Abstract
ATP is emerging as an ubiquitous extracellular messenger. However, measurement of ATP concentrations in the pericellular space is problematic. To this aim, we have engineered a firefly luciferase-folate receptor chimeric protein that retains the N-terminal leader sequence and the C-terminal GPI anchor of the folate receptor. This chimeric protein, named plasma membrane luciferase (pmeLUC), is targeted and localized to the outer aspect of the plasma membrane. PmeLUC is sensitive to ATP in the low micromolar to millimolar level and is insensitive to all other nucleotides. To identify pathways for nonlytic ATP release, we transfected pmeLUC into cells expressing the recombinant or native P2X7 receptor (P2X7R). Both cell types release large amounts of ATP (100–200 μM) in response to P2X7R activation. This novel approach unveils a hitherto unsuspected nonlytic pathway for the release of large amounts of ATP that might contribute to spreading activation and recruitment of immune cells at inflammatory sites.
INTRODUCTION
The very low extracellular levels under quiescent conditions, the quick increases caused by many different stimuli, the fast degradation in the extracellular space, and the presence of specific receptors make ATP an ideal extracellular messenger (Zimmermann, 2000; Burnstock, 2004). It is generally acknowledged that ATP is secreted by many different cell types by as yet poorly characterized nonlytic mechanisms (Bodin and Burnstock, 2001; Lazarowski et al., 2003). However, full appreciation of the role of ATP as an extracellular signal has been hampered by lack of proper probes for accurate measurement of the extracellular concentration. The prototypic ATP probe is firefly luciferase, a bioluminescent ATP-dependent enzyme that can detect ATP in the pico–millimolar range. Luciferase is mostly used to assay the ATP concentration in cell-free supernatants after cell or tissue stimulation. This procedure, albeit technically very simple, involves manipulations that can cause cell rupture or unwanted stimulation (sampling, centrifugation, recovery of the supernatants). Furthermore, these off-line measurements do not allow detection of rapidly changing localized ATP transients close to the surface of the plasma membrane. Previous observations have clearly shown that ATP levels measured in the proximity of the plasma membrane surface can be up to 10- to 20-fold higher than those measured in the bulk solution by the soluble luciferase assay (Beigi et al., 1999).
To provide a simple and reliable tool to measure ATP in the pericellular space, we have generated a novel probe by appending to luciferase the targeting sequences (leader sequence and GPI anchor) derived from the folate receptor. This chimeric protein, encoded by an appropriately designed cDNA, is targeted to the plasma membrane and detects ATP in the extracellular milieu close to the cell surface. Thanks to this novel probe, we have also been able to identify a novel pathway for ATP release.
MATERIALS AND METHODS
Reagents
Benzoyl ATP (BzATP), oxidized ATP (oATP), DMEM, DMEM-F12, and MEM nonessential amino acid solution 100× were from Sigma-Aldrich (St. Louis, MO). ATP, ADP, UTP, UDP, and GTP were purchased from Boehringer-Roche Diagnostics (Mannheim, Germany). Luciferin used for ATP measurements with pmeLUC was from DUCHEFA Biochemie (Amsterdam, The Netherlands). Luciferin-luciferase solutions for ATP measurements with the Firezyme luminometer were from Promega (Madison, WI). Dithiothreitol (DTT) was purchased from Merck (Darmstadt, Germany). All experiments were performed in a saline solution containing: 135 mM NaCl, 5 mM KCl, 0.5 mM KH2PO4, 1 mM MgSO4, 1 mM CaCl2, 5.5 mM glucose, and 20 mM HEPES, pH 7.4, at 37°C.
Engineering of pmeLUC
Plasma membrane luciferase (pmeLUC) was obtained as follows: luciferase cDNA was amplified from a modified pGL3 plasmid (kind gift of Dr. Guy Rutter, University of Bristol, United Kingdom) using the following primers: 5′-CCC TGC AGA TGG AAG ACG CCA AAA ACA TAA AGA AAG G-3′ (corresponding to the sequence encoding amino acids 1–9 of luciferase; PstI site underlined) and 5′-GCT GCA GCC ACG GCG ATC TTT CCG CCC TTC TTG G-3′ (including amino acids 542–549 of luciferase cDNA without the stop codon; PstI site underlined). The PCR product was transferred to pBSK+ vector (Stratagene, La Jolla, CA), digested with the enzyme PstI and inserted in the right frame between a PstI fragment encoding the complete N-terminal leader sequence of the human folate receptor (26 aa) fused with myc tag (10 aa) and a PstI fragment of the GPI anchor protein (28 aa), to generate the construct shown in Figure 1A. The whole final construct was excised by a NotI/XhoI or XbaI digestion and cloned into the expression vectors pcDNA3 or VR1012, respectively. The clone was checked by sequence analysis carried out on service at the BioMolecular Research sequencing core of the CRIBI-University of Padova.
