The cells (2 × 105) were infected with T. cruzi trypomastigotes as described in Materials and Methods. Cell extracts (100 μg protein/lane) were resolved on 12.5% SDS-PAGE, and Western blots were probed with anti-c-FLIP antibody specific for amino acid residues 2–17 and with anti-FADD antibody. Actin was used for loading control. (B) Cell extracts were immunoprecipitated (IP) using anti-c-FLIP mAb (Dave-2). Precipitated samples were analyzed by Western blotting with anti-c-FLIP antibody described in A. Cellular extracts (CE) were also analyzed by Western blotting (10 μg protein/lane). Numbers on the right indicate kilodaltons.
Posttranscriptional Up-regulation of c-FLIP Protein in T. cruzi–infected Cells
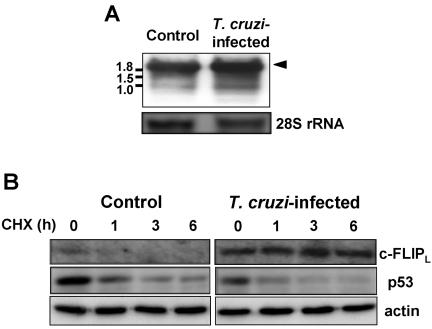
We examined whether the increase in c-FLIPL protein was due to the elevation of the corresponding mRNA in T. cruzi–infected cells. Northern blots showed that T. cruzi–infected cells had a level of c-FLIP mRNA (2.1 kb) nearly equivalent to that in uninfected cells (Figure 2A). In infected cells, mRNA of ∼1 kb was slightly up-regulated, but we did not elucidate whether this mRNA was translated. Importantly, c-FLIPL mRNA level does not parallel the increased protein level (see Figure 1).
Figure 2.
Expression of c-FLIP mRNA and effect of cycloheximide treatment. (A) Total RNA (2.5 μg/lane) was resolved on 1% agarose gel, Northern-blotted, and probed for c-FLIP. The band corresponding to c-FLIPL mRNA is indicated by arrowhead. Numbers on the left indicate kilobases. (B) Cells (70–80% confluent) were incubated with 100 μM cycloheximide for the indicated time, and the expression of c-FLIPL and p53 was analyzed by Western blotting. Actin was used for loading control.
Because c-FLIP is a short-lived protein, inhibitors of protein synthesis lower its expression level in a variety of cell lines (Imanishi et al., 2000a). In contrast to uninfected cells, T. cruzi–infected cells treated with cycloheximide did not show a rapid decrease in c-FLIPL protein (Figure 2B). The level of expression of p53, another short-lived protein regulated by the ubiquitin-proteasome pathway (Zhang et al., 2004), however, was rapidly decreased by cycloheximide treatment in both T. cruzi–infected and uninfected cells, indicating that T. cruzi infection selectively inhibits the degradation of c-FLIPL protein. Taken together, these results indicate that c-FLIPL is posttranscriptionally up-regulated by T. cruzi infection.
c-FLIP Is Also Up-regulated in T. cruzi–infected Cardiomyocytes In Vivo
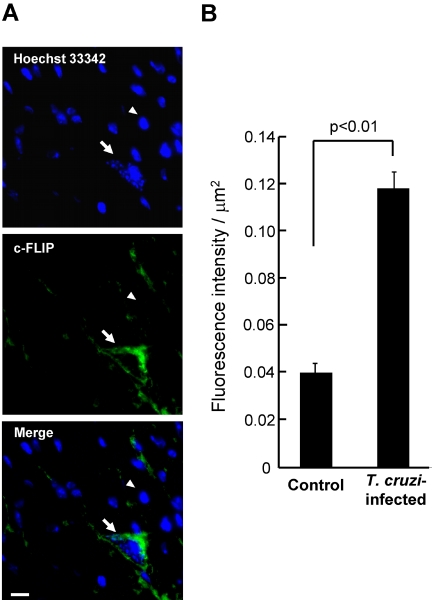
We also examined whether c-FLIP protein is increased in T. cruzi–infected mouse heart muscle cells, in which Chagasic myocarditis and amastigotes are often observed (Figure 3A). The monoclonal anti-c-FLIP antibody (G-11), with specificity for c-FLIP in immunohistochemical analysis (Kim and Seong, 2003; Mathas et al., 2004), revealed strong staining throughout the cytoplasm of an amastigote-dwelling cardiomyocyte, with obscure staining in uninfected cells. The fluorescence intensity of 58 each of T. cruzi–infected and uninfected cells was measured for statistical evaluation (Figure 3B). Up-regulation of c-FLIP was observed in all T. cruzi–infected cells, showing the fluorescence intensity significantly (p < 0.01) higher in the infected than in the uninfected cells. These results indicate that c-FLIP is also up-regulated in T. cruzi–infected cells in vivo when compared with uninfected cells.
