Abstract
Arg8-vasopressin (AVP) promotes the differentiation of myogenic cell lines and mouse primary satellite cells by mechanisms involving the transcriptional activation of myogenic bHLH regulatory factors and myocyte enhancer factor 2 (MEF2). We here report that AVP treatment of L6 cells results in the activation of calcineurin-dependent differentiation, increased expression of MEF2 and GATA2, and nuclear translocation of the calcineurin target NFATc1. Interaction of these three factors occurs at MEF2 sites of muscle specific genes. The different kinetics of AVP-dependent expression of early (myogenin) and late (MCK) muscle-specific genes correlate with different acetylation levels of histones at their MEF2 sites. The cooperative role of calcineurin and Ca2+/calmodulin-dependent kinase (CaMK) in AVP-dependent differentiation is demonstrated by the effect of inhibitors of the two pathways. We show here, for the first time, that AVP, a “novel” myogenesis promoting factor, activates both the calcineurin and the CaMK pathways, whose combined activation leads to the formation of multifactor complexes and is required for the full expression of the differentiated phenotype. Although MEF2–NFATc1 complexes appear to regulate the expression of an early muscle-specific gene product (myogenin), the activation of late muscle-specific gene expression (MCK) involves the formation of complexes including GATA2.
INTRODUCTION
An important role in the development of skeletal muscle is played by the expression and activity of myogenic regulatory factors (MRFs; Myf-5, MyoD, myogenin, and MRF4), muscle-restricted members of the large super family of basic helix-loop-helix (bHLH) transcription factors. Functionally, the MRFs act as heterodimers interacting with ubiquitous bHLH proteins, known as E-proteins, and in a combinatorial manner with other myogenic transcription factors such as MEF2 (Molkentin and Olson, 1996).
Also the histone acetyltransferases and histone deacetylases (HDACs) play important roles in transcriptional activation and silencing of muscle-specific genes, establishing a molecular paradigm of transcriptional regulation based on modulation of chromatin structure by reversible acetylation of histone tails (McKinsey et al., 2001, 2002b; Kouzarides, 2000). Recent work on HDACs indicated that some members of the class II HDACs (HDACs 4-5-7) are specifically expressed in skeletal muscle cells, interact with MEF2 and specifically inhibit skeletal myoblast differentiation by repressing MEF2 activity on muscle specific genes (Miska et al., 1999, 2001).
Physiologically, myogenic differentiation is regulated by hormones and growth factors (Olson, 1992). TGFβ and FGF inhibit differentiation (Olson et al., 1986; Clegg et al., 1987), whereas IGFs are potent inducers of myogenic proliferation, differentiation, and hypertrophy (Florini et al., 1991a, 1991b; Rosenthal and Cheng, 1995; Engert et al., 1996; Musaro and Rosenthal, 1999; Musaro et al., 1999).
We previously showed that the neurohypophyseal nonapeptide Arg8-vasopressin (AVP) and related peptides constitute a novel family of positive regulators of terminal differentiation of myogenic cell lines (L5 and L6) and primary satellite cells (Teti et al., 1993; Nervi et al., 1995; Minotti et al., 1998). Our findings and those of other laboratories (Mangiacapra et al., 1992; Smith et al., 1992; Breton et al., 2002) support the hypothesis that the AVP or a related peptide plays a physiological role in myogenesis. By interacting with V1 type receptors, AVP induces activation of phospholipases C and D, regulates cAMP levels, increases cytosolic Ca2+ concentration and up-regulates Myf-5 and myogenin expression (Nervi et al., 1995; Naro et al., 1997; Naro et al., 1999; Coletti et al., 2000). Furthermore we reported that the AVP-dependent myogenic differentiation involves the activation of the CaMK signaling pathway (Scicchitano et al., 2002). In particular, we demonstrated that AVP-mediated myogenic differentiation is dependent on nuclear export of the histone deacetylase 4 (HDAC4), leading to activation of MEF2 transcription factor at critical DNA binding sites present on target genes such as myogenin (Scicchitano et al., 2002). Noticeably, the pharmacological inhibition of the CaMK pathway did not completely inhibit the AVP-induced myogenic differentiation, suggesting that other pathways might be involved in the induction and/or maintenance of the differentiated muscle phenotype.
Recent studies (Musaro et al., 1999; Olson and Williams, 2000; Friday et al., 2003; Glass, 2003), have implicated an additional calcium-dependent signaling pathway in cardiac and skeletal muscle differentiation and hypertrophy. Calcineurin (CnA), a serine/threonine phosphatase activated by Ca2+/calmodulin, is responsible for transducing environmental signals that control gene expression in several biological processes (Crabtree, 1999) and has been recently implicated in hypertrophic cardiomyopathies (Braz et al., 2003), skeletal muscle differentiation and hypertrophy (Molkentin et al., 1998; Musaro et al., 1999; Olson and Williams, 2000), and specification of slow muscle phenotype (Wu et al., 2000; Serrano et al., 2001). At the molecular level calcineurin activates the transcription of the nuclear factor of activated T-cell (NFAT) family comprising NFATc1, NFATc2, NFATc3, and NFATc4. After dephosphorylation, NFAT translocates to the nucleus and activates target genes (Batiuk and Halloran, 1997). Interestingly the interaction of the zinc-finger protein GATA4 and MEF2, with members of the NFAT family, activate the transcription of cardiac-specific genes (Molkentin et al., 1998; Wu et al., 2000). These findings prompted us to investigate whether the calcineurin-NFAT pathway and the recruitment at specific target promoters of GATA2 proteins (the GATA isoform expressed in the skeletal muscle; Musaro et al., 1999; Paul and Rosenthal, 2002; Sakuma et al., 2003) are involved in the AVP-induced myogenic differentiation and the role of MEF2 in this pathway. Indeed, we show for the first time that both the CaMK and the calcineurin pathways are activated in AVP-stimulated myoblasts and that their interaction leads to the formation of multifactor complexes on specific sites of the regulatory regions of muscle specific genes. Furthermore, by exploitation of genetic and pharmacological manipulations, we demonstrate that the activation of both pathways is required for the full expression of the differentiated phenotype.
MATERIALS AND METHODS
Cell Cultures
L6 rat myogenic cells were seeded at 12.000/cm2 and cultured in DMEM supplemented with 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (DMEM), and 10% heat-inactivated fetal bovine serum (FBS; growth medium [GM]). Twenty-four hours after plating, cultures were extensively washed with DMEM, shifted to serum-free medium consisting of DMEM supplemented with 1% fatty acid-free bovine serum albumin (BSA, Sigma, St. Louis, MO; Minotti et al., 1998), and treated with synthetic AVP (Sigma) for different time points. When appropriate, different concentration of cyclosporin A (CsA) and/or KN62, as detailed in the text, were added 20 min before of the beginning AVP treatment.
RT-PCR
Total RNA was prepared from L6 cells using Trizol Reagent (Invitrogen, Carlsbad, CA), following the manufacturer's protocol.
