Abstract
Cell cycle progression is dependent on the nuclear localization and transcriptional effects of activated extracellular signal-regulated kinase (ERK)1 and ERK2 mitogen-activated protein (MAP) kinases (ERK1/2). Phosphoprotein enriched in astrocytes (PEA-15) binds ERK1/2 and inhibits their nuclear localization, thus blocking cell proliferation. Here, we report that phosphorylation of PEA-15 blocks its interaction with ERK1/2 in vitro and in vivo and that phosphorylation of both Ser104 and Ser116 is required for this effect. Using phosphomimetic and nonphosphorylatable mutants of PEA-15, we found that PEA-15 phosphorylation abrogates its capacity to block the nuclear localization and transcriptional activities of ERK1/2; this phosphorylation therefore enables the proliferation of cells that express high levels of PEA-15. Additionally, we report that PEA-15 phosphorylation can modulate nontranscriptional activities of ERK1/2, such as the modulation of the affinity of integrin adhesion receptors. Finally, we used a novel anti-phospho-specific PEA-15 antibody to establish that PEA-15 is phosphorylated in situ in normal mammary epithelium. These results define a novel posttranslational mechanism for controlling the subcellular localization of ERK1/2 and for specifying the output of MAP kinase signaling.
INTRODUCTION
The mitogen-activated protein (MAP) kinases extracellular signal-regulated kinase (ERK)1 and ERK2 (hereafter referred to as ERK1/2), play a critical role in cell cycle progression by phosphorylating transcription factors such as Elk-1 (Gille et al., 1995). To phosphorylate these transcription factors, ERK1/2 must enter the nucleus. The nuclear import and export of ERK1/2 are regulated by a variety of protein–protein interactions that can therefore serve as important control points in cell proliferation. Indeed, several proteins have been identified that can bind to ERK1/2 and prevent its nuclear accumulation, including β-arrestin, calponin, mitogen-activated protein kinase phosphatase-3, dominant negative mitogen-activated protein kinase kinase (MEK), and phosphoprotein enriched in astrocytes (PEA-15) (Menice et al., 1997; Camps et al., 1998; Dang et al., 1998; Formstecher et al., 2001; Robinson et al., 2002; Tohgo et al., 2002; Whitehurst et al., 2004). Among these, PEA-15, a small death effector domain (DED)-containing protein, sequesters ERK in the cytoplasm, thereby inhibiting cell proliferation in a variety of cell types and contributing to cellular senescence (Formstecher et al., 2001; Gaumont-Leclerc et al., 2004). Importantly, PEA-15 binding does not interfere with ERK1/2 activation in vitro or in vivo nor does it inhibit ERK1/2's capacity to phosphorylate substrates, including nuclear substrate in vitro (Formstecher et al., 2001; Gaumont-Leclerc et al., 2004). PEA-15 alters ERK signaling by retaining ERK1/2 in the cytoplasm by blocking nuclear import and by promoting nuclear export (Formstecher et al., 2001; Whitehurst et al., 2004). Thus, PEA-15 is a protein that controls cell proliferation by preventing ERK1/2 accumulation in the nucleus.
Numerous biological functions have been ascribed to PEA-15 since its discovery in astrocytes (Araujo et al., 1993). Its amino acid sequence is completely conserved in human, mouse, rat, and hamster and is widely expressed in tissues, including brain, breast, lung, and prostate (Araujo et al., 1993; Danziger et al., 1995; Estelles et al., 1996). PEA-15 expression is increased in type II diabetes and its overexpression in fibroblasts or transgenic mice inhibits glucose transport (Condorelli et al., 1998). Furthermore, the presence of a DED in PEA-15 suggests a regulatory role in apoptosis; in some systems, it can protect cells from receptor-mediated apoptosis (i.e., tumor necrosis factor-related apoptosis-inducing ligand, Fas, and tumor necrosis factor [TNF]-α) (Condorelli et al., 1999; Estelles et al., 1999; Kitsberg et al., 1999; Zvalova et al., 2001; Renault et al., 2003; Sharif et al., 2003). PEA-15 also binds to and regulates the expression of phospholipase D1 and the activity of p90 ribosomal S6 kinase 2 (Zhang et al., 2000; Vaidyanathan and Ramos, 2003). PEA-15 has been ascribed a role in a variety of diseases, including squamous cell carcinoma, glioma, breast cancer, astrogliosis, and diabetes (Bera et al., 1994; Hwang et al., 1997; Condorelli et al., 1998; Glienke et al., 2000; Tsukamoto et al., 2000; Dong et al., 2001; Embury et al., 2001; Underhill et al., 2001; Sharif et al., 2004). Thus, PEA-15 is a multifunctional protein with roles in multiple physiological and pathological processes.
As noted above, there is compelling evidence that PEA-15 expression leads to inhibition of cell proliferation by binding to ERK1/2 to prevent their transcriptional activities (Formstecher et al., 2001; Gaumont-Leclerc et al., 2004). Yet, PEA-15 is expressed in certain tumor cells lines that proliferate rapidly and cultured astrocytes continue to proliferate while expressing the protein at high levels (Araujo et al., 1993; Estelles et al., 1996). Furthermore, PEA-15 binds to the kinase-insert domain of ERK2; this interaction blocks the ability of MEK1 to activate ERK2 (Whitehurst et al., 2004). Paradoxically, over expression of PEA-15 leads to MEK1- and MEK2-dependent increases in ERK1/2 activation (Ramos et al., 2000). The capacity of PEA-15 to stimulate ERK1/2 activation and its presence in rapidly dividing cells suggest that the interaction of PEA-15 with ERK1/2 may be subject to regulation by posttranslational modifications of one of the proteins.
The structure of PEA-15 suggests that phosphorylation could regulate its binding to ERK1/2. In cultured astrocytes, PEA-15 is phosphorylated on two Ser residues, Ser104 and Ser116. Protein kinase C (PKC) phosphorylates Ser104 and calcium/calmodulin kinase (CamKII) or Akt phosphorylate Ser116 (Araujo et al., 1993; Danziger et al., 1995; Kubes et al., 1998). PEA-15 is composed of the N-terminal DED and a C terminus that forms a less structured “tail” (Hill et al., 2002). The C-terminal tail contains both phosphorylation sites in proximity to residues required for ERK1/2 binding (Ramos et al., 1998, 2000; Formstecher et al., 2001; Hill et al., 2002; Chou et al., 2003). Furthermore, phosphorylation of Ser116 regulates the antiapoptotic function of PEA-15 and modulates its targeting to the death inducing signaling complex (DISC) (Condorelli et al., 1999; Trencia et al., 2003). In this study, we analyzed the effect of phosphorylation on ERK1/2 binding and on cell proliferation. Here, we report that phosphorylation of PEA-15 blocks its interaction with ERK1/2 in vitro and in vivo and that phosphorylation of both Ser104 and Ser116 is required for this effect. Using phosphomimetic and nonphosphorylatable mutants of PEA-15, we found that PEA-15 phosphorylation abrogates its capacity to block the nuclear localization and transcriptional activities of ERK1/2, thus enabling the proliferation of cells that express high levels of PEA-15. We also report that PEA-15 phosphorylation can modulate nontranscriptional activities of ERK1/2, such as the regulation of the affinity of integrin adhesion receptors (Chou et al., 2003). Finally, we used a phospho-specific anti-PEA-15 antibody to establish that PEA-15 is phosphorylated in tumor cells and in situ in normal tissue. Thus, these studies define a novel posttranslational mechanism for controlling the subcellular localization of ERK1/2 and for specifying the biological consequences of the MAP kinase signaling cascade.
MATERIALS AND METHODS
Cell Lines
Chinese hamster ovary (CHO) cells, glioma CRL-1620 cells (glioma 1620), NIH 3T3, and MB-MDA-231 breast cancer cells (MDA-231) were obtained from American Type Culture Collection (Manassas, VA). All cells were cultured in DMEM with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1% l-glutamine, and 1% nonessential amino acids (all from Invitrogen, Carlsbad, CA).