Figure 3.
Immunohistochemical detection of c-FLIP in the cardiomyocytes of mice infected with T. cruzi. (A) Thin sections prepared from T. cruzi–infected mouse hearts were stained with Hoechst 33342 and with anti-c-FLIP mAb (G-11). Details are described in Materials and Methods. Fluorescence images and their merger in the same field in a typical section are shown. The arrow points toward a T. cruzi-dwelling cardiomyocyte. The arrowhead points at a typical uninfected cell. Bar in Merge, 10 μm. (B) The fluorescence intensity of 58 each of T. cruzi–infected and uninfected heart muscle cells was measured for a quantitation of expression level of c-FLIP. Statistical significance was assessed by Student's t test.
The fluorescence intensity of the infected cardiomyocytes in vivo did not clearly parallel in vitro studies (see Figure 1A), probably because of the lightly and heavily infected host cells in vivo and in vitro, respectively, with average parasite numbers of 5.5 and 15–20 per infected cell. Additionally, the T. cruzi–infected heart muscle cell, cut into a thin section, may exhibit a partial fluorescence staining of the whole cell. These results are consistent with a suggestion that the degree of the fluorescence intensity in host cells in vivo is lower than that of Western blot in vitro.
Signals were also detected near the cells lacking the parasite infection in the section shown in Figure 3A. Importantly, because these cells are not stained throughout the cytoplasm, the fluorescence would not be c-FLIP signal. We experienced that the mouse blood vessel was nonspecifically stained with anti-c-FLIP antibody (G-11). Therefore, it may be possible that blood vessels near uninfected cells were nonspecifically detected with this antibody. Alternatively, it may be possible that, because heart muscle cells are very long, a part of infected cells, whose nuclei and infected parasites were not seen, was stained with the antibody.
T. cruzi Infection Inhibits Fas-stimulated Procaspase-8 Activation by Recruitment of c-FLIPL into the DISC
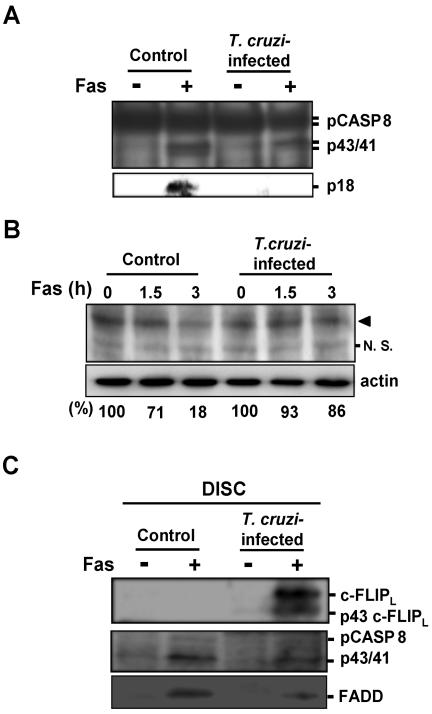
At the DISC, procaspase-8 is cleaved and converted to p43/41 fragments, and these are further processed to active caspase-8, a heterotetramer composed of two p18 and two p10 polypeptides (Medema et al., 1997). In the presence of a large amount of c-FLIPL, procaspase-8 and c-FLIPL are recruited into the DISC, the p43/41 cleavage products of caspase-8 and p43 cleavage product of c-FLIPL are generated, and then the cleavage intermediates remain bound to the DISC and can no longer be replaced by procaspase-8 (Scaffidi et al., 1999; Krueger et al., 2001). Because c-FLIPL protein is highly expressed in T. cruzi–infected cells, we examined procaspase-8 processing in infected and control cells following Fas stimulation (Figures 4, A and B).