RT-PCR was performed using 1 μg of total RNA reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (M-MLV RT; Invitrogen). PCR reactions were carried out in a final volume of 50 μl in a buffer containing 1 μl of RT reaction, 200 μM dNTP, 1.5 mM MgCl2, 0.2 μM of each primer, and 1 U of Taq-DNA polymerase (Invitrogen). The PCR products were analyzed in 2% agarose gel. The following specific primers were used: MEF2A: forward: 5′-AAGAAAATACAAATCACACGC-3′, reverse: 5′-AATCACTATCTTCATTTAGTT-3′, MEF2C: forward: 5′-AGTACACCGAGTACAACGAGC-3′, reverse: 5′-GCCTGTGTTACCTGCACTTGG-3′; MEF2D: forward: 5′-ATGGGGAGGAAAAAGATTCAG -3′, reverse: 5′-AAGGGATGATGTCACCAGGGA-3′; myogenin: forward: 5′-CTGGGGACCCCTGAGCATTG-3′, reverse: 5-ATCGCGCTCCTCCTGGTTGA-3′; NFATc1: forward: 5′-CATGCGAGCCATCATCGACTGTGCTGGGATCCTGA-3′, reverse: 5′-ATTGGCAGGAAGGTACGTGAAACG-3′; GATA2: forward: 5′-ACACACCACCCAATACCCACCTAT-3′, reverse: 5′-CCTAGCCCATGGCAGTCACCATGCT-3′; MCK: forward: 5′-GATGTCATCCAGACTGGGGTGGACAACC-3′, reverse: 5′-TGAACTCGCCCGTCAGGCTGTTGAG-3′; and MHC: forward: 5′-AGGGAGCTTGAAAACGAGGT-3′, reverse: 5′-GCTTCCTCCAGCTCGTGCTG-3′.
The following oligonucleotides were used to detect glyceraldehyde 3-phosphate dehydrogenase transcript (used as internal control): GAPDH: forward: 5′-AACATCAAATGGGGTGAGGCC-3′, reverse: 5′-GTTGTCATGGATGACCTTGGC-3′.
Plasmid Construction and Transfection
The pMyo84-luciferase was derived from the pMyo84-CAT plasmid (Edmondson et al., 1992). PCR-based strategy was used because of the absence of compatible restriction sites in the different pMyo84-CAT plasmids and pGl2-Basic (Promega, Madison, WI) polylinkers. Briefly, the myogenin 84-base pairs fragment was obtained by PCR from the original plasmid using oligonucleotides containing the appropriate restriction sites. The PCR product were purified, digested, and subcloned in the pGl2-Basic vector. The constructs were analyzed by sequencing to avoid PCR-introduced mutation.
For transient transfections, L6 cells were plated in six-well 35-mm dishes at a density of 2.5 × 105 cells/well in DMEM supplemented with 10% FBS. Twenty-four hours later, cells were transfected by using the lipid-based reagent Fugene 6 (Roche Molecular Biochemicals, Indianapolis, IN) following the manufacturer's instructions, using 1 μg of each of the following reporter plasmids/plate: pMyo84-luc (which contains the 84 base pairs myogenin promoter) and pMCK-luc, which contains 301 base pairs mouse MCK enhancer upstream of the 246 base pairs basal promoter (Sternberg et al., 1988). Where indicated 1 μg of the dominant negative construct of calcineurin, CnA-KO (Muramatsu and Kincaid, 1996), kindly provided by Dr. Muramatsu, was cotransfected. The plasmid encoding β-galactosidase under the control of the cytomegalovirus (CMV) promoter, CMV-βgal, was included to monitor transfection efficiency and for normalization of reactions. At 24 h after the start of transfection, the transfection mixture was removed and replaced with DMEM + 1% bovine serum albumin and the cells were treated with or without 0.1 μM AVP for 24 h. Cells were then washed twice with phosphate-buffered saline (PBS) and scraped in 1× reporter lysis buffer (Promega). Luciferase activity was determined with a luciferase assay kit (Promega).
Electrophoretic Mobility Shift Assays
Cells were plated in 150-mm dishes (2.5 × 106 cells/dish) in DMEM supplemented with 10% fetal calf serum (FCS). After 24 h the cells were shifted in serum-free medium and, as appropriate, treated for 3 d with 0.1 μM AVP in the presence or in the absence of the following inhibitors: 3 μM CsA, 8 μM KN62, or both. Cells were then scraped in PBS and centrifuged (3000 × g, 15 min at 4°C), and the cell pellets were resuspended in 5 volumes of 0.3 M sucrose in Buffer A (10 mM HEPES-KOH, pH 7.9,10 mM KCl, 1.5 mM MgCl2, 0.1 mM EGTA, 0.5 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) containing protease inhibitors (Complete Mini, Roche Molecular Biochemicals). After 10 min on ice, the cells were homogenized by 10 strokes in a Dounce homogenizer. Nuclei were pelleted by centrifugation (4.500 × g, 15 min at 4°C) and resuspended in three volumes of Buffer B (400 mM NaCl, 10 mM HEPES-KOH, pH 7.9, 1.5 mM MgCl2, 0.1 mM EGTA, 0.5 mM DTT, 5% glycerol, 0.5 mM PMSF) containing protease inhibitors (Complete Mini). Nuclei were homogenized by 10 strokes in a Dounce and then rocked for 30 min at 4°C. Samples were centrifuged at 100,000 × g for 30 min at 4°C, and the supernatant, containing nuclear proteins, was dialyzed overnight versus Buffer C (20 mM HEPES-KOH, pH 7.9, 75 mM NaCl, 0.1 mM EDTA, 0.5 mM DTT, 20% glycerol, 0.5 mM PMSF). Dialyzed samples were centrifuged (25,000 × g, 15 min at 4°C) to remove any precipitated proteins, and the nuclear extracts concentration was determined according to the Bradford protocol (Bradford, 1976). dsDNA oligonucleotides were end-labeled using 32P-ATP and T4 polynucleotide kinase (Roche Molecular Biochemicals) following the manufacturer's protocol. Nuclear extracts (15 μg) were incubated with 1 μg of poly dI/dC for 15 min at room temperature in a final volume of 20 μl in the presence of Buffer D (10 mM Tris-HCl, pH 7.5, 50 mM KCl, 5 mM MgCl2, 1 mM DTT, 1 mM EDTA, 15% glycerol). Labeled oligonucleotides (40,00 cpm) were added in the presence or in the absence of 100-fold excess of cold competitor oligonucleotides or of antibodies anti-MEF2 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-GATA2 (Santa Cruz) or anti-NFATc1 (ABR) for 30 min. Samples were separated on a 4% nondenaturing polyacrylamide gel in 0.5× TBE buffer run at 100 V. Gels were dried down and visualized by autoradiography. dsDNA oligonucleotides used in these experiments were derived from MEF2 (5′-AAGCTCGCTCTAAAAATAACCCTGTCCCTGGT-3′) and E-box (5′-TTTAACCCAGACATGTGGCTGCCCC-3′) consensus sequence on MCK promoter.
Immunofluorescence
Cells were fixed for 10 min in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100 in PBS for 2 min, and incubated for 30 min with PBS containing 5% BSA. Cells were then incubated overnight at 4°C with the selected primary antibody at the appropriate dilution. Antibodies used were as follows: monoclonal anti-F5D (kindly provided by Dr W. E. Wright, University of Texas, Dallas TX), monoclonal anti-MF20, polyclonal anti-MEF2 (Santa Cruz Biotechnology), monoclonal anti-GATA2 (Santa Cruz Biotechnology), monoclonal anti-NFATc1 (ABR), polyclonal anti-HA (Sigma). Cells were then washed with PBS containing 1% BSA and incubated for 60 min with the appropriate secondary antibody. Secondary antibodies used were as follows: TRITC goat anti-rabbit IgG (H+L) Conjugate (ZyMax Grade, Zymed Laboratories, South San Francisco, CA), TRITC-goat anti-mouse IgG (H+L) conjugate (ZyMax Grade, Zymed Laboratories), and FITC-goat anti-rabbit IgG (Sigma). After washing in 1% BSA in PBS the cells were mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA) and examined in a Zeiss Axioplan fluoromicroscope (Thornwood, NY).