Antibodies and Immunohistochemistry
Rabbit polyclonal anti-PEA-15 (3099) was raised against a synthetic peptide containing the C-terminal 14 amino acids (EEEIIKLAPPPKKA) of PEA-15 as described previously (Ramos et al., 2000). Anti-PEA-15 (4513) was raised against a glutathione S-transferase-PEA-15 fusion protein (GST-PEA-15). Anti-PEA-15 (4513) was absorbed with GST-agarose, adjusted to pH 8.0 in 100 mM Tris, and bound to immobilized GST-PEA-15. Affinity-purified anti-PEA-15 (4513) was eluted using 100 mM glycine (pH 3.0) and neutralized with 1 M Tris (pH 8.0). Anti-PEA-15 phospho-S116 (p-PEA-15), which recognizes PEA-15 when it is phosphorylated at Ser116, was produced at BioSource International (Camarillo, CA). Antiserum was generated using a chemically synthesized phosphorylated peptide (IRQP[pS]EEEIIKL) coupled to keyhole limpet hemocyanin and injected into specific pathogen-free rabbits. The resulting phosphorylation site-specific antibody was purified using both negative and positive peptide affinity purification. The rabbit antibody against lamins A/C was a gift from Dr. Larry Gerace (The Scripps Research Institute, La Jolla, CA). Antibodies against Influenza hemagglutinin (HA) tag and Rho GDI were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FLAG antibody was obtained from Sigma-Aldrich (St. Louis, MO). Unless otherwise indicated, all antibodies were used at a 1:1000 dilution. Anti-rabbit and anti-mouse horseradish peroxidase (HRP)-conjugated antibodies (BioSource International) and SuperSignal Pico Chemiluminescent Substrate (Pierce Chemical, Rockford, IL) were used for Western blot detection.
Immunohistochemistry was performed on Formalin-fixed/paraffin-embedded breast tissue sections by immunoperoxidase staining. Paraffin-embedded sections were deparaffinized and then microwaved for 10 min in citrate buffer (10 mM sodium citrate, 0.05% Tween 20, pH 6.0). After cooling, endogenous peroxidase was blocked with 0.3% H2O2 in phosphate-buffered saline (PBS) (1.7 mM KH2PO4, 5.2 mM Na2HPO4 150 mM NaCl, pH 7.4) for 10 min. Further blocking was done in 1% bovine serum albumin (BSA) (Sigma-Aldrich) in PBS for 20 min. Primary antibodies were incubated on the slides overnight at 4°C. Affinity purified anti-PEA-15 (4513) was used at 0.1 μg/μl, and anti-p-PEA-15 was used at a 1:25 dilution. Equivalent amounts of control rabbit IgG and 1% BSA were used as a control. After washing in PBS, goat anti-rabbit HRP secondary antibodies (1:500) were added to the slides for 30 min at room temperature. Staining was then developed with aminoethylcarbazole chromagen. Slides were counterstained with Mayer's hematoxylin. Dilutions and staining conditions were validated on fixed MB-MDA-231 and CRL 1620 cell lines before performing immunohistochemistry on breast tissue.
cDNA Constructs
PEA-15 cDNA expression constructs used in this work have been described previously (Chou et al., 2003). PEA-15 cDNA was expressed from two vectors, pCDNA3.1(+) (Invitrogen) for eukaryotic expression and pGEX2T (Amersham Biosciences UK, Little Chalfont, Buckinghamshire, United Kingdom) for in vitro protein production from bacterial cells. The PEA-15 mutant L123R was initially described in Hill et al. (2002). All PEA-15 constructs in pCDNA3 included a C-terminal HA tag. Additional PEA-15 mutants S104D, S116D, S104A, and S116A, were generated with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) by using wild-type pGEX2T-PEA-15 or pCDNA3-PEA-15 as the template. All plasmid constructs were verified by DNA sequencing. The pEGFP-C1 vector was obtained from BD Biosciences Clontech (Palo Alto, CA).
In Vitro Protein Production
BL21 competent bacteria were transfected with pGEX2T-PEA-15 plasmids (wild-type or mutant) and induced to express protein with 1 mM isopropyl β-d-thiogalactoside for 2–3 h. The bacteria were lysed in a PBS buffer containing 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 2 mM dithiothreitol (DTT), 5 μg/ml aprotinin, and 0.5 mM leupeptin. Wild-type and mutant GST-PEA-15 were then enriched from total bacterial lysate by binding to glutathione-Sepharose 4B beads (Amersham Biosciences UK). Bead-bound GST-PEA-15 was washed with PBS and eluted using 20 mM glutathione. After dialysis against PBS, purified GST-PEA-15 was stored at –70°C.
In Vitro Phosphorylation and ERK Binding
NIH 3T3 cells were lysed in a kinase active buffer (1% NP-40, 25 mM Tris HCl, pH 7.4, 150 mM NaCl, 1 mM NaF, 1 mM EGTA, 2 mM DTT, 3 mM MgCl2, 2 mM CaCl2, 1 mM NaVO4, EDTA-free protease inhibitor cocktail [Roche Diagnostics, Mannheim, Germany]) to which 1 mM ATP with 0.022% [γ-32P]ATP was added. One hundred micrograms of total cell lysate in 100 μl was then incubated with Sepharose-immobilized GST-PEA-15 for 2 h at 37°C in the presence or absence of lipid activator [50 μg/ml l-α-phosphatidyl-l-serine sodium salt (Sigma-Aldrich) and 50 μM phorbol 12-myristate 13-acetate (PMA; EMD Biosciences, La Jolla, CA) in 25 mM Tris, pH 7.4. In some cases, PKC or CamKII were inhibited by addition of 1 μM bisindoylmaleimide I (Bis) or 10 μM KN-62 (EMD Biosciences), respectively.
At the end of the incubation, beads were sedimented by centrifugation at 22,000 × g and washed three times with PBS. The bead bound PEA-15 was solubilized by digestion of the immobilized GST-PEA-15 fusion protein with thrombin (100 U/ml) in a buffer containing 100 mM Tris, pH 8.0, 150 mM NaCl, 2.5 mM CaCl2. Eluted proteins were resolved by SDS-PAGE under reducing conditions and transferred to nitrocellulose membranes. Incorporation of 32P was assayed by autoradiography. The blots were stained with antibodies reactive with ERK1/2 (Santa Cruz Biotechnology) to detect bound ERK1/2 and antibodies against PEA-15 to assay loading of the affinity matrix.
Coimmunoprecipitation
CHO cells were cotransfected with 0.5 μg of pCDNA3-FLAG-ERK2 and either 1.5 μg of pCDNA3-HA-PEA-15, pCDNA3-HA-PEA-15 mutants, or empty vector. Transient transfections were carried out using Lipofectamine and Plus reagents as per manufacturer's protocol (Invitrogen). Cells were harvested 24 h posttransfection and scraped into 1 ml of lysis buffer (20 mM HEPES, pH 7.4, 2 mM EGTA, 2 mM MgCl2, 2 mM NaVO4, protease inhibitors cocktail). Total cell lysates were homogenized by 20 serial passages through a 27-gauge needle and then centrifuged at 22,000 × g for 10 min. The supernatant was precleared with 20 μl of protein G-Sepharose beads (∼50% slurry; Amersham Biosciences UK) at 4°C for 30 min. For each condition, 2 μg of anti-HA antibody and 20 μl of protein G-Sepharose beads were added to 500 μg of cleared lysate, and incubated for 2 h at 4°C. Immunoprecipitates were washed three times in lysis buffer and solubilized in 5× SDS-PAGE sample buffer [10% (wt/vol) SDS, 250 mM Tris, pH 6.8, 500 mM DTT, 50% glycerol, 1 mg/ml bromphenol blue]. Western blotting with anti-FLAG was used to assay ERK coimmunoprecipitation and with anti-HA to detect immunopre-cipitated PEA-15. Separately, 25 μg of total cell lysate was immunoblotted for FLAG-ERK and HA-PEA-15 to verify comparable expression in all samples.