Figure 4.
Processing of procaspase-8 and c-FLIPL in T. cruzi–infected cells. (A) Top: cells were incubated in the presence or absence of anti-Fas antibody for 5 h, and the cell lysates were immunoprecipitated with anti-caspase-8 mAb (12F5). The precipitated protein was analyzed by Western blotting with rabbit anti-caspase-8 polyclonal antibody (GD-13). Bottom: cell extracts (200 μg protein/lane) were fractionated on 12.5% SDS-PAGE and Western-blotted with anti-caspase-8 antibody (GD-13). p18 form processed from p43/41 form of caspase-8 is shown. (B) Cells were stimulated with anti-Fas antibody for 0, 1.5, and 3 h. Each cell extract (55 μg protein/lane) was fractionated on 15% SDS-PAGE and Western-blotted with anti-caspase-8 antibody. Arrowhead indicates the position of pro-caspase-8. Western blots of the panel were scanned using RFLP-scan (Scanalytics, Billerica, MA) and quantified for procaspase-8. The levels of procaspase-8 are shown at the bottom. N.S., nonspecific cross-reactive band. (C) T. cruzi–infected and uninfected cells were stimulated with anti-Fas antibody or were left untreated. After lysis of these cells, DISC or unstimulated Fas was immunoprecipitated by anti-Fas antibody (APO1–3) and analyzed by Western blotting using anti-caspase-8 antibody (GD-13), anti-c-FLIP antibody described in Figure 1A, and anti-FADD antibody. The positions of the proteins and the respective cleavage fragments are indicated.
Five hours after Fas stimulation of control and infected cells, followed by immunoprecipitation with a caspase-8-specific antibody (12F5), Western blot visualized with an anti-caspase-8 antibody (GD-13) showed that p43/41 could be detected in both control cells and T. cruzi–infected cells (Figure 4A, top panel). However, in control cells, but not in infected cells, p18 was produced (Figure 4A, bottom panel). Then, we monitored processing of procaspase-8 in control and T. cruzi–infected cells after Fas stimulation (Figure 4B). The anti-caspase-8 antibody we used (GD-13) does not differentiate between procaspase-8a (55 kDa) and -8b (53 kDa; Scaffidi et al., 1997; indicated by arrowhead). We found that, 3 h after Fas stimulation, only 14% of procaspase-8 was processed in T. cruzi–infected cells, whereas 82% was processed in control cells. These results indicate that procaspase-8 is recruited into the DISC, but that activation of the cytoplasmic procaspase-8 pool is strongly prevented in infected cells, upon Fas stimulation.
Next, we analyzed the DISC composition in T. cruzi–infected cells to know whether c-FLIPL is recruited into the DISC (Figure 4C). Both full-length c-FLIPL and p43 cleavage fragment of c-FLIPL were detected in Fas-stimulated T. cruzi–infected cells. In control cells, we could not detect both c-FLIPL and p43 c-FLIPL after Fas stimulation, which may be due to the low expression of c-FLIPL. Procaspase-8 was hardly detectable both in infected and control cells, but p43/p41 cleavage fragments of caspase-8 were detected in both infected and control cells after Fas stimulation. It has been shown that recruitment of FADD into the DISC is reduced in cells overexpressing c-FLIPL (Krueger et al., 2001). Consistent with this, FADD detected in our experiment was reduced in infected cells when compared with control cells. Taken together, our results indicate that T. cruzi infection inhibits the activation of procaspase-8 into caspase-8, due to the recruitment of c-FLIPL to the DISC, eventually leading to the inhibition of Fas-mediated apoptosis.