Immunoblotting Analysis
For total homogenates cells were lysed with RIPA buffer (10 mM Tris-HCl, pH 7.5, 10 mM EDTA, 0.5 M NaCl, 0.5% NaDoc, 1% NP40) containing protease inhibitors cocktail (Mini Complete, Roche Molecular Biochemicals). Nuclear and cytosolic extracts were prepared as described for electrophoretic mobility shift assay (EMSA). Equal amount of proteins determined according to the Bradford protocol (Bradford, 1976; 15–20 μg) were separated by SDS-PAGE and transferred electrophoretically to Hybond C extramembrane (Amersham, Piscataway, NJ). Nonspecific binding was blocked in TBST containing 5% nonfat milk for 1 h and then the membrane was incubated overnight in 0.5% nonfat milk in TBST containing primary antibodies. The primaries antibodies used were the same mentioned above except for the monoclonal anti α-tubulin (Sigma). Blots were then washed extensively in TBST and then incubated with either goat anti-mouse HRP-conjugated or goat anti-rabbit HRP-conjugated secondary antibody in TBST containing 0.5% nonfat milk. Blots were washed in TBST and antibody binding was detected using Super Signal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL).
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation assays (ChIP) were performed using DNA extracts of L6 cells cultured for 24 h in DMEM supplemented 10% FCS, shifted in serum-free medium, and induced to differentiate by AVP treatment for different time points. Where indicated CsA or KN62 or both were added 20 min before of AVP addition. Equal amounts of chromatin of each sample (normalized by ethidium bromide staining of DNA) were immunoprecipitated with anti-acetyl histone H4 (Upstate Biotechnology, Lake Placid, NY) or with one of the following antibodies: anti-HDAC4, anti-NFATc1, anti-GATA2, or anti-MEF2. DNA fragments present in the immunoprecipitated were subjected to 28 cycles of PCR with primers specific to amplify sequences spanning the essential MEF2-binding site in the MCK enhancer (forward: 5′-CTGTAGACATGGAGAAGCTTGC3′; reverse: 5′-GTTTGAGAACCAGGTTCAGTTTC-3′) or myogenin promoter (forward: 5′-GAATCACATGTAATCCACTGGA-3′; reverse: 5′-ACGCCAACTGCTGGGTGCCA-3′). As a control for the DNA content, PCR reactions were also performed on chromatin samples before immunoprecipitation (input). Parallel extracts were exposed to normal rabbit serum (nonimmune) to control for nonspecific precipitation of chromatin.
RESULTS
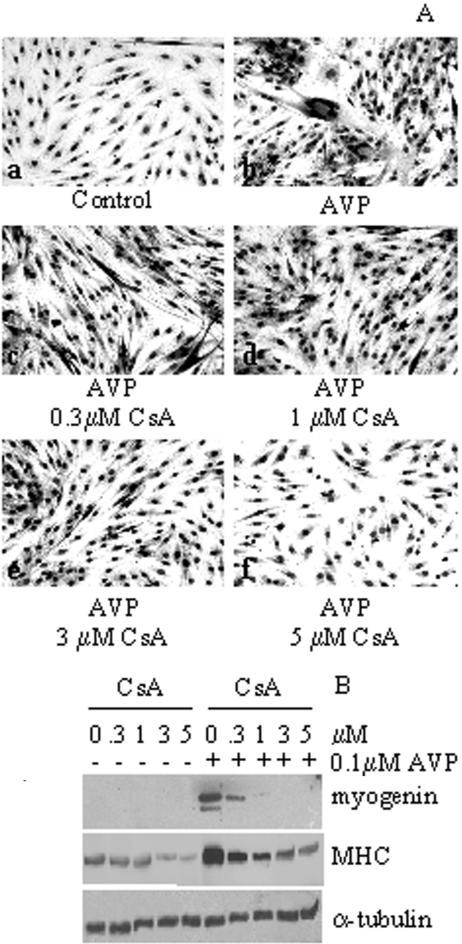
Calcineurin-mediated Signaling Pathway Is Involved in AVP-dependent Myogenic Differentiation. We previously showed that the CaMK signaling pathway is partially involved in AVP-dependent myogenic differentiation (Scicchitano et al., 2002). We therefore investigated the involvement of calcineurin signaling, a pathway-modulating calcium-dependent protein phosphorylation. To this purpose L6 myoblasts were treated with 0.1 μM AVP, in the presence and absence of different concentrations of the calcineurin inhibitor cyclosporine A (CsA), in serum-free medium (Figure 1, A and B). AVP treatment induced the formation of large myotubes, whereas very few, small myotubes were present in the control cultures (Figure 1A). CsA addition dramatically decreased, in a dose-dependent manner, the significant level of morphological differentiation induced by AVP.
Figure 1.
CsA treatment inhibits AVP-dependent myogenic differentiation. L6 cells were plated in GM. After 24 h, when the cells were subconfluent, the cultures were shifted to serum-free medium and treated or not with 0.1 μM AVP for 5 d in the presence of different concentrations of CsA. (A) Morphological differentiation of L6 cells were evaluated by Wright-Giemsa staining: large, multinucleated myotubes are formed in the presence of AVP (b), which are dramatically decreased in the presence of CsA in a dose-dependent manner (c–f). (B) Western blot analysis of whole L6 cell extracts showing that the presence of increasing concentrations of CsA strongly reduces the high level of the myogenin and MHC expression induced by AVP. Expression of α-tubulin is used to verify equal loading of the samples. The data are representative of three independent experiments.
Western blot analysis showed that the expression of both myogenin and MHC, two markers of terminal myogenic differentiation, was strongly induced by AVP treatment and displayed a dose-dependent reduction in response to increasing concentrations of CsA (Figure 1B).
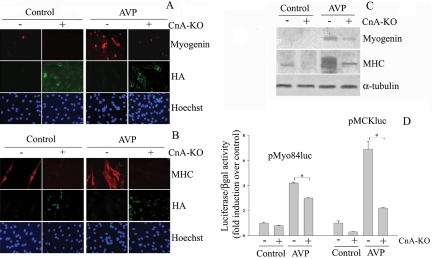
Because the above-mentioned data were generated with a pharmacological inhibitor and could potentially be due to nonspecific effects of the drug (as reviewed in Wilkins and Molkentin, 2002), we examined the effect of genetic ablation of CnA activity by transient transfection of the CnA dominant negative construct CnA-KO (Muramatsu and Kincaid, 1996), which contains the HA epitope at its amino terminus in proliferating L6 cells. CnA-KO and mock-transfected L6 cells were further cultivated for 3 d in the presence or absence of 0.1 μM AVP. Immunofluorescence (Figure 2, A and B) and Western blotting (Figure 2C) analysis revealed that the expression levels of myogenin and MHC proteins were strongly induced in the presence of AVP and were significantly down-regulated by forced-expression of CnA-KO.
Figure 2.
Expression of the CnA dominant negative construct drastically inhibits the AVP-stimulated expression of myogenin and MHC and the activity of muscle-specific regulatory elements. (A–C) L6 cells were plated in GM and after 24 h were transiently transfected with either the HA-tagged CnA dominant negative construct, CnA-KO (+) or an empty vector (–). After an additional 24 h the cultures were shifted to serum-free medium and treated or not with 0.1 μM AVP for 3 d. The immunofluorescence analysis shows that the presence of the CnA-KO, revealed by the anti-HA antibody, drastically reduces the AVP-dependent induction of the myogenin (A) and the MHC (B) proteins. Note that no expression of myogenic markers occurs in HA-positive cells. (C) The inhibitory effect of CnA-KO on myogenin and MHC expression is confirmed by Western blot analysis of whole cell extracts. (D) L6 cells were transiently cotransfected with the CnA-KO construct in conjunction with either the pMyo84-Luc or the MCK-Luc (see Materials and Methods). Twenty-four hours after transfection the cells were shifted from GM to the serum-free medium and treated or not with 0.1 μM AVP for an additional 24 h. The luciferase reporter gene assay shows that AVP treatment exerts a strong stimulation of the activity of both promoters, cotransfection with CnA-KO inhibits the transactivating activity of the MCK enhancer and, to a lesser extent, that of the myogenin promoter (p < 0.05). Luciferase activity was determined and normalized to the β-gal activity as described in Materials and Methods. Statistical analysis was performed by t test on data obtained from four independent experiments.