Subcellular Fractionation
CHO cells were transfected with cDNA encoding wild-type PEA-15, phosphomimetic mutants, or empty vector in a 3:1 ratio to HA-tagged ERK2. Cells were allowed to recover from transfection by allowing growth in complete media for 24 h. Next, cells were maintained in serum-free media for an additional 24 h. Cells were stimulated for 3 h with 10% FBS and then harvested and suspended at 5 × 106 cells/ml in fractionation buffer (20 mM HEPES, pH 7.5, 1.5 mM MgCl2, 5 mM KCl, 1 mM DTT, 1 mM NaVO4, with Complete EDTA Free protease inhibitor cocktail [Roche Diagnostics]). After incubation on ice for 20 min, cells were homogenized by shearing through a 23-gauge needle. Twenty-five microliters of total cell lysate was saved, and the remaining sample was centrifuged at 3000 × g to sediment the nuclei. The supernatant was then spun at 20,800 × g for 30 min at 4°C to separate the sedimented membrane fraction from the soluble cytosolic fraction. The nuclear pellet was washed two times with fractionation buffer, resuspended in 250 μl, and loaded onto a 250-μl cushion formed by 1 M sucrose in fractionation buffer, and centrifuged at 2250 × g for 10 min at 4°C. The pellet was then extracted into 250 μl of fractionation buffer containing 1% NP-40. Total, cytosolic, and nuclear fractions were resolved by SDS-PAGE and analyzed by Western blotting.
Serum Response Element reporter assay
CHO cells were transfected with 1.0 μg of wild-type pCDNA3-HA-PEA-15, 0.33 μg of pSRE-Luc (BD Biosciences Clontech), and 0.33 μg of pRL-TK (Promega, Madison, WI). Cells were grown in complete medium for 24 h and then shifted to serum-free media for 24 h. Cells were then stimulated by addition 10% FBS for 3 h, harvested, and resuspended in 250 μl of passive lysis buffer (supplied with dual luciferase assay kit; Promega). Twenty microliters of total lysate was placed in one well of a 96-well plate, and 100 μl of luciferase assay reagent (dual-luciferase reporter assay kit; Promega) was added. Firefly luciferase activity was assayed by measuring light emission using a 96-well plate Lucy 2 Luminometer (Anthos Labtec Instruments, Salzburg, Austria) with 1-s integration. One hundred microliters Stop-and-glo reagent (dual-luciferase reporter assay kit; Promega) was added to stop firefly luciferase activity and assay Renilla luciferase activity to correct for transfection efficiency. All conditions were assayed in triplicate in each experiment, and each experiment was performed in triplicate.
Cell Proliferation
CHO cells were transfected with 1.0 μg of pCDNA3-HA-PEA-15 and 0.33 μg of pEGFP-C1 (GFP) vector as a transfection reporter. Cells were grown in complete medium for 24 h and then shifted to serum-free media for 24 h. Bromodeoxyuridine (BrdU, 10 μM; BD Biosciences PharMingen, San Diego, CA) was added and 15 min later 10% FBS was added. Cells were harvested after 45 min, resuspended in 50 μl of ice-cold PBS, and fixed by addition of 1 ml of 1% formaldehyde in PBS for 2 min at room temperature. After washing one time with PBS, the cells were resuspended in 100 μl of PBS and incubated with 1 ml of cold 70% ethanol for 5 min on ice. The cells were then washed two times with PBS, resuspended in 50 μl of PBS, and incubated with 20 U of DNase I (Roche Diagnostics) for 30 min. To detect BrdU, 1 μl of anti-BrdU antibody (BD Biosciences PharMingen) was added for 30 min at room temperature. After one wash with PBS, 1 μl of phycoerythrin-conjugated F(ab′)2 goat anti-mouse IgG (BD Biosciences PharMingen) was added for 30 min at room temperature. Cells were analyzed by flow cytometry for BrdU incorporation by using green fluorescent protein (GFP) expression as a marker for transfection. The percentage of change in BrdU incorporation was calculated by comparing the geometric mean fluorescence intensity of the BrdU staining of each sample to serum-starved vector-transfected cells. Whole cell lysates were analyzed for transfected protein expression by Western blotting.
Flow Cytometry
Analytical two-color flow cytometry was carried out as described previously (Chou et al., 2003). Briefly, CHO cells were transfected with 0.1 μg of pEGFP as transfection marker and a combination of 1.5 μg of pCDNA1-Raf-CAAX with 1.5 μg of various pCDNA3-HA-PEA-15 constructs. Transient transfections were carried out using Lipofectamine and Plus reagents using the manufacturer's protocol. After 24 h in normal growth media, cells were harvested and analyzed for transfection efficiency (GFP) and integrin binding to 3Fn-(9-11). Integrin activation was quantified as an activation index (AI) as defined in Chou et al. (2003). The percentage of reversal of suppression was calculated as described previously (Chou et al., 2003).
RESULTS AND DISCUSSION
PEA-15 Phosphorylation Blocks ERK1/2 Binding In Vitro
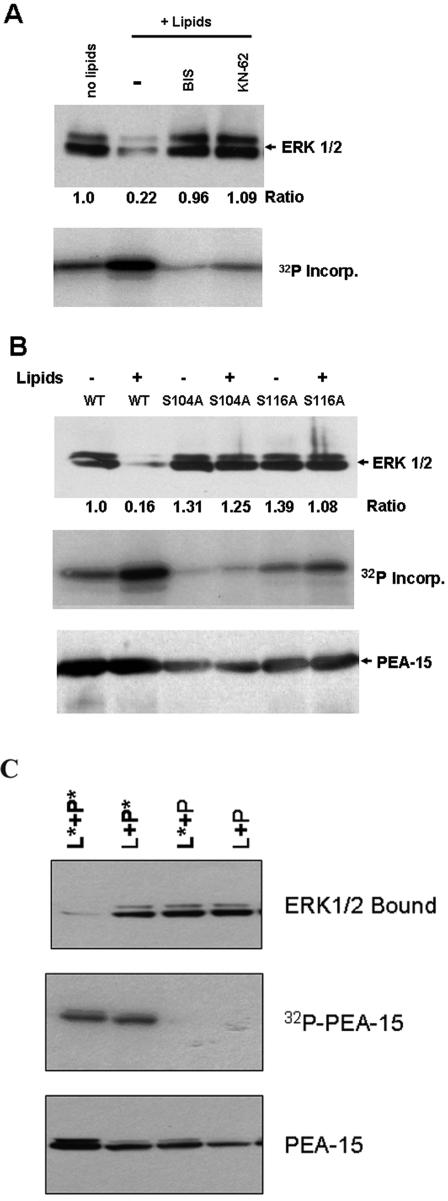
PEA-15 binding to ERK1/2 is in part mediated by the tail of PEA-15, a region that contains both major phosphorylation sites Ser104 and Ser116 (Araujo et al., 1993; Kubes et al., 1998; Trencia et al., 2003). We therefore phosphorylated GST-PEA-15 in vitro and examined effects on ERK binding. When GST-PEA-15 was incubated with cell lysate in the presence of activating lipids (PMA and phosphatidyl serine), it was phosphorylated and lost the capacity to bind to ERK1 and ERK2 (Figure 1A). Inhibitors of either PKC or CamKII (Bis or KN-62, respectively) reduced PEA-15 phosphorylation by 75 and 60%, and restored the ability of PEA-15 to bind ERK1/2. This restoration of ERK1/2 binding with only partial loss in phosphorylation suggests that phosphorylation at both sites is required to block the interaction of ERK1/2 with PEA-15.
Figure 1.