c-FLIP Knock-down with siRNA Restores Fas-mediated Apoptosis in T. cruzi–infected Cells
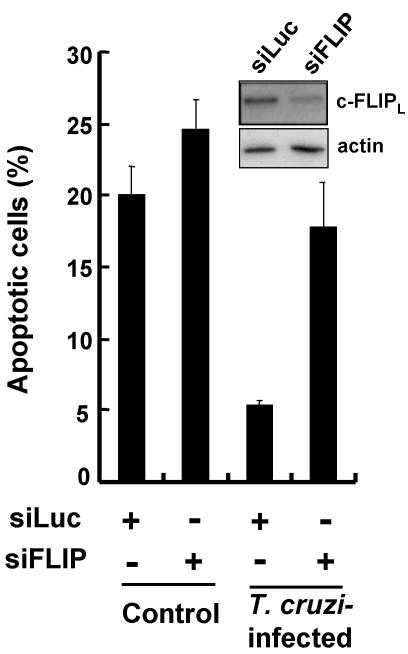
A selective c-FLIP knock-down with siRNA was carried out to clarify the crucial role of this protein in the inhibition of Fas-mediated apoptosis in T. cruzi–infected cells. Transfection of c-FLIP-specific siRNA (siFLIP) reduced the levels of c-FLIPL protein in infected cells when compared with a negative control siRNA specific for luciferase (siLuc; Figure 5, inset).
Figure 5.
c-FLIP knock-down and recovery of apoptosis in T. cruzi–infected cells. Preparation of siLuc (negative control) and si-FLIP, transfection of these siRNAs into cells, T. cruzi infection, and Fas stimulation were carried out as described in Materials and Methods. siRNA-transfected cells (70–80% confluent), which were subsequently infected with T. cruzi and incubated for 24 h, were stimulated for 5 h with anti-Fas antibody. The percentage of apoptotic cells was determined. In each experiment, more than 350 uninfected or 250 T. cruzi–infected cells were examined. The values are means of three separate experiments and the bars represent standard deviations. The inset shows the expression level of c-FLIPL and actin in T. cruzi–infected cells (15 μg protein/lane). Because of the low expression level of c-FLIPL in control cells, the protein was not detected under the conditions examined.
When we tested the effects of RNA interference against c-FLIP on the rate of apoptosis induced by Fas stimulation, we found that the control siRNA (siLuc) induced the apoptosis of 20.0 and 5.4% of the uninfected and T. cruzi–infected cell samples, respectively (Figure 5), indicating that T. cruzi infection strongly inhibited Fas-mediated apoptosis (Nakajima-Shimada et al., 2000); the difference is statistically significant (p < 0.01). In contrast, siFLIP resulted in a markedly and significantly increased apoptosis (from 5.4 to 17.8%) in T. cruzi–infected cells (p < 0.05), with a slight increase in apoptosis (from 20.0 to 24.6%) in uninfected cells (p = 0.188). Importantly, the rate of apoptosis was not significantly different between the infected cells transfected with siFLIP and the uninfected cells transfected with siLuc or siFLIP. The result implies that c-FLIP knock-down in T. cruzi–infected cells yielded the recovery of Fas-mediated apoptosis to a level equivalent to that in uninfected cells. From these findings, we conclude that c-FLIPL protein plays a key role in the inhibition of Fas-mediated apoptosis in T. cruzi–infected cells.
DISCUSSION
Inhibition of death receptor–mediated apoptosis is highly likely to play an important role for the survival of the intracellular protozoan parasite, T. cruzi, in infected cells. We have shown here that T. cruzi posttranscriptionally up-regulates and exploits the host protein, c-FLIP, to interfere with Fas-mediated host cell apoptosis. This is the first report showing a crucial molecule for a eukaryotic intracellular pathogen, which is much more complex an organism than virus or bacteria, to inhibit death-inducing signal. Concerning the death receptors and their signals examined to date, c-FLIP displays a protective role against apoptosis mediated by all these receptors (Thome and Tschopp, 2001). Therefore, the host c-FLIP would be a good target to be “manipulated” by intracellular protozoan parasites.
Several viruses encode v-FLIP (Thome and Tschopp, 2001), ensuring their survival and propagation. However, a BLAST search of TcruziDB, an integrated genome database for T. cruzi, shows that there is no FLIP homologue in the T. cruzi genome (Luchtan et al., 2004). The method by which the parasite benefits from exploitation of host c-FLIP, but not from exploitation of own FLIP homologue, is not yet known. Perhaps c-FLIP in infected cells is unable to become a target of CD8+ cytotoxic T lymphocytes, or posttranscriptional up-regulation of c-FLIP is more efficient and effective than expression of its own FLIP homologue in the inhibition of death-inducing signals.