We then investigated the ability of the CnA-dominant negative construct to modify the AVP response of promoters of genes expressed at early and late stages of myogenesis, such as myogenin and MCK, upon transient cotransfection into L6 cells (Figure 2D). To this purpose, we used two reporter constructs: 1) the pMyo84-luc containing the 84-base pair sequence preceding the transcription initiation site presenting the MEF2 and E-box binding sites of the human myogenin promoter, cloned 5′ to the luciferase reporter gene; 2) pMCK-luc containing the 301-base pair mouse MCK enhancer upstream of the 246-base pair basal promoter and luciferase also presenting an MEF2 and E-box binding sites (Sternberg et al., 1988). Figure 2D shows that AVP treatment strongly stimulated the pMyo84-luc and pMCK-luc activities of about four- and sevenfold, respectively. In the presence of CnA-KO the magnitude of the AVP response of the pMyo84-luc and MCK-luc activities was reduced by ∼25 and 75%, respectively, thus suggesting that both these promoters are regulated by AVP with a mechanism also involving CnA activation.
AVP Modulates the Expression of Genes Involved in the Calcineurin-signaling Pathway at Different Stages of Myogenesis
To assess the physiological pathway of the CnA-mediated events in the AVP-dependent differentiation, we investigated the activation of specific genes involved in the CnA pathway, such as MEF2, NFATc1, and GATA-2 (Musaro et al., 1999; Friday et al., 2000, 2003; Olson and Williams, 2000; McKinsey et al., 2002a) in relation to myogenin and MCK gene expression.
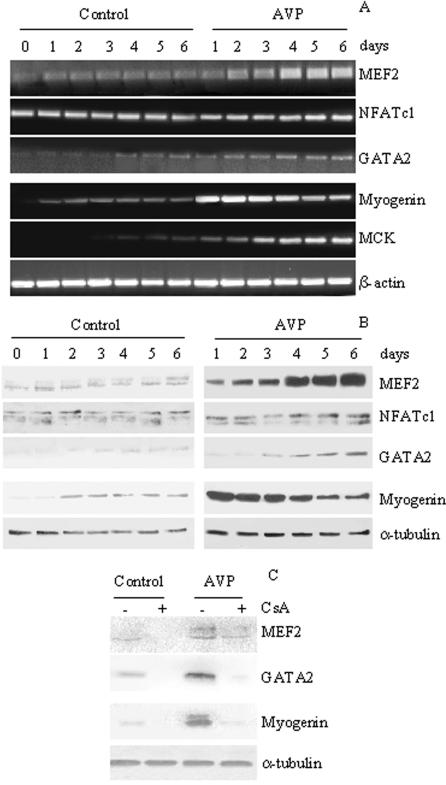
RT-PCR (Figure 3A) and Western blotting (Figure 3B) analysis, performed over a 6-d time course using specific antibodies, revealed that the time course of the effect of AVP is different for the three responding factors. MEF2, weakly expressed at all experimental time points in control cells, was significantly increased at 48 h, and subsequent time points, of AVP treatment. The expression of the CnA-target gene, NFATc1, was not modulated at any experimental time points either in the absence or in the presence of AVP (Figure 3, A and B), a result that is consistent with the evidence that CnA induces NFAT nuclear translocation rather than the modulation of NFAT expression (see below; Rao et al., 1997). The expression of GATA2, constantly low although increasing with time in control cells, was stimulated at 3 d and subsequent time points in AVP-treated cells (Figure 3, A and B). The expression of myogenin and MCK was also analyzed in the same samples to assess the degree of differentiation (Figure 3, A and B). Both myogenin and MCK were expressed at low levels in control L6 cells after shifting to serum-free medium, and their expression was dramatically increased in AVP-treated cells, as early as 24 h for myogenin, and only at later time points for MCK.
Figure 3.
AVP-dependent modulation of calcineurin target gene expression. Twenty-four hours after plating in GM, L6 cells were shifted to serum-free medium and treated or not with 0.1 μM AVP. RNA (A) and whole cell extracts (B) were collected at different times in the absence and in the presence of AVP and analyzed for MEF2, NFATc1, and GATA2 expression. The expression levels of myogenin and MCK were evaluated as markers of differentiation. MEF2 expression was not modulated in control cultures, but appears up-regulated as early as 24 h of AVP treatment. GATA2 expression was weakly detectable after 3 d in the control culture and was up-regulated after 3 d of AVP treatment. The expression of the CnA-target gene, NFATc1, was not modulated both in the control or in the presence of AVP. (C) Addition of 3 μM CsA 20 min before that of AVP blocked myogenin, GATA2 and, partially, MEF2 expression, in samples collected 3 d after the beginning of AVP treatment. β-actin was used as internal control for the RT-PCR analysis and the α-tubulin antibody was used to verify the equal loading of the samples for the Western blot analysis. The data are representative of at least two independent experiments.
CsA treatment inhibited the expression of the three transcription factors both in control and in AVP-treated cells (Figure 3C), consistently with data indicating a calcineurin control of their expression (Musaro et al., 1999; Friday et al., 2000, 2003).
AVP Induces the Accumulation of MEF2, GATA2, and NFATc1 into L6 Cells Nuclei
Calcineurin regulates the activity of both NFAT and MEF2 transcription factors (Crabtree, 1999; Wu et al., 2000; Youn et al., 2000), and functional interaction between members of the MEF2 and GATA families has been shown to play a key role in cardiac and skeletal muscle (Morin et al., 2000; Sakuma et al., 2003). To investigate whether AVP induces the formation of specific active multifactor complexes comprising GATA2, MEF2, and NFATc1 at the promoter region of muscle genes, we initially studied the subcellular localization of these factors in L6 cells in response to AVP treatment.
Western blotting of nuclear and cytosolic fractions (Figure 4) and immunofluorescence analysis (Figure 5, A and B) revealed that AVP treatment (3 d) induced the accumulation of MEF2 and GATA2 proteins into the nucleus of L6 cells and the translocation of NFATc1 from the cytosol to the nucleus. Notably MEF2-GATA2 and MEF2-NFATc1 colocalized in the same subsets of nuclei in AVP treated cultures (Figure 5, A and B). Interestingly, L6 cells also showed a characteristic organization of the nuclei, forming nuclear rings, which represent a morphological marker of muscle hypertrophy/maturation in vitro (Musaro et al., 1999; Musaro and Rosenthal, 1999; Figure 5, A and B and Figure 1).
Figure 4.
AVP treatment induces nuclear accumulation of MEF2, GATA2, and NFATc1. After 24 h in GM, L6 cells were shifted in serum-free medium and treated or not with 0.1 μM AVP for 3 d. Western blot of the nuclear and cytosolic fractions shows the accumulation of MEF2 and GATA2 proteins in the nuclei of L6 cells in the presence of AVP and the nuclear translocation from the cytosol to the nucleus of NFATc1 during AVP-dependent myogenic differentiation. Red Ponceau staining was used to verify equal loading of the samples.
Figure 5.
AVP treatment induces MEF2/GATA2 and MEF2/NFATc1 colocalization. After 24 h in GM, L6 cells were shifted in serum-free medium and treated or not with 0.1 μM AVP for 3 d and then processed for immunofluorescence analysis. The AVP treatment induces the colocalization of MEF2/GATA2 (A) and of MEF2/NFATc1 (B) in the nuclei of L6 cells.