Phosphorylation of PEA-15 blocks binding to ERK1/2. Recombinant GST-PEA-15 was immobilized on glutathione-Sepharose beads and incubated with lysates of NIH 3T3 cells in kinase active buffer containing 10 μCi of [γ-32P]ATP in the presence or absence of 50 mg/ml phosphatidyl serine and 100 nM phorbol myristate acetate (lipids) at 37°C for 2 h. The beads were washed, and bound proteins were eluted in sample buffer, separated by SDS-PAGE, and immunoblotted with anti-ERK1/2 to assess ERK binding and with anti-PEA-15 to assess the retention of PEA-15 on the beads. Incorporation of 32P into PEA-15 was detected by autoradiography of the dried blots. The ERK1/2 blots were quantified by densitometric scanning, and the ERK bound was divided by that bound to wild-type PEA-15 to obtain the indicated ratios. (A) Phosphorylation of wild-type PEA-15 in the presence of lipid activators blocked the interaction of ERK1/2 with PEA-15. Treatment with PKC (1 μM Bis) or CamKII (10 μM KN-62) inhibitors blocked phosphorylation of wild-type PEA-15 and restored the interaction of ERK1/2 with PEA-15. (B) Mutation of PEA-15 Ser104 or Ser116 phosphorylation sites to Ala blocked phosphorylation and preserved the ability of PEA-15 to bind ERK1/2. (C) Phosphorylation of PEA-15 is necessary but not sufficient to inhibit ERK1/2 binding. Recombinant GST-PEA-15 was incubated with lysates of NIH 3T3 cells in the presence of absence of lipid activators, as described in A. Lysate and PEA-15 were then separated by centrifugation and subsequently recombined for binding assays. When lipid activator-treated lysate was added to modified PEA-15 (L*+P*) ERK1/2 binding was strongly inhibited. In contrast, combinations in which either the lysate or the PEA-15 had been incubated in the absence of activators (L*+P, L+P*) bound ERK1/2 to the same extent as combinations in which no lipid activator was added (L+P).
To confirm that PEA-15 phosphorylation was responsible for the inhibition of ERK1/2 binding, we individually changed each of the phosphorylated serine residues to alanine. Mutation of either Ser104 or Ser116 reduced in vitro phosphorylation and prevented the loss of ERK1/2 binding (Figure 1B). This result is consistent with previous studies that suggested cooperative phosphorylation at the two sites (Kubes et al., 1998). These experiments demonstrate that PEA-15 phosphorylation inhibits ERK1/2 binding and suggest that phosphorylation at both Ser104 and Ser116 is required for this inhibition.
PEA-15 phosphorylation in vitro with purified PKC in combination with CamKII failed to inhibit ERK1/2 binding (our unpublished data), suggesting that PEA-15 phosphorylation, although necessary, was not sufficient to inhibit ERK1/2 binding. To address this possibility, we incubated GST-PEA-15 with cell lysate in the presence of activating lipids (PMA and phosphatidyl serine); the phosphorylated GST-PEA-15 and the lysate were then separated by centrifugation. Recombination of the modified lysate with the phosphorylated PEA-15 resulted in little binding of ERK1/2 (Figure 1C). In sharp contrast, ERK1/2 bound strongly when unmodified cell lysate was added to the phosphorylated PEA-15. Furthermore, the modified lysate still contained ERK1/2 capable of binding PEA-15, as evidenced by robust binding when modified lysate was added to unmodified GST-PEA-15 (Figure 1C). Autoradiography of the modified lysate revealed the presence of several newly phosphorylated species; however, there was no increase in phospho-ERK1/2 as judged by immunoblotting (our unpublished data). Thus, phosphorylation of PEA-15 is required but not sufficient for disruption of the interaction of PEA-15 with ERK1/2.
Phosphomimetic Mutations in PEA-15 Disrupt Its Interaction with ERK1/2 In Vivo
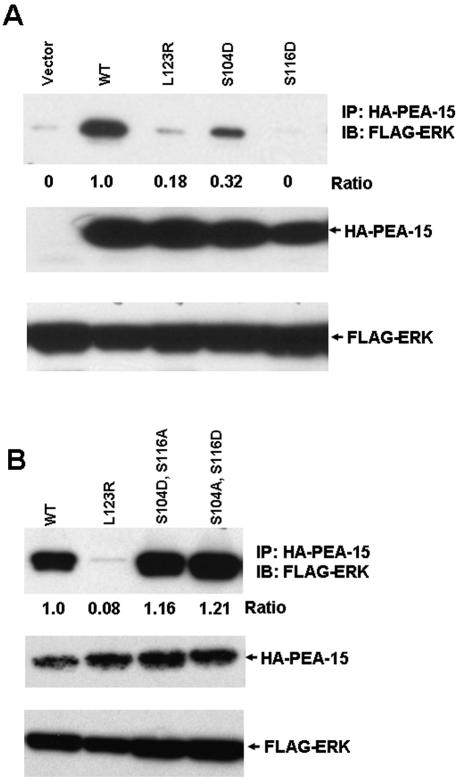
The foregoing experiments established that PEA-15 phosphorylation can disrupt its interaction with ERK1/2 in vitro. To assess the effect of PEA-15 phosphorylation on ERK2 interactions and functions in vivo, we introduced phosphomimetic Asp mutations into Ser104 and Ser116. These HA-tagged mutants were expressed in CHO cells, and their ability to coimmunoprecipitate with FLAG-tagged ERK2 was assayed. Wild-type PEA-15 coimmunoprecipitated with FLAG-ERK2; however, PEA-15 (S104D) or PEA-15 (S116D) exhibited markedly reduced coimmunoprecipitation (Figure 2A). Quantification of the data revealed that there was no detectable coprecipitation of the S116D mutant and nearly a 70% reduction of coprecipitation with the S104D mutant. For comparison, there was 85% reduction of coprecipitation with PEA-15 (L123R), a mutant known to block ERK1/2 binding (Hill et al., 2002). In each of these mutants, one of the phosphorylatable Ser residues remained, suggesting that the other Ser residue might have become phosphorylated in vivo, leading to the inhibition of ERK binding. To directly test this idea, we created double mutants [PEA-15 (S104D, S116A) and PEA-15 (S104A, S116D)]. Each of these mutants coprecipitated with FLAG-ERK to a slightly greater extent than wild-type PEA-15 (116 and 121% of wild-type, respectively) (Figure 2B). Combined with the previous experiments, these results establish that phosphorylation of PEA-15 at Ser104 and Ser116 lead to disruption of its interaction with ERK1 and ERK2 and that preventing phosphorylation at either site is sufficient to maintain ERK1/2 association.
Figure 2.
Phosphomimetic PEA-15 mutations block its association with ERK2 in cells. CHO cells were cotransfected with cDNAs encoding FLAG-tagged ERK2, and HA-tagged wild-type PEA-15 or the indicated PEA-15 mutants. Lysates of these cells were immunoprecipitated with anti-HA antibody, and the immunoprecipitates were fractionated by SDS-PAGE and immunoblotted with anti-FLAG antibody to detect coprecipitated ERK2. These immunoprecipitates also were blotted with anti-PEA-15 to verify consistent immunoprecipitation. The whole cell lysates were blotted with anti-FLAG to verify expression of the recombinant ERK2. The blots were quantified by densitometric scanning and the quantity of coprecipitated ERK2 was divided by ERK2 precipitated with wild-type PEA-15 to obtain the indicated ratios. (A) Phosphomimetic mutations (S104D and S116D) in PEA-15 reduce interaction with ERK2. Note that the S116D mutant blocked coprecipitation of ERK2 with PEA-15 to a greater extent than the L123R mutant, previously reported to disrupt the PEA-15-ERK interaction (Hill et al., 2002). The S104D mutant also inhibited the PEA-15–ERK2 interaction but to a lesser extent than either of the other two mutants. (B) Phosphorylation of both Ser104 and Ser116 is required to block interaction with ERK1/2. The free Ser on each Asp mutant was mutated to a nonphosphorylatable Ala, creating double mutants PEA-15(S104A, S116D) and PEA-15(S104D, S116A). Each of these double mutants restored the interaction of PEA-15 with ERK1/2.