Some tumor cells highly express c-FLIP and its level has correlated with cellular resistance to death receptor–mediated apoptosis (Igney and Krammer, 2002). Using siRNA specific for c-FLIP, it was recently shown that this protein is a key molecule in death receptor resistance in Hodgkin/Reed-Sternberg cells (Mathas et al., 2004). However, c-FLIP expression is reportedly regulated by transcriptional mechanism for most of these cells. By contrast, T. cruzi posttranscriptionally stabilizes the host c-FLIP protein (see Figure 2B), a unique strategy that the parasite takes advantage of the short-lived nature of the target protein.
T. cruzi infection has been shown to protect mammalian cells from apoptotic death caused by growth factor deprivation (Clark and Kuhn, 1999; Chuenkova and Pereira, 2000; Chuenkova et al., 2001). This phenomenon, however, has not been investigated from the viewpoint of inhibition of death receptor–mediated apoptosis. Interestingly, T. cruzi transsialidase activates phosphatidylinositol 3-kinase (PI3K)/Akt protein kinase signaling, which is utilized as a survival pathway in a variety of cell types (Chuenkova et al., 2001). Additionally, c-FLIP expression depends on the PI3K/Akt protein kinase activity in many cell lines, where its up-regulation may take place at a transcriptional level (Panka et al., 2001). By contrast, our data clearly show that c-FLIP, at least c-FLIPL, is up-regulated posttranscriptionally in T. cruzi–infected cells, implying that its up-regulation is independent on PI3K/Akt protein kinase signaling. Indeed, treatment of T. cruzi–infected cells with LY294002, a specific and permeable PI3K inhibitor, at 50 μM for 24 h did not affect c-FLIP expression or sensitivity to Fas stimulation (our unpublished data).
How T. cruzi selectively inhibits the degradation of c-FLIP protein is an important issue. As a short-lived protein, c-FLIP level would be regulated by the ubiquitin-proteasome pathway, in which the specific ubiquitin ligase (E3) is involved. In general, however, identification of physiologically functional E3 is important, but difficult, in that the endogenous expression level of c-FLIP is very low (Scaffidi et al., 1999), and in that c-FLIP interact with various key proteins, including procaspase-8, FADD, TNFR-associated factors 1 and 2, and Rip and Raf kinases (Kataoka et al., 2000). Because of these factors, it would therefore be difficult to pinpoint the role of the E3 associated with the posttranscriptional up-regulation of c-FLIP. Nevertheless, we believe that further molecular and cellular pursuit of insight into how T. cruzi stabilizes c-FLIP contributes to identify the specific E3.
Although we found that c-FLIP was highly expressed in infected cardiomyocytes in vivo, it is not clear that the protein is the only molecule that inhibits death receptor–mediated apoptosis in vivo. Because c-FLIP knock-out mice do not survive past day 10.5 of embryogenesis (Yeh et al., 2000), the role of c-FLIP in adult mammals is not well understood. However, it has been shown that c-FLIP is highly expressed in the adult human and murine heart when compared with other organs and suggested that down-regulation of c-FLIP sensitized cardiac myocytes to apoptotic death (Rasper et al., 1998). Furthermore, c-FLIP is abundant in normal cardiomyocytes from failing human hearts, but the protein is absent from apoptotic cardiac myocytes (Imanishi et al., 2000b). It is, therefore, strongly suggested that c-FLIP functions as a strong antiapoptotic factor in T. cruzi–dwelling cardiomyocytes in hearts from infected animals. Because the differences in biological features of T. cruzi amastigotes in acute and chronic phase of infection are poorly understood, whether the chronic stage amastigotes also up-regulate c-FLIP is interesting. However, these amastigotes could not be observed by standard histochemical techniques because of the very low parasite density. To address this question, we need experimental techniques that allow more exhaustive analysis of a whole mouse heart and we need more detailed information about the cell biological features of the chronic stage amastigotes (e.g., their method of replication and the spot(s) they preferably persist). On the other hand, it has been proposed that inadequate clearance of the acute phase parasites due to a substandard immune response leads to chronic phase of Chagas' disease. Therefore, c-FLIP may be a clue to understanding how the intracellular parasite persists in mammalian cells, eventually giving rise to pathogenicity at the molecular level.