Changes of Histone Acetylation Status of Chromatin Surrounding the MEF2 Sites of Muscle Genes Occur at Different Stages of AVP-induced Myogenesis
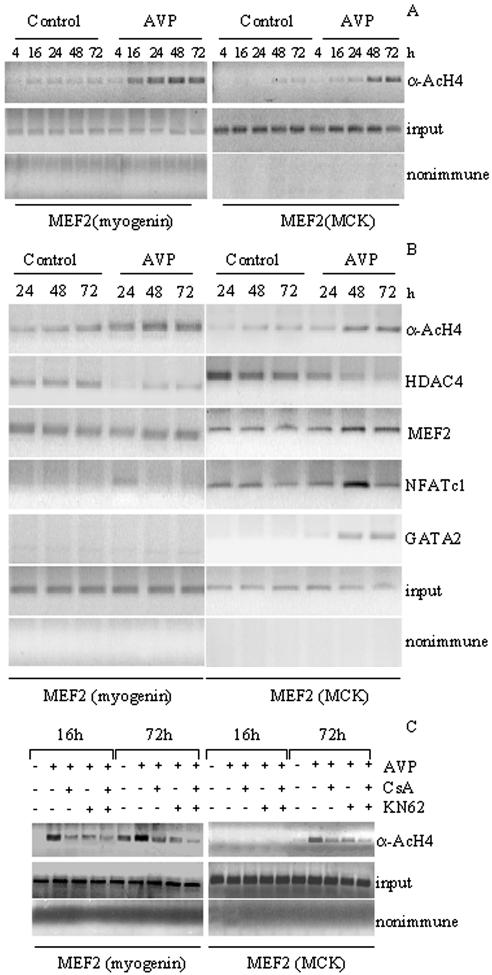
To verify whether AVP treatment affects histone acetylation at the MEF2 sites of responsive genes activated in different phases of myogenesis, ChIP assays were performed on L6 cells cultures at different time points after AVP treatment. DNA extracts were immunoprecipitated with an antibody specific for hyperacetylated histone H4 (α-AcH4; Figure 6A) and analyzed by PCR using primers designed for the specific amplification of regions containing the MEF2 binding sites present on the myogenin promoter or the MCK enhancer. Histone-H4 at the MEF2 sites on either myogenin or MCK remained mostly hypoacetylated in untreated L6 cells during the 72-h time course (Figure 6A). Histone H4 hyperacetylation at the MEF2 site of the myogenin promoter was detectable at early times (16 h) of AVP treatment, whereas histone H4 hyperacetylation at the MEF2 site of the MCK enhancer was detected after 48 h of AVP addition. These results are in agreement with the kinetics of the induction of myogenin and MCK mRNA transcripts by AVP (Figure 3A). Thus, AVP-dependent enhancement of histone H4 acetylation at the MEF2 sites containing regions on either the myogenin promoter or the MCK enhancer may play a role in the activation of muscle gene expression.
Figure 6.
AVP treatment induces histone H4 acetylation and formation of multifactor complexes at MEF2 sites of muscle genes with a mechanism involving both CaMK and calcineurin. ChIP assays were performed on DNA extracts prepared from L6 cells cultured in serum-free medium in the presence or in the absence of 0.1 μM AVP for the times indicated. 3 μM CsA, 8 μM KN62 or both were added as appropriate. DNA extracts were immunoprecipitated with the acetylated histone H4 (α-AcH4)-specific antibody (A–C) or with antibodies against MEF2, HDAC4, NFATc1, and GATA2 (B). Parallel extracts were exposed to preimmune rabbit serum (nonimmune) to control nonspecific precipitation of chromatin. Immunoprecipitated DNA was analyzed by PCR using primers designed to amplify sequences spanning the MEF2-binding site of the myogenin promoter and of the MCK enhancer (A–C). Input represents the DNA control in which PCR amplification was performed before immunoprecipitation, to confirm that equivalent amounts of DNA were present in each sample (A–C). The data are representative of at least two independent experiments.
AVP Promotes the Formation of Specific Protein Complexes at the MEF2 Sites on the Myogenin Promoter and the MCK Enhancer
We then evaluated by ChIP assays the presence of HDAC4, NFATc1, and GATA2 with MEF2 at its sites on the myogenin promoter or on the MCK enhancer, also in relation with the local acetylation status, at different stages of AVP-induced myogenesis (Figure 6B). L6 cells were treated with AVP or saline for 24, 48, and 72 h and chromatin fragments were immunoprecipitated with anti-hyperacetylated histone H4, anti-MEF2, anti-HDAC4, anti-GATA2 and anti-NFATc1 antibodies. DNA from the immunoprecipitate was then amplified by PCR using primers to recognize the specific MEF2 sites containing regions of both the myogenin promoter and the MCK enhancer. ChIP analysis (Figure 6B) showed that HDAC4 was present at the MEF2 site of the myogenin promoter in untreated cells at all examined time points, whereas it was significantly reduced at all times of AVP treatment, in agreement with our previous data showing AVP-dependent HDAC4 nuclear export and induction of myogenin expression (Scicchitano et al., 2002). HDAC4 presence at the MEF2 site of the MCK enhancer followed a similar behavior, although with a delay compared with the myogenin promoter. In all cases the behavior of HDAC4 is confirmed by the specular changes of histone H4 acetylation. MEF2 is present on the promoters of both genes at all time points both in control and AVP-treated cells. In agreement with the different kinetics of expression of myogenin and MCK, NFATc1 presence at the MEF2 site of the myogenin promoter was undetectable in control samples and occurred at 24 h of AVP treatment. On the other hand NFATc1 was present at the MEF2 site of the MCK promoter at all examined time points, in samples from both control and AVP-treated cell. Notably, GATA2, whose expression was induced at 48–72 h of AVP treatment (Figure 3, A and B), was present at 48–72 h of AVP addition only on the MEF2 site of the MCK promoter, but not on that of the myogenin promoter.
These results suggest that a specific and sequential recruitment of HDAC4, NFATc1, and GATA2 at the MEF2 binding sites present on the myogenin promoter and the MCK enhancer is induced by AVP in L6 cells and is consistent with the temporal activation of the transcription of these genes.
We have previously shown (Scicchitano et al., 2002) that KN62 and KN93, two inhibitors of CaMK signaling (Ramirez et al., 1997), inhibited, although partially, the ability of AVP to induce myogenic differentiation in L6 cells. We therefore investigated by ChIP assay the inhibitory effect of CsA and KN62 on the AVP-dependent changes of the chromatin acetylation status at MEF2 site containing regions on the myogenin promoter and on the MCK enhancer (Figure 6C). As shown in Figure 6C the amount of hyperacetylated histone H4 associated with these regulatory regions on myogenin and MCK genes observed in the presence of AVP, was partially reduced by either CsA or KN62 treatment, whereas was further reduced by the combined presence of the two inhibitors.