Phosphorylation of PEA-15 Prevents Inhibition of Cell Proliferation
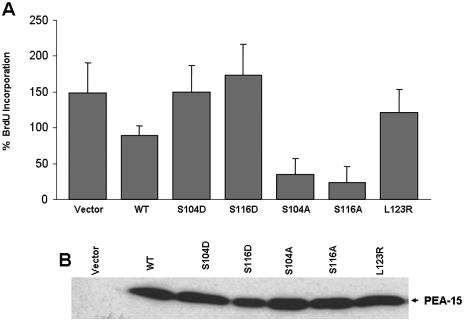
We previously reported that PEA-15 expression inhibits cell proliferation (Formstecher et al., 2001). Because PEA-15 is widely expressed, we hypothesized that PEA-15 phosphorylation might alter its capacity to inhibit cell proliferation. To assess the effect of PEA-15 phosphorylation on proliferation, cells were transfected with PEA-15 or PEA-15 (S104D) or (S116D) mutants, and the incorporation of BrdU was measured to assess DNA synthesis. Expression of wild-type PEA-15 led to a 50% reduction in BrdU incorporation compared with vector-transfected cells (Figure 3A). This effect was completely reversed by the S104D or S116D mutation, indicating that phosphorylation of PEA-15 reverses its inhibition of cell proliferation. PEA-15 wild-type and mutants were expressed at similar levels (Figure 3B). Furthermore, transfection of cells with nonphosphorylatable PEA-15 (S104A or S116A) mutants led to an even more profound (75–85%) suppression of DNA synthesis (Figure 3A). This latter result suggests that endogenous kinases phosphorylated transfected PEA-15, thereby attenuating its effect on proliferation; however, the fact that wild-type PEA-15 suppressed proliferation indicates that under these conditions of serum stimulation, PEA-15 is not fully phosphorylated. Thus, the capacity of PEA-15 to inhibit cell proliferation is regulated by its phosphorylation.
Figure 3.
PEA-15 phosphorylation prevents its inhibition of cell proliferation. (A) Nonphosphorylatable PEA-15 mutants suppress cell proliferation more than wild type; phosphomimetic mutants fail to suppress. CHO cells were transfected with cDNAs encoding HA-PEA-15 or the indicated PEA-15 mutants. After 24 h in growth media, the cells were placed in 0.5% serum for an additional 24 h. BrdU (10 μM) was added to the medium, followed by 10% serum to stimulate cell proliferation. After 1 h, the cells were harvested, and BrdU incorporation was assayed by staining with anti-BrdU and quantified using flow cytometry. Data were acquired as geometric mean fluorescence intensity of BrdU staining and are expressed as percentage of BrdU incorporation, where 100% is basal BrdU incorporation of vector-transfected serum-starved cells. Transfection with PEA-15 blocked serum stimulation of proliferation. In contrast, both phosphomimetic mutants (S104D, S116D) did not block cell proliferation. Both nonphosphorylatable mutants (S104A, S116A) reduced proliferation less than that seen in the absence of serum. (B) Expression of transfected PEA-15 variants. Lysates of the cells described in A were fractionated by SDS-PAGE and immunoblotted with PEA-15 antibody to detect transfected PEA-15.
PEA-15 Phosphorylation Reverses the Biological Consequences of Its Interaction with ERK1/2
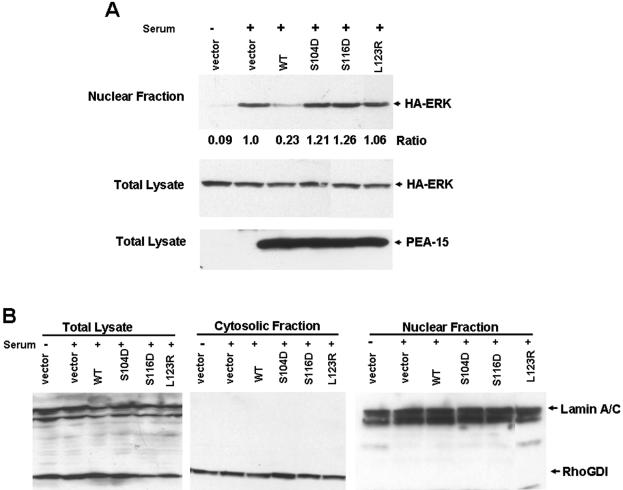
The foregoing experiments established that PEA-15 phosphorylation blocks its capacity to interact with ERK1/2 and to inhibit cell proliferation. Cell proliferation, in part, depends on ERK1/2 entering the nucleus where they can phosphorylate transcription such as Elk-1, c-myc, c-fos, and c-jun, resulting in cell cycle progression (Whitmarsh and Davis, 1996). PEA-15 expression blocks nuclear accumulation of activated ERK1/2, promoting its retention in the cytosol; this effect is dependent on its ability to bind ERK1/2 (Formstecher et al., 2001; Whitehurst et al., 2004). We therefore examined the effect of the phosphomimetic mutations on PEA-15's capacity to inhibit ERK2 nuclear translocation and ERK-dependent transcription. We transfected cells with PEA-15 or phosphomimetic mutants and examined serum-stimulated ERK2 nuclear translocation. Serum stimulation induced nuclear accumulation of ERK2, which was almost completely blocked in cells transfected with PEA-15 (Figure 4A). In contrast, PEA-15 (S104D) or (S116D) mutants did not block nuclear accumulation of ERK (Figure 4A). In each case, similar levels of HA-ERK2 and HA-PEA-15 expression were observed (Figure 4A, bottom). Nuclear fractions were free from the cytosolic marker Rho GDI, and cytosolic fractions were free from nuclear markers (lamins A/C) (Figure 4B). Thus, PEA-15 phosphorylation inhibits its capacity to block nuclear accumulation of ERK2.
Figure 4.
PEA-15 phosphorylation prevents inhibition of nuclear translocation of ERK1/2. (A) Phosphomimetic mutations (S104D, and S116D) in PEA-15 do not block nuclear import of ERK2. CHO cells were cotransfected with HA tagged PEA-15 wild-type or mutants and HA-ERK2, grown in 10% serum for 24 h, followed by serum-free media for 24 h. The cells were then stimulated with 10% serum for 3 h, disrupted by osmotic shock, and fractionated into nuclear and cytoplasmic fractions. Each fraction or the total cell lysate was separated by SDS-PAGE and immunoblotted with anti-HA to detect ERK2 and PEA-15. Wild-type PEA-15 blocked nuclear accumulation of ERK2, whereas phosphomimetic PEA-15 (S104D and S116D) did not (top). Whole cell lysates also were assayed for HA-ERK and PEA-15 expression (middle and bottom). (B) Validation of subcellular fractionation. Whole cell lysates or nuclear and cytosolic fraction from A were separated by SDS-PAGE and immunoblotted with antibodies reactive for the cytosolic marker RhoGDI (bottom left) and the nuclear marker lamin A/C (bottom right).
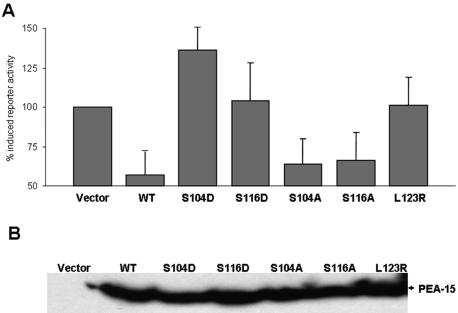
After translocation to the nucleus, activation of the c-fos transcription factor by ERK1/2 is required for transcription of genes that contain the c-fos serum response element (SRE) (Gille et al., 1995). Using an SRE-luciferase reporter assay, we compared the effects of PEA-15 and phosphomimetic mutants on ERK1/2-dependent transcription. PEA-15 dramatically reduced serum-induced SRE reporter activity (Figure 5A). This is consistent with other studies that report a decrease in Elk-1 reporter activity induced by PEA-15 (Formstecher et al., 2001). In contrast, PEA-15 (S104D) and (S116D) failed to block SRE activity (Figure 5A). As before, the double mutants (S014D, S116A) and (S104A, S116D) maintained the capacity to block SRE reporter activity (Figure 5A). PEA-15 wild-type and mutants were expressed at similar levels (Figure 5B). Thus, as with blockade of ERK1/2 binding, phosphorylation of both Ser residues is required to abolish the capacity of PEA-15 to block ERK2 nuclear translocation and ERK1/2-dependent transcription.
Figure 5.