Acknowledgments
We thank S. Nakamura for immunohistochemical assistance. This work was supported by Grant-in-Aids for Scientific Research (15019099, 14570221, 15390138, and 17390123) from the Ministry of Education, Science, Sports, Culture, and Technology (ESSCT) of Japan. T.A. is supported by a Grant-in-Aid for the 21st Century COE Research from the Ministry of ESSCT of Japan.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–12–1051) on May 25, 2005.
Abbreviations used: c-FLIP, cellular FLICE inhibitory protein; c-FLIPL, c-FLIP long; DISC, death-inducing signaling complex; FADD, Fas-associated death domain-containing protein; siRNA, small interfering RNA oligoribonucleotide.
References
- Benedict, C. A., Norris, P. S., and Ware, C. F. (2002). To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 3, 1013–1018. [DOI] [PubMed] [Google Scholar]
- Beverley, S. M. (1996). Hijacking the cell: parasites in the driver's seat. Cell 87, 787–789. [DOI] [PubMed] [Google Scholar]
- Brener, Z. (1973). Biology of Trypanosoma cruzi. Annu. Rev. Microbiol. 27, 347–382. [DOI] [PubMed] [Google Scholar]
- Chuenkova, M. V., Furnari, F. B., Cavenee, W. K., and Pereira, M. A. (2001). Trypanosoma cruzi trans-sialidase: a potent and specific survival factor for human Schwann cells by means of phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. USA 98, 9936–9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuenkova, M. V., and Pereira, M. A. (2000). A trypanosomal protein synergizes with the cytokines ciliary neurotrophic factor and leukemia inhibitory factor to prevent apoptosis of neuronal cells. Mol. Biol. Cell 11, 1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, R. K., and Kuhn, R. E. (1999). Trypanosoma cruzi does not induce apoptosis in murine fibroblasts. Parasitology 118(Pt 2), 167–175. [DOI] [PubMed] [Google Scholar]
- Heussler, V. T., Küenzi, P., and Rottenberg, S. (2001). Inhibition of apoptosis by intracellular protozoan parasites. Int. J. Parasitol. 31, 1166–1176. [DOI] [PubMed] [Google Scholar]
- Higuchi Mde, L., Benvenuti, L. A., Martins Reis, M., and Metzger, M. (2003). Pathophysiology of the heart in Chagas' disease: current status and new developments. Cardiovasc. Res. 60, 96–107. [DOI] [PubMed] [Google Scholar]
- Igney, F. H., and Krammer, P. H. (2002). Death and anti-death: tumour resistance to apoptosis. Nat. Rev. Cancer 2, 277–288. [DOI] [PubMed] [Google Scholar]
- Imanishi, T., Hano, T., Nishio, I., Liles, W. C., Schwartz, S. M., and Han, D. K. (2000a). Transition of apoptotic resistant vascular smooth muscle cells to troptotic sensitive state is correlated with downregulation of c-FLIP. J. Vasc. Res. 37, 523–531. [DOI] [PubMed] [Google Scholar]
- Imanishi, T. et al. (2000b). Cellular FLIP is expressed in cardiomyocytes and down-regulated in TUNEL-positive grafted cardiac tissues. Cardiovasc. Res. 48, 101–110. [DOI] [PubMed] [Google Scholar]
- James, E. R., and Green, D. R. (2004). Manipulation of apoptosis in the host-parasite interaction. Trends Parasitol. 20, 280–287. [DOI] [PubMed] [Google Scholar]
- Kataoka, T. et al. (2000). The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr. Biol. 10, 640–648. [DOI] [PubMed] [Google Scholar]
- Kim, K. H., and Seong, B. L. (2003). Pro-apoptotic function of HBV X protein is mediated by interaction with c-FLIP and enhancement of death-inducing signal. EMBO J. 22, 2104–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger, A., Schmitz, I., Baumann, S., Krammer, P. H., and Kirchhoff, S. (2001). Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J. Biol. Chem. 276, 20633–20640. [DOI] [PubMed] [Google Scholar]
- Locksley, R. M., Killeen, N., and Lenardo, M. J. (2001). The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104, 487–501. [DOI] [PubMed] [Google Scholar]