The AVP-dependent formation of multifactor complexes requires the MEF2 site and it is mediated by the activation of both CaMK and calcineurin pathways
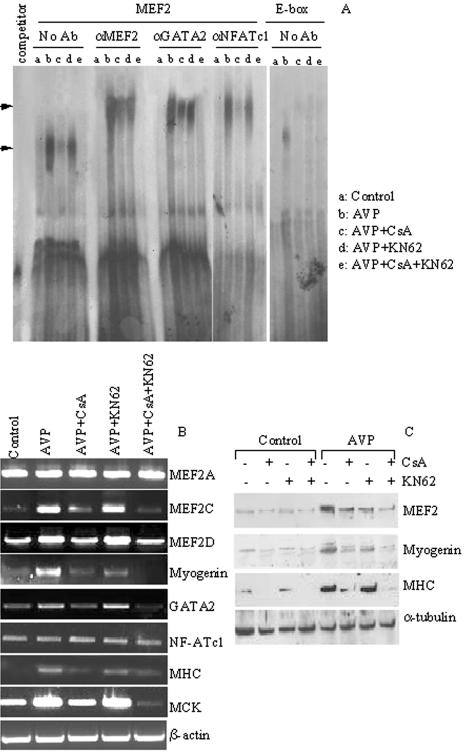
To investigate the direct role of MEF2 in the formation of specific multifactor complexes and in the recruitment of these factors to promoters containing MEF2 sites, we performed EMSA experiments using nuclear extracts from L6 cells treated or not with AVP for 3 d and with oligonucleotides representing the MEF2 consensus sequence present on the MCK promoter (Figure 7). This experimental time point was chosen based on the presence of each factor on the MEF2 site of the MCK promoter. Figure 7A shows that AVP induced the formation of specific complexes (lane b) that were efficiently competed by an excess of unlabeled oligonucleotide (“competitor” lane). These complexes were supershifted in the presence of antibodies against MEF2, GATA2, and NFATc1, thus indicating the presence of all these proteins at this specific MEF2 site. Indeed, these complexes were not formed when an oligonucleotide for mutated MEF2 was used (unpublished data). The protein: DNA complexes were specifically induced by AVP treatment because they were not present in untreated L6 cells (lane a; Figure 7A).
Figure 7.
(A) AVP treatment induces the CaMK- and calcineurin-mediated formation of specific nuclear complexes at the MEF2-binding site of the MCK promoter. EMSA analysis was performed using nuclear extracts from control L6 cells (a), or from cells treated with 0.1 μM AVP for 3 d in the absence (b) or in the presence of the following inhibitors: 3 μM CsA (c), 8 μM KN62 (d), or both (e). 32P-labeled oligonucleotides, corresponding to the MEF2 site or to the E-box site of the MCK promoter, were used as probes. Competition assays were performed by adding a 100-fold molar excess of unlabeled MEF2 specific oligonucleotide (“competitor”) to the reaction mixture containing the extracts from cells treated with AVP. Supershift assays were performed by preincubating the reaction mixture of the nuclear extracts with antibodies against MEF2, GATA2, and NFATc1, as indicated. Arrows indicate shifted complexes. (B and C) The CaMK and calcineurin pathways are involved in the AVP-dependent gene expression. L6 cells were plated in GM. After 24 h the cultures were shifted to serum-free medium and treated or not for 3 d with 0.1 μM AVP in the presence or in the absence of the following inhibitors: 3 μM CsA, 8 μM KN62, or both. (B) One microgram of total RNA of each sample was subjected to RT-PCR using specific primers as indicated in the figure. β-actin was amplified as an internal control. Ethidium bromide staining of the RT-PCR products is shown. (C) Western blot analysis was performed using antibodies against MEF2, myogenin, and MHC. α-tubulin antibody was used to verify equal loading of the samples.
The effect of calcineurin and CaMK inhibition on the formation of the MEF2-GATA2-NFATc1:DNA complexes was analyzed by using CsA and KN62 singularly or in combination. CsA treatment partially inhibited the complexes formed at the MEF2 site by MEF2 and GATA2 and completely blocked the recruitment of NFATc1 (lane c; Figure 7A). In contrast KN62 only slightly affected the formation of the MEF2-GATA2-NFATc1: DNA complexes (lane d; Figure 7A), but strongly potentiated the inhibitory effect of CsA when the two inhibitors were used simultaneously (lane e; Figure 7A). These experiments provide evidence that MEF2, GATA2, and NF-ATc1 contribute to the same transcriptional complex at the MEF2 site on the MCK promoter and indicate that the CnA and the CaMK pathways cooperate in mediating the inductive role of AVP on muscle differentiation. The specificity of such protein:DNA complexes was demonstrated using oligonucleotides representing the E1-box, rather than the MEF2 site, of the MCK promoter (Figure 7, right panel). Unlike the AVP-dependent complexes on the MEF2 site of the MCK promoter, those on the E-box were very low and in fact disappeared with CsA and KN62 treatments (lanes c–e). This result indicates the importance of the MEF2 site, compared with the E-box, with respect to AVP-dependent regulation.
To clarify the role of the CaMK and CnA pathways during AVP-induced muscle differentiation, we performed experiments to analyze the effect of these agents on the expression of specific genes associated with the myogenic program, such as myogenin, MEF2A, C, D, GATA2, MHC, and MCK. RT-PCR and Western blotting analysis (Figure 7, B and C) revealed that although AVP treatment strongly induced the expression levels of the examined genes (except for MEF2A and NFATc1), their expression levels were negatively modulated by the inhibition of the CnA pathway and, at a lower extent, of the CaMK pathway. Moreover the combined treatment with CsA and KN62 more drastically inhibited muscle gene expression further confirming the cooperative effect of the CaMK and CnA signal transduction pathways in the myogenic differentiation program activated by AVP.
DISCUSSION
The results presented in this article show, for the first time, that AVP, a “novel” myogenesis promoting factor, activates both the calcineurin and the CaMK pathways, whose combined activation leads to the formation of transcription factor complexes and is required for the full expression of the differentiated phenotype.
The physiological relevance of the effect of AVP (or a vasopressin-like peptide) on myogenesis is indicated by several findings: 1) high levels of immunoreactive AVP are present in extracts of human embryonic skeletal muscle, declining as gestational age increases (Smith et al., 1992); 2) a vasopressin-like peptide is present in the mammalian sympathetic nervous system, a finding that may cast light on the question of the origin of AVP during skeletal muscle development (Hanley et al., 1984); 3) functional oxytocin receptors (which belong to the AVP receptors family) are expressed in human satellite cells (Breton et al., 2002). Thus, it is intriguing to speculate that during embryonic development (and possibly during postnatal growth and repair) AVP or a related peptide participates in the physiological control of myogenesis (and in the control of muscle homeostasis).
We previously reported that AVP treatment activates the CaMK signaling pathway, which in turn induces cytosolic compartmentalization of the histone deacetylase 4, a mechanism related to the transcriptional activation of MEF2 (Scicchitano et al., 2002). However, CaMK alone is not able to sustain the entire myogenic program activated by AVP, because inhibition of this pathway does not result in complete inhibition of muscle differentiation. These evidences suggest that additional pathways are involved in the promotion and maintenance of muscle differentiation induced by AVP.
By exploitation of genetic ad pharmacological manipulations we demonstrate for the first time that: 1) calcineurin is a downstream effector in AVP-dependent myogenic differentiation; 2) AVP treatment results in the calcineurin-dependent up-regulation of myogenin, MEF2, and GATA2 expression and of MEF2 and GATA2 nuclear content.
The lack of effect of AVP on the expression of NFATc1 is in agreement with data showing that calcineurin is involved in NFATc1 nuclear translocation (Rao et al., 1997; Molkentin et al., 1998) but not in its expression. In fact, in L6 cells, NFATc1 translocates from the cytosol to the nuclear compartment as a result of AVP treatment.
A large body of evidence demonstrates that the decision of a myoblast to differentiate is dictated by a balance of positive and negative influences on the transcriptional activity of MEF2. Consistently with the findings that HDAC4 represses muscle transcription by deacetylating core histones associated with regulatory regions of muscle genes (Miska et al., 1999, 2001), we show that the level of acetylated histone H4 associated with the myogenin promoter and MCK enhancer, both of which are directly regulated by MEF2 (Gossett et al., 1989; Edmondson et al., 1992), increases in AVP-dependent myogenesis.