PEA-15 phosphomimetic mutants do not block ERK-dependent transcription. (A) Phosphomimetic mutations (S104D and S116D) in PEA-15 do not block SRE luciferase reporter activity. CHO cells were cotransfected with PEA-15 or the indicated mutants, a SRE-dependent luciferase reporter construct (pSRE-Luc), and a constitutive Renilla reporter (pRL-TK). Cells were grown in 10% serum for 24 h, serum-free media for 24 h, and then stimulated with 10% serum for 3 h. ERK nuclear activity was assayed as a function of SRE luciferase reporter activity. SRE reporter activity was corrected for transfection efficiency by the cotransfected Renilla reporter activity. Results are reported as the percentage of increase in SRE reporter activity compared with vector-transfected serum-starved cells. Wild-type PEA-15 and both nonphosphorylatable mutants (S0104A, S116A) markedly suppressed SRE-dependent transcription. In contrast, the phosphomimetic PEA-15 mutants (S104D, S116D) did not inhibit SRE reporter activity. Data depicted are the mean ± SE of four determinations. (B) Expression of transfected PEA-15. Lysates of cells described in A were fractionated by SDS-PAGE and immunoblotted with anti-PEA-15 antibody to detect transfected PEA-15.
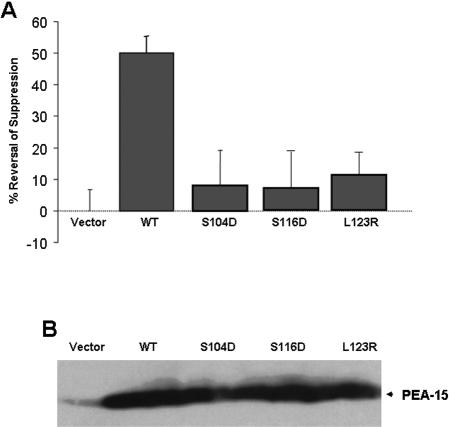
In addition to its transcriptional activities, ERK1 and ERK2 have a variety of transcription-independent effects on cells (Cobb et al., 1994; Johnson and Lapadat, 2002; Reddy et al., 2003; Roux and Blenis, 2004). Among these are the capacity of activated ERK1/2 to markedly reduce the affinity of integrin adhesion receptors (Hughes et al., 1997). PEA-15 blocks ERK-dependent suppression of integrin activation by binding to ERK1/2 (Chou et al., 2003). Therefore, we sought to investigate the effect of phosphomimetic mutations of PEA-15 on integrin suppression. CHO cells were transfected with Raf-CAAX to activate ERK1/2 and suppress integrin activation and were cotransfected with PEA-15 or PEA-15 mutants. After 24 h, the cells were detached and the binding of soluble cell binding domain of fibronectin [3Fn(9-11)] was measured to assess the activation of integrin α5β1. Transfection with Raf-CAAX markedly suppressed the binding of 3Fn(9-11), and this suppression was reversed by transfection with wild-type PEA-15. In sharp contrast, the phosphomimetic mutations in PEA-15 (S104D or S116D) abolished this effect (Figure 6A). PEA-15 wild-type and mutants were expressed at similar levels (Figure 6B). Thus, PEA-15 phosphorylation also prevents its effects on the nontranscriptional activities of ERK 1/2.
Figure 6.
Phosphomimetic PEA-15 mutants do not reverse integrin suppression. (A) Effect of PEA-15 wild-type and mutants on Raf-CAAX–induced integrin suppression. CHO cells were cotransfected with cDNA encoding a constitutively active Raf kinase (Raf-CAAX) in combination with PEA-15 or the indicated mutants. After 24 h, the cells were detached, and the binding of soluble cell binding domain of Fn [3Fn(9-11)] binding was measured to assess the affinity state of integrin α5β1. This was quantified as an AI, where AI = 100 * (F –Fo)/(Fm –Fo). F represents the geometric mean fluorescence (GMF) of 3Fn-(9-11) binding alone, Fo is the GMF of 3Fn-(9-11) binding in the presence of 10 mM EDTA, and Fm is the GMF of 3Fn-(9-11) binding in the presence of 9EG7. The percentage of suppression was calculated as 100 * (AIM –AIT)/AIM, where AIM is the activation index of the vector transfected cells, and AIT is the activation index in the presence of a Raf-CAAX or Raf-CAXX + PEA-15 WT or mutants. The percentage of reversal of suppression was calculated as 100 * (AI –AIR)/AI, in which AI is the activation index of the control cells, and AIR is the activation index in the presence of a transfected PEA-15 WT or mutants. Wild-type PEA-15 reversed Raf-CAAX–induced integrin suppression >50%, whereas PEA-15 phosphomimetic mutants (S104D, S116D) failed to do so at all. (B) Expression of transfected PEA-15 variants. Lysates of cells described in A were fractionated by SDS-PAGE and immunoblotted with PEA-15 antibody to detect transfected PEA-15.
PEA-15 Is Phosphorylated In Vivo
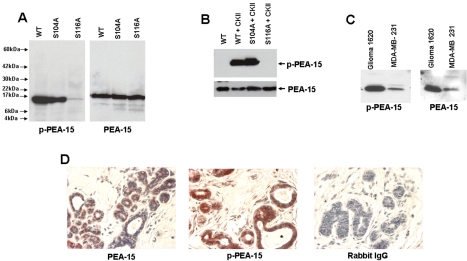
PEA-15 is widely expressed in a variety of tissues and is phosphorylated in lysates of astrocytes (Araujo et al., 1993). We used an antibody directed against a peptide derived from the human PEA-15 sequence corresponding to amino acids 112–123 which Ser116 was phosphorylated to examine in vivo PEA-15 phosphorylation (p-PEA-15). This antibody was PEA-15-specific; it recognized a single band with mobility of authentic PEA-15 in PEA-15-transfected CHO cells. In contrast the anti-p-PEA-15 failed to react with the PEA-15 (S116A) (Figure 7A), but it still reacted with the PEA-15 (S105A and S105D) mutants (our unpublished data). The anti-p-PEA-15 failed to react with purified recombinant PEA-15; however, phosphorylation with purified CamKII resulted in strong reactivity with this antibody. Reactivity was not observed when PEA-15 (S116A) was treated with CamKII under identical conditions (Figure 7B). Thus, the anti-p-PEA-15 is both PEA-15 specific and phosphorylation specific.
Figure 7.
PEA-15 is phosphorylated in cells and tissues. (A) Phospho-specific anti-PEA-15 (anti-p-PEA-15) is PEA-15 specific. CHO cells were transfected with cDNAs encoding PEA-15 or the indicated mutants. After 48 h, the cells were lysed, fractionated by SDS-PAGE, and analyzed by immunoblotting with either anti-phospho-PEA-15 (p-PEA-15) or an anti-PEA-15 antibody (PEA-15) that reacts with both phosphorylated and unphosphorylated PEA-15. Note that both antibodies reacted with a polypeptide of molecular weight of ∼15 k and no other cellular polypeptides. This polypeptide was absent from immunoblots of untransfected CHO cells (our unpublished data). Mutation of Ser116 to Ala abolished reactivity of the p-PEA-15 antibody. (B) Anti-p-PEA-15 is phospho-specific. Purified recombinant PEA-15 or the indicated mutants were phosphorylated in vitro with purified CamKII, fractionated by SDS-PAGE, and immunoblotted with anti-p-PEA-15 or anti-PEA-15. Anti-p-PEA-15 reacted with phosphorylated but not unphosphorylated PEA-15. Reactivity was abolished by mutating the CamKII phosphorylation site, Ser116, to Ala. (C) Phosphorylation of PEA-15 in cultured cells. The indicated tumor cell lines were lysed in sample buffer, fractionated by SDS-PAGE, and immunoblotted with either anti-PEA-15 or anti-p-PEA-15. Note that PEA-15 is expressed and phosphorylated in both cell lines. (D) PEA-15 is phosphorylated in situ in normal breast epithelium. Sections of normal mammary tissue were immuno peroxidase stained for PEA-15 (left) or phospho-PEA-15 (middle) and counterstained with Mayer's hematoxylin. Preimmune rabbit IgG was used as a staining control (right).
Having established the specificity of the antibody, we examined the phosphorylation status of PEA-15 in selected tumor cell lines. The 1620 glioma cell line and MB-MDA-231 breast cancer cell lines both expressed PEA-15 that was reactive with anti-p-PEA-15 (Figure 7C). We next sought to assess the potential presence and phosphorylation state of PEA-15 in normal epithelial cells. Previous studies have shown that PEA-15 protein is expressed in normal brain and mammary tissue (Bera et al., 1994; Danziger et al., 1995; Estelles et al., 1996; Hwang et al., 1997; Kubes et al., 1998; Ramos et al., 2000; Tsukamoto et al., 2000; Sharif et al., 2004). Immunohistochemical staining of normal breast tissue revealed the presence of PEA-15 in mammary epithelium and to a much lesser extent in the mammary stroma (Figure 7D). These mammary epithelial cells were strongly reactive with anti-p-PEA-15, establishing that PEA-15 is phosphorylated in situ in normal mammary epithelium. Thus, PEA-15 is phosphorylated in vivo in cell lines and tissues.