- Luchtan, M., Warade, C., Weatherly, D. B., Degrave, W. M., Tarleton, R. L., and Kissinger, J. C. (2004). TcruziDB: an integrated Trypanosoma cruzi genome resource. Nucleic Acids Res. 32 Database issue, D344–D346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathas, S. et al. (2004). c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J. Exp. Med. 199, 1041–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema, J. P., Scaffidi, C., Kischkel, F. C., Shevchenko, A., Mann, M., Krammer, P. H., and Peter, M. E. (1997). FLICE is activated by association with the CD95 death-inducing signaling complex (DISC). EMBO J. 16, 2794–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel, C. M., and Lazdins, J. (2003). Chagas disease. Nat. Rev. Microbiol. 1, 14–15. [DOI] [PubMed] [Google Scholar]
- Moss, J. E., Aliprantis, A. O., and Zychlinsky, A. (1999). The regulation of apoptosis by microbial pathogens. Int. Rev. Cytol. 187, 203–259. [DOI] [PubMed] [Google Scholar]
- Nakajima-Shimada, J., Zou, C., Takagi, M., Umeda, M., Nara, T., and Aoki, T. (2000). Inhibition of Fas-mediated apoptosis by Trypanosoma cruzi infection. Biochim. Biophys. Acta 1475, 175–183. [DOI] [PubMed] [Google Scholar]
- Panka, D. J., Mano, T., Suhara, T., Walsh, K., and Mier, J. W. (2001). Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J. Biol. Chem. 276, 6893–6896. [DOI] [PubMed] [Google Scholar]
- Rasper, D. M. et al. (1998). Cell death attenuation by `Usurpin,' a mammalian DED-caspase homologue that precludes caspase-8 recruitment and activation by the CD-95 (Fas, APO-1) receptor complex. Cell Death Differ. 5, 271–288. [DOI] [PubMed] [Google Scholar]
- Ren, Y., and Savill, J. (1998). Apoptosis: the importance of being eaten. Cell Death Differ. 5, 563–568. [DOI] [PubMed] [Google Scholar]
- Rottenberg, M. E., Bakhiet, M., Olsson, T., Kristensson, K., Mak, T., Wigzell, H., and Örn, A. (1993). Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect. Immun. 61, 5129–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi, C., Medema, J. P., Krammer, P. H., and Peter, M. E. (1997). FLICE is predominantly expressed as two functionally active isoforms, caspase-8/a and caspase-8/b. J. Biol. Chem. 272, 26953–26958. [DOI] [PubMed] [Google Scholar]
- Scaffidi, C., Schmitz, I., Krammer, P. H., and Peter, M. E. (1999). The role of c-FLIP in modulation of CD95-induced apoptosis. J. Biol. Chem. 274, 1541–1548. [DOI] [PubMed] [Google Scholar]
- Tarleton, R. L., Koller, B. H., Latour, A., and Postan, M. (1992). Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356, 338–340. [DOI] [PubMed] [Google Scholar]
- Tarleton, R. L., and Zhang, L. (1999). Chagas disease etiology: autoimmunity or parasite persistence? Parasitol. Today 15, 94–99. [DOI] [PubMed] [Google Scholar]
- Thome, M., and Tschopp, J. (2001). Regulation of lymphocyte proliferation and death by FLIP. Nat. Rev. Immunol. 1, 50–58. [DOI] [PubMed] [Google Scholar]
- Vaux, D. L., Haecker, G., and Strasser, A. (1994). An evolutionary perspective on apoptosis. Cell 76, 777–779. [DOI] [PubMed] [Google Scholar]
- Yang, D., Buchholz, F., Huang, Z., Goga, A., Chen, C. Y., Brodsky, F. M., and Bishop, J. M. (2002). Short RNA duplexes produced by hydrolysis with Escherichia coli RNase III mediate effective RNA interference in mammalian cells. Proc. Natl. Acad. Sci. USA 99, 9942–9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, W. C. et al. (2000). Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity 12, 633–642. [DOI] [PubMed] [Google Scholar]
- Zhang, H. G., Wang, J., Yang, X., Hsu, H. C., and Mountz, J. D. (2004). Regulation of apoptosis proteins in cancer cells by ubiquitin. Oncogene 23, 2009–2015. [DOI] [PubMed] [Google Scholar]