In particular the faster acetylation of myogenin-MEF2 site (≥16 h), compared with that of MCK-MEF2 (≥48 h), is consistent with a faster expression response to AVP of myogenin, compared with MCK. With respect to the molecular mechanism by which AVP induces the signals leading to myogenic differentiation and maturation, our results support the following model: in untreated L6 cells HDAC4 is associated with MEF2, which in turn is present on its site on both the myogenin and the MCK regulatory regions (Scicchitano et al., 2002); conversely, during the first 24 h of AVP treatment, HDAC4 translocates to the cytosol and the NFATc1 transcription factor translocates to the nucleus upon dephosphorylation by calcineurin. In the nucleus NFATc1 associates with MEF2 and stimulates the expression of myogenin.
AVP-dependent histone H4 acetylation on the MEF2 site of the MCK enhancer occurs with a slower time course than that of the myogenin promoter. HDAC4 association with the MEF2 site of MCK enhancer slowly decreases and NFATc1 accumulates at the same site more persistently than in the case of the myogenin promoter. Interestingly, the maximal level of MCK-MEF2 chromatin acetylation (48–72 h) coincides with the presence of GATA2 on this region, consistent with the evidence that GATA2 is involved in muscle maturation and hypertrophy (Musaro et al., 1999). The AVP-dependent high level of acetylated histone H4 associated with MEF2 sites of the myogenin promoter and of the MCK enhancer, is partially reduced by the inhibition of either the CaMK or the calcineurin pathway and is further reduced by the combined inhibition of these two pathways.
An important characteristic of our model is that AVP regulates, at the transcriptional level, not only the expression of early differentiation markers, such as myogenin, but also that of late differentiation markers, such as MCK. Moreover, calcineurin is involved in the transcriptional regulation of the expression of both myogenin and MCK. The different time courses of the expression of myogenin and MCK in response to AVP treatment, reflect a different recruitment of HDAC4, NFATc1, and GATA2 by MEF2 on MEF2 sites of the myogenin and, respectively, of the MCK regulatory regions.
By EMSA we further demonstrate that, in AVP stimulated L6 cells, MEF2 plays a key role in the formation of protein: DNA complexes comprising MEF2, GATA2, and NFATc1 on the MEF2 site present on muscle-specific genes, such as MCK. The complex is site-specific, because the oligonucleotide representing the E1-box site in the MCK enhancer binds AVP-stimulated protein:DNA complexes at much lower levels. Furthermore, no complexes are formed when the oligonucleotide for mutated MEF2 is used. Additional interactions may also contribute to MEF2/NFATc1/GATA2 complex activity, including the MRFs or accessory protein(s) such as the transcriptional coactivator p300, which has been previously demonstrated to bind both MEF2 and NFATc1 (Sartorelli et al., 1997; Garcia-Rodriguez and Rao, 1998).
Interestingly, the formation of multifactor complexes is the result of a combinatorial activation of the CaMK and the calcineurin pathways by AVP, as demonstrated by the total absence of protein:DNA complexes when these two pathways are simultaneously inhibited. Further, the drastic effect of calcineurin inhibition on the expression levels of regulatory genes such as myogenin and, at a lower extent, MEF2, and of structural genes such as myosin and MCK, is potentiated by the concomitant inhibition of the CaMK pathway. Thus, CaMK and calcineurin act cooperatively, activating transcriptional targets in the AVP-dependent myogenic differentiation.
Our findings therefore, show that AVP is a novel myogenic factor that induces both the CaMK and the calcineurin pathways during L6 myoblasts differentiation. Thus skeletal muscle shares with cardiac muscle elements of a common signaling system, in which CaMK and calcineurin regulate muscle hypertrophy and regeneration processes by controlling combinatorial association of MEF2, NFAT, and GATA transcription factors (Molkentin et al., 1998; Wu et al., 2000). In this context AVP might represent an interesting candidate to develop new therapeutic strategies to delay the onset, or slow down the progression, of muscle wasting and loss of muscle power, a major cause of disability resulting from aging, disuse, and neuromuscular disorders.
Acknowledgments
We thank Thomas Rando and Marina Bouchè for critical reading of the manuscript. We also thank Angelica Toschi and Carla Ramina for excellent technical help. B.M.S. was supported by funds of the University of Rome “La Sapienza” (Progetti di Ateneo). This work was partially supported by the Italian Ministry of University and Research (COFIN and FIRB programs), by the Ministry of Health (IRCS programs), by Telethon-Italy, and by the Centro di Eccellenza BEMM (Molecular Biology and Medicine), “La Sapienza” University.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–01–0055) on June 1, 2005.
Abbreviations used: AVP, arg8-vasopressin; CaMK, calcium/calmodulin-dependent kinase; CSA, cyclosporin A; HDAC, histone deacetylase; MCK, muscle creatine kinase; MEF2, myocyte enhancer factor 2; MHC, myosin heavy chain; NFAT, nuclear factor of activated T-cells.
References
- Batiuk, T. D., and Halloran, P. F. (1997). The downstream consequences of calcineurin inhibition. Transplant. Proc. 29, 1239–1240. [DOI] [PubMed] [Google Scholar]
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Braz, J. C. et al. (2003). Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Invest. 111, 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton, C., Haenggeli, C., Barberis, C., Heitz, F., Bader, C. R., Bernheim, L., and Tribollet, E. (2002). Presence of functional oxytocin receptors in cultured human myoblasts. J. Clin. Endocrinol. Metab. 87, 1415–1418. [DOI] [PubMed] [Google Scholar]
- Clegg, C. H., Linkhart, T. A., Olwin, B. B., and Hauschka, S. D. (1987). Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J. Cell Biol. 105, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletti, D., Silvestroni, L., Naro, F., Molinaro, M., Adamo, S., and Palleschi, S. (2000). Vesicle-mediated phosphatidylcholine reapposition to the plasma membrane following hormone-induced phospholipase D activation. Exp. Cell Res. 256, 94–104. [DOI] [PubMed] [Google Scholar]
- Crabtree, G. R. (1999). Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell 96, 611–614. [DOI] [PubMed] [Google Scholar]
- Edmondson, D. G., Cheng, T. C., Cserjesi, P., Chakraborty, T., and Olson, E. N. (1992). Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol. Cell. Biol. 12, 3665–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert, J. C., Berglund, E. B., and Rosenthal, N. (1996). Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J. Cell Biol. 135, 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini, J. R., Ewton, D. Z., and Magri, K. A. (1991a). Hormones, growth factors, and myogenic differentiation. Annu. Rev. Physiol. 53, 201–216. [DOI] [PubMed] [Google Scholar]
- Florini, J. R., Ewton, D. Z., and Roof, S. L. (1991b). Insulin-like growth factor-I stimulates terminal myogenic differentiation by induction of myogenin gene expression. Mol. Endocrinol. 5, 718–724. [DOI] [PubMed] [Google Scholar]
- Friday, B. B., Horsley, V., and Pavlath, G. K. (2000). Calcineurin activity is required for the initiation of skeletal muscle differentiation. J. Cell Biol. 149, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday, B. B., Mitchell, P. O., Kegley, K. M., and Pavlath, G. K. (2003). Calcineurin initiates skeletal muscle differentiation by activating MEF2 and MyoD. Differentiation 71, 217–227. [DOI] [PubMed] [Google Scholar]