Our studies establish that PEA-15 phosphorylation blocks its binding to ERK1/2. The ERK2 binding surface of PEA-15 includes part of the N-terminal DED and of the C-terminal “tail” (Hill et al., 2002). Both phosphorylation sites are within the C-terminal tail, suggesting that phosphorylation might sterically hinder ERK1/2 binding. The unphosphorylated tail of PEA-15 is unstructured and phosphorylation may alter its conformation to block ERK1/2 binding (Hill et al., 2002). Interestingly, phosphorylation of PEA-15 stimulates its recruitment to the DISC, an event presumably mediated by the interaction of its DED with DISC components such as FADD (Gille et al., 1995; Camps et al., 1998; Dang et al., 1998). Because ERK1/2 also interacts with the DED of PEA-15, our results raise the possibility that ERK1/2 binding blocks FADD binding to PEA-15. PEA-15 phosphorylation, by displacing ERK1/2, may make the DED available for binding to FADD, enabling PEA-15 recruitment to the DISC and thus blocking apoptosis (Menice et al., 1997; Formstecher et al., 2001; Robinson et al., 2002).
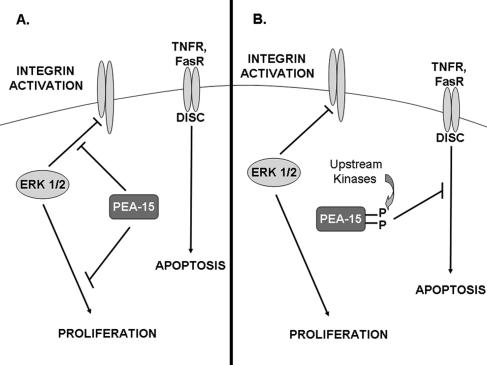
PEA-15 is a downstream effector of multiple kinases including PKC, Akt, and CamKII. As reported here, PEA-15 phosphorylation can regulate the proliferation of the cells that express it. When PEA-15 is unphosphorylated, its expression leads to Ras activation and therefore ERK1/2 activation (Ramos et al., 2000). As shown here, only unphosphorylated PEA-15 binds ERK1/2 and blocks its nuclear translocation and therefore cell proliferation. We found that when PEA-15 is phosphorylated, it loses the capacity to bind ERK1/2 and block proliferation; simultaneously, it gains the capacity to enter the DISC and to inhibit apoptosis (Kitsberg et al., 1999; Hao et al., 2001; Trencia et al., 2003). Protein phosphorylation can be dynamic and reversible; hence, PEA-15 phosphorylation may serve to regulate the shuttling of PEA-15 among its many binding partners (Figure 8), thereby controlling cell proliferation and survival.
Figure 8.
Effects of PEA-15 phosphorylation on cellular functions. (A) Unphosphorylated PEA-15 binds activated ERK1/2 and blocks cell proliferation and reverses integrin suppression. Unphosphorylated PEA-15 binds activated ERK1/2 and prevents their nuclear accumulation, thereby preventing cell proliferation. Additionally, PEA-15 reverses activated ERK1/2-mediated integrin suppression. (B) Phosphorylated PEA-15 does not block cell proliferation or reverse integrin suppression, but it is recruited to the DISC to block apoptosis. Kinases such as PKC, CamKII, or Akt can phosphorylate PEA-15. Phosphorylated PEA-15 does not bind activated ERK1/2 and therefore has no effect on cell proliferation or integrin activation. In contrast, phosphorylated PEA-15 is recruited to the DISC, where it can block TNF receptor- or Fas receptor-mediated apoptosis.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04–11–1007) on May 25, 2005.
Abbreviations used: DED, death effector domain; DISC, death inducing signaling complex; ERK1/2, extracellular signal-regulated kinase 1 and 2; MAP, mitogen-activated protein kinase; PEA-15, phosphoprotein enriched in astrocytes.
References
- Araujo, H., Danziger, N., Cordier, J., Glowinski, J., and Chneiweiss, H. (1993). Characterization of PEA-15, a major substrate for PKC in astrocytes. J. Biol. Chem. 268, 5911–5920. [PubMed] [Google Scholar]
- Bera, T. K., Guzman, R. C., Miyamoto, S., Panda, D. K., Sasaki, M., Hanyu, K., Enami, J., and Nandi, S. (1994). Identification of a mammary transforming gene (MAT1) associated with mouse mammary carcinogenesis. Proc. Natl. Acad. Sci. USA 91, 9789–9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps, M., Nichols, A., Gillieron, C., Antonsson, B., Muda, M., Chabert, C., Boschert, U., and Arkinstall, S. (1998). Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280, 1262–1265. [DOI] [PubMed] [Google Scholar]
- Chou, F. L., Hill, J. M., Hsieh, J. C., Pouyssegur, J., Brunet, A., Glading, A., Uberall, F., Ramos, J. W., Werner, M. H., and Ginsberg, M. H. (2003). PEA-15 binding to ERK1/2 MAP kinases is required for its modulation of integrin activation. J. Biol. Chem. [DOI] [PubMed]
- Cobb, M. H., Hepler, J. E., Cheng, M., and Robbins, D. (1994). The mitogen-activated protein kinases, ERK1 and ERK2. Semin. Cancer Biol. 5, 261–268. [PubMed] [Google Scholar]
- Condorelli, G., Vigliotta, G., Cafieri, A., Trencia, A., Andalo, P., Oriente, F., Miele, C., Caruso, M., Formisano, P., and Beguinot, F. (1999). PED/PEA-15, an anti-apoptotic molecule that regulates FAS/TNFR1-induced apoptosis. Oncogene 18, 4409–4415. [DOI] [PubMed] [Google Scholar]
- Condorelli, G., et al. (1998). PED/PEA-15 gene controls glucose transport and is overexpressed in type 2 diabetes mellitus. EMBO J 17, 3858–3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang, A., Frost, J. A., and Cobb, M. H. (1998). The MEK1 proline-rich insert is required for efficient activation of the mitogen-activated protein kinases ERK1 and ERK2 in mammalian cells. J. Biol. Chem. 273, 19909–19913. [DOI] [PubMed] [Google Scholar]
- Danziger, N., Yokoyama, M., Jay, T., Cordier, J., Glowinski, J., and Chneiweiss, H. (1995). Cellular expression, developmental regulation, and phylogenic conservation of PEA-15, the astrocytic major phosphoprotein and PKC substrate. J. Neurochem. 64, 1016–1025. [DOI] [PubMed] [Google Scholar]
- Dong, G., Loukinova, E., Chen, Z., Gangi, L., Chanturita, T. I., Liu, E. T., and Van Waes, C. (2001). Molecular profiling of transformed and metastatic murine squamous carcinoma cells by differential display and cDNA microarray reveals altered expression of multiple genes related to growth, apoptosis, angiogenesis, and the NF-κB signal pathway. Cancer Res. 61, 4797–4808. [PubMed] [Google Scholar]
- Embury, J., Klein, D., Pileggi, A., Ribeiro, M., Jayaraman, S., Molano, R. D., Fraker, C., Kenyon, N., Ricordi, C., Inverardi, L., and Pastori, R. L. (2001). Proteins linked to a protein transduction domain efficiently transduce pancreatic islets. Diabetes 50, 1706–1713. [DOI] [PubMed] [Google Scholar]
- Estelles, A., Charlton, C. A., and Blau, H. M. (1999). The phosphoprotein protein PEA-15 inhibits Fas- but increases TNF-R1-mediated caspase-8 activity and apoptosis. Dev. Biol. 216, 16–28. [DOI] [PubMed] [Google Scholar]
- Estelles, A., Yokoyama, M., Nothias, F., Vincent, J. D., Glowinski, J., Vernier, P., and Chneiweiss, H. (1996). The major astrocytic phosphoprotein PEA-15 is encoded by two mRNAs conserved on their full length in mouse and human. J. Biol. Chem. 271, 14800–14806. [DOI] [PubMed] [Google Scholar]
- Formstecher, E., et al. (2001). PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev. Cell 1, 239–250. [DOI] [PubMed] [Google Scholar]