- Garcia-Rodriguez, C., and Rao, A. (1998). Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP). J. Exp. Med. 187, 2031–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass, D. J. (2003). Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat. Cell Biol. 5, 87–90. [DOI] [PubMed] [Google Scholar]
- Gossett, L. A., Kelvin, D. J., Sternberg, E. A., and Olson, E. N. (1989). A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol. Cell. Biol. 9, 5022–5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley, M. R., Benton, H. P., Lightman, S. L., Todd, K., Bone, E. A., Fretten, P., Palmer, S., Kirk, C. J., and Michell, R. H. (1984). A vasopressin-like peptide in the mammalian sympathetic nervous system. Nature 309, 258–261. [DOI] [PubMed] [Google Scholar]
- Kouzarides, T. (2000). Acetylation: a regulatory modification to rival phosphorylation? EMBO J. 19, 1176–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiacapra, F. J., Roof, S. L., Ewton, D. Z., and Florini, J. R. (1992). Paradoxical decrease in myf-5 messenger RNA levels during induction of myogenic differentiation by insulin-like growth factors. Mol. Endocrinol. 6, 2038–2044. [DOI] [PubMed] [Google Scholar]
- McKinsey, T. A., Zhang, C. L., and Olson, E. N. (2001). Control of muscle development by dueling HATs and HDACs. Curr. Opin. Genet. Dev. 11, 497–504. [DOI] [PubMed] [Google Scholar]
- McKinsey, T. A., Zhang, C. L., and Olson, E. N. (2002a). MEF 2, a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem. Sci. 27, 40–47. [DOI] [PubMed] [Google Scholar]
- McKinsey, T. A., Zhang, C. L., and Olson, E. N. (2002b). Signaling chromatin to make muscle. Curr. Opin. Cell Biol. 14, 763–772. [DOI] [PubMed] [Google Scholar]
- Minotti, S., Scicchitano, B. M., Nervi, C., Scarpa, S., Lucarelli, M., Molinaro, M., and Adamo, S. (1998). Vasopressin and insulin-like growth factors synergistically induce myogenesis in serum-free medium. Cell Growth Differ. 9, 155–163. [PubMed] [Google Scholar]
- Miska, E. A., Karlsson, C., Langley, E., Nielsen, S. J., Pines, J., and Kouzarides, T. (1999). HDAC4 deacetylase associates with and represses the MEF2 transcription factor. EMBO J. 18, 5099–5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska, E. A., Langley, E., Wolf, D., Karlsson, C., Pines, J., and Kouzarides, T. (2001). Differential localization of HDAC4 orchestrates muscle differentiation. Nucleic Acids Res. 29, 3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin, J. D., Lu, J. R., Antos, C. L., Markham, B., Richardson, J., Robbins, J., Grant, S. R., and Olson, E. N. (1998). A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93, 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin, J. D., and Olson, E. N. (1996). Defining the regulatory networks for muscle development. Curr. Opin. Genet. Dev. 6, 445–453. [DOI] [PubMed] [Google Scholar]
- Morin, S., Charron, F., Robitaille, L., and Nemer, M. (2000). GATA-dependent recruitment of MEF2 proteins to target promoters. EMBO J. 19, 2046–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu, T., and Kincaid, R. L. (1996). Inhibition of NF-AT signal transduction events by a dominant-negative form of calcineurin. Biochem. Biophys. Res. Commun. 218, 466–472. [DOI] [PubMed] [Google Scholar]
- Musaro, A., McCullagh, K. J., Naya, F. J., Olson, E. N., and Rosenthal, N. (1999). IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400, 581–585. [DOI] [PubMed] [Google Scholar]
- Musaro, A., and Rosenthal, N. (1999). Maturation of the myogenic program is induced by postmitotic expression of insulin-like growth factor I. Mol. Cell. Biol. 19, 3115–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naro, F., Donchenko, V., Minotti, S., Zolla, L., Molinaro, M., and Adamo, S. (1997). Role of phospholipase C and D signalling pathways in vasopressin-dependent myogenic differentiation. J. Cell. Physiol. 171, 34–42. [DOI] [PubMed] [Google Scholar]
- Naro, F., Sette, C., Vicini, E., De Arcangelis, V., Grange, M., Conti, M., Lagarde, M., Molinaro, M., Adamo, S., and Nemoz, G. (1999). Involvement of type 4 cAMP-phosphodiesterase in the myogenic differentiation of L6 cells. Mol. Biol. Cell 10, 4355–4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi, C., Benedetti, L., Minasi, A., Molinaro, M., and Adamo, S. (1995). Arginine-vasopressin induces differentiation of skeletal myogenic cells and up-regulation of myogenin and Myf-5. Cell Growth Differ. 6, 81–89. [PubMed] [Google Scholar]
- Olson, E. N. (1992). Interplay between proliferation and differentiation within the myogenic lineage. Dev. Biol. 154, 261–272. [DOI] [PubMed] [Google Scholar]
- Olson, E. N., Sternberg, E., Hu, J. S., Spizz, G., and Wilcox, C. (1986). Regulation of myogenic differentiation by type beta transforming growth factor. J. Cell Biol. 103, 1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, E. N., and Williams, R. S. (2000). Remodeling muscles with calcineurin. Bioessays 22, 510–519. [DOI] [PubMed] [Google Scholar]
- Paul, A. C., and Rosenthal, N. (2002). Different modes of hypertrophy in skeletal muscle fibers. J. Cell Biol. 156, 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez, M. T., Zhao, X. L., Schulman, H., and Brown, J. H. (1997). The nuclear deltaB isoform of Ca2+/calmodulin-dependent protein kinase II regulates atrial natriuretic factor gene expression in ventricular myocytes. J. Biol. Chem. 272, 31203–31208. [DOI] [PubMed] [Google Scholar]
- Rao, A., Luo, C., and Hogan, P. G. (1997). Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747. [DOI] [PubMed] [Google Scholar]
- Rosenthal, S. M., and Cheng, Z. Q. (1995). Opposing early and late effects of insulin-like growth factor I on differentiation and the cell cycle regulatory retinoblastoma protein in skeletal myoblasts. Proc. Natl. Acad. Sci. USA 92, 10307–10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma, K., Nishikawa, J., Nakao, R., Watanabe, K., Totsuka, T., Nakano, H., Sano, M., and Yasuhara, M. (2003). Calcineurin is a potent regulator for skeletal muscle regeneration by association with NFATc1 and GATA-2. Acta Neuropathol. (Berl) 105, 271–280. [DOI] [PubMed] [Google Scholar]
- Sartorelli, V., Huang, J., Hamamori, Y., and Kedes, L. (1997). Molecular mechanisms of myogenic coactivation by p 300, direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol. Cell. Biol. 17, 1010–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano, B. M., Spath, L., Musaro, A., Molinaro, M., Adamo, S., and Nervi, C. (2002). AVP induces myogenesis through the transcriptional activation of the myocyte enhancer factor 2. Mol. Endocrinol. 16, 1407–1416. [DOI] [PubMed] [Google Scholar]
- Serrano, A. L., Murgia, M., Pallafacchina, G., Calabria, E., Coniglio, P., Lomo, T., and Schiaffino, S. (2001). Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc. Natl. Acad. Sci. USA 98, 13108–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A., Stephen, R. I., Arkley, M. M., and McIntosh, N. (1992). Immunoreactive arginine vasopressin in human fetal and neonatal skeletal muscle. Early Hum. Dev. 28, 215–222. [DOI] [PubMed] [Google Scholar]
- Sternberg, E. A., Spizz, G., Perry, W. M., Vizard, D., Weil, T., and Olson, E. N. (1988). Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol. Cell. Biol. 8, 2896–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti, A., Naro, F., Molinaro, M., and Adamo, S. (1993). Transduction of arginine vasopressin signal in skeletal myogenic cells. Am. J. Physiol 265, C113–C121. [DOI] [PubMed] [Google Scholar]
- Wilkins, B. J., and Molkentin, J. D. (2002). Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J. Physiol 541, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H. et al. (2000). MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 19, 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn, H. D., Chatila, T. A., and Liu, J. O. (2000). Integration of calcineurin and MEF2 signals by the coactivator p300 during T-cell apoptosis. EMBO J. 19, 4323–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]