- Gaumont-Leclerc, M. F., Mukhopadhyay, U. K., Goumard, S., and Ferbeyre, G. (2004). PEA-15 is inhibited by adenovirus E1A and plays a role in ERK nuclear export and Ras-induced senescence. J. Biol. Chem. 279, 46802–46809. [DOI] [PubMed] [Google Scholar]
- Gille, H., Kortenjann, M., Thomae, O., Moomaw, C., Slaughter, C., Cobb, M. H., and Shaw, P. E. (1995). ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 14, 951–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glienke, J., Schmitt, A. O., Pilarsky, C., Hinzmann, B., Weiss, B., Rosenthal, A., and Thierauch, K. H. (2000). Differential gene expression by endothelial cells in distinct angiogenic states. Eur. J. Biochem. 267, 2820–2830. [DOI] [PubMed] [Google Scholar]
- Hao, C., Beguinot, F., Condorelli, G., Trencia, A., Van Meir, E. G., Yong, V. W., Parney, I. F., Roa, W. H., and Petruk, K. C. (2001). Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in human malignant glioma cells. Cancer Res. 61, 1162–1170. [PubMed] [Google Scholar]
- Hill, J. M., Vaidyanathan, H., Ramos, J. W., Ginsberg, M. H., and Werner, M. H. (2002). Recognition of ERK MAP kinase by PEA-15 reveals a common docking site within the death domain and death effector domain. EMBO J. 21, 6494–6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, P. E., Renshaw, M. W., Pfaff, M., Forsyth, J., Keivens, V. M., Schwartz, M. A., and Ginsberg, M. H. (1997). Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell 88, 521–530. [DOI] [PubMed] [Google Scholar]
- Hwang, S., Kuo, W. L., Cochran, J. F., Guzman, R. C., Tsukamoto, T., Bandyopadhyay, G., Myambo, K., and Collins, C. C. (1997). Assignment of HMAT1, the human homolog of the murine mammary transforming gene (MAT1) associated with tumorigenesis, to 1q21.1, a region frequently gained in human breast cancers. Genomics 42, 540–542. [DOI] [PubMed] [Google Scholar]
- Johnson, G. L., and Lapadat, R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912. [DOI] [PubMed] [Google Scholar]
- Kitsberg, D., Formstecher, E., Fauquet, M., Kubes, M., Cordier, J., Canton, B., Pan, G., Rolli, M., Glowinski, J., and Chneiweiss, H. (1999). Knock-out of the neural death effector domain protein PEA-15 demonstrates that its expression protects astrocytes from TNFα-induced apoptosis. J. Neurosci. 19, 8244–8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes, M., Cordier, J., Glowinski, J., Girault, J. A., and Chneiweiss, H. (1998). Endothelin induces a calcium-dependent phosphorylation of PEA-15 in intact astrocytes: identification of Ser104 and Ser116 phosphorylated, respectively, by PKC and calcium/calmodulin kinase II in vitro. J. Neurochem. 71, 1307–1314. [DOI] [PubMed] [Google Scholar]
- Menice, C. B., Hulvershorn, J., Adam, L. P., Wang, C. A., and Morgan, K. G. (1997). Calponin and mitogen-activated protein kinase signaling in differentiated vascular smooth muscle. J. Biol. Chem. 272, 25157–25161. [DOI] [PubMed] [Google Scholar]
- Ramos, J. W., Hughes, P. E., Renshaw, M. W., Schwartz, M. A., Formstecher, E., Chneiweiss, H., and Ginsberg, M. H. (2000). Death effector domain protein PEA-15 potentiates Ras activation of extracellular signal receptor-activated kinase by an adhesion-independent mechanism. Mol. Biol. Cell 11, 2863–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos, J. W., Kojima, T. K., Hughes, P. E., Fenczik, C. A., and Ginsberg, M. H. (1998). The death effector domain of PEA-15 is involved in its regulation of integrin activation. J. Biol. Chem. 273, 33897–33900. [DOI] [PubMed] [Google Scholar]
- Reddy, K. B., Nabha, S. M., and Atanaskova, N. (2003). Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 22, 395–403. [DOI] [PubMed] [Google Scholar]
- Renault, F., Formstecher, E., Callebaut, I., Junier, M. P., and Chneiweiss, H. (2003). The multifunctional protein PEA-15 is involved in the control of apoptosis and cell cycle in astrocytes. Biochem. Pharmacol. 66, 1581–1588. [DOI] [PubMed] [Google Scholar]
- Robinson, F. L., Whitehurst, A. W., Raman, M., and Cobb, M. H. (2002). Identification of novel point mutations in ERK2 that selectively disrupt binding to MEK1. J. Biol. Chem. 277, 14844–14852. [DOI] [PubMed] [Google Scholar]
- Roux, P. P., and Blenis, J. (2004). ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif, A., Canton, B., Junier, M. P., and Chneiweiss, H. (2003). PEA-15 modulates TNFα intracellular signaling in astrocytes. Ann. N.Y. Acad. Sci. 1010, 43–50. [DOI] [PubMed] [Google Scholar]
- Sharif, A., Renault, F., Beuvon, F., Castellanos, R., Canton, B., Barbeito, L., Junier, M. P., and Chneiweiss, H. (2004). The expression of PEA-15 (phosphoprotein enriched in astrocytes of 15 kDa) defines subpopulations of astrocytes and neurons throughout the adult mouse brain. Neuroscience 126, 263–275. [DOI] [PubMed] [Google Scholar]
- Tohgo, A., Pierce, K. L., Choy, E. W., Lefkowitz, R. J., and Luttrell, L. M. (2002). β-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J. Biol. Chem. 277, 9429–9436. [DOI] [PubMed] [Google Scholar]
- Trencia, A., et al. (2003). Protein kinase B/Akt binds and phosphorylates PED/PEA-15, stabilizing its antiapoptotic action. Mol. Cell Biol. 23, 4511–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto, T., et al. (2000). Expression of MAT1/PEA-15 mRNA isoforms during physiological and neoplastic changes in the mouse mammary gland. Cancer Lett. 149, 105–113. [DOI] [PubMed] [Google Scholar]
- Underhill, D. A., Vogan, K. J., Underhill, T. M., and Gros, P. (2001). Identification of a novel, alternatively spliced isoform and single nucleotide polymorphisms in the murine Pea-15 gene. Mamm. Genome 12, 172–174. [DOI] [PubMed] [Google Scholar]
- Vaidyanathan, H., and Ramos, J. W. (2003). RSK2 activity is regulated by its interaction with PEA-15. J. Biol. Chem. 278, 32367–32372. [DOI] [PubMed] [Google Scholar]
- Whitehurst, A. W., Robinson, F. L., Moore, M. S., and Cobb, M. H. (2004). The death effector domain protein PEA-15 prevents nuclear entry of ERK2 by inhibiting required interactions. J. Biol. Chem. 279, 12840–12847. [DOI] [PubMed] [Google Scholar]
- Whitmarsh, A. J., and Davis, R. J. (1996). Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74, 589–607. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Redina, O., Altshuller, Y. M., Yamazaki, M., Ramos, J., Chneiweiss, H., Kanaho, Y., and Frohman, M. A. (2000). Regulation of expression of phospholipase D1 and D2 by PEA-15, a novel protein that interacts with them. J. Biol. Chem. 275, 35224–35232. [DOI] [PubMed] [Google Scholar]
- Zvalova, D., Formstecher, E., Fauquet, M., Canton, B., and Chneiweiss, H. (2001). Keeping TNF-induced apoptosis under control in astrocytes: PEA-15 as a `double key' on caspase-dependent and MAP-kinase-dependent pathways. Prog. Brain Res. 132, 455–467. [DOI] [PubMed] [Google Scholar]