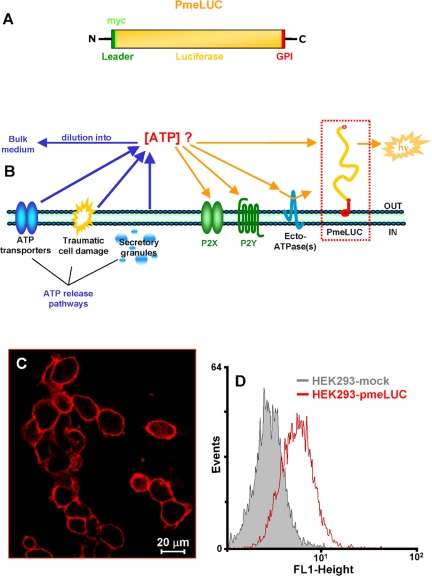
Figure 1.
Structure and localization of the pmeLUC construct. The structure comprising the full-length coding sequence of luciferase inserted in-frame between the N-terminal, leader sequence (26 aa) and the C-terminal GPI anchor (28 aa) of the folate receptor (A). Schematic rendering of the plasma membrane localization of pmeLUC (B). (C) Immunofluorescence and (D) FACS analysis of HEK293 cells transfected with pmeLUC (HEK293-pmeLUC) or with the empty vector (HEK293-mock)
Cell Transfection
HEK293 cells were cultured in DMEM-F12 (Sigma-Aldrich). Media were complemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen, San Giuliano Milanese, Italy). ACN neuroblastoma cells were cultured in DMEM supplemented with MEM nonessential amino acid solution 100× (Sigma-Aldrich). HEK293 cells were transfected with the calcium phosphate method. Cells transiently expressing the pmeLUC construct were assayed 36 h after transfection. We also generated clones stably expressing pmeLUC or the P2X7R by culture of the transfected cells in the presence of G418 (0.8 mg/ml, added 48 h after transfection) for 3 wk. Stable P2X7- or pmeLUC-expressing clones were kept in the continuous presence of 0.2 mg/ml G418 sulfate (Geneticin; Calbiochem, La Jolla, CA). ACN cells were transfected with pmeLUC by Lipofectamine (Invitrogen) and tested 24 h after transfection. Briefly, cells were incubated in 250 μl serum-free transfection medium (OPTIMEM) in the presence of Lipofectamine-plus-DNA (0.4 μg per well). After a 3-h incubation, 1 ml of DMEM plus 10% FBS supplemented with MEM nonessential amino acid solution 100× (Sigma-Aldrich) was added. Cells were assayed 24 h after transfection. To allow a high level of plasma membrane expression of the transfected constructs, cells were incubated overnight in the presence of 1 mM DTT (Mezghrani et al., 2001). Furthermore, they were also kept at room temperature (21°C) for 2 h before transfer into the thermostated luminometer chamber. These treatments that did not perturb luciferase activity or P2X7 function maximized pmeLUC surface expression by improving transport to the plasma membrane and slowing down recycling.
Immunofluorescence
HEK293 cells, seeded onto 24-mm coverslips, were fixed with 4% formaldehyde in phosphate-buffered saline (PBS) solution for 30 min, permeabilized with 0.2% Triton X-100 for 5 min at room temperature, rinsed three times with PBS, and incubated for 30 min with 0.2% gelatin in PBS to block nonspecific binding sites. Immunostaining was carried out for 1 h at 37°C with a commercial monoclonal antibody (mAb) against the c-myc epitope tag (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:100 dilution in 0.2% gelatin in PBS. Immunodetection was carried out using Texas Red-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) used at 1:50 dilution in 0.2% gelatin in PBS. After immunostaining, cells were imaged with a Zeiss LSM 510 Confocal Laser Scanning Microscope (Thornwood, NY).
ATP Measurement
ATP was measured in the custom-made luminometer described by Rizzuto and coworkers (Brini et al., 1999; Jouaville et al., 1999). For experiments, cells plated onto 13-mm coverslips were placed in a 37°C thermostatted chamber (diameter 15 mm, height 2 mm) and perfused with a saline solution supplemented with luciferin at a concentration of 5 μM. The chamber was held in a photomultiplier kept in a dark refrigerated (4°C) box. Light emission was detected by a Thorn EMI photon counting board installed in an IBM-compatible computer. The board allowed storing of the data in the computer memory for further analysis. During the experiments the thermostatted chamber was continuously perfused with buffer by means of a Gilson peristaltic pump. Alternatively, ATP was measured in the supernatants using soluble luciferase in a Firezyme luminometer as previously described (Baricordi et al., 1999; Solini et al., 2004).
FACS Analysis
Nonpermeabilized HEK293 cells stably transfected with pmeLUC or with the empty vector were labeled with the mouse mAb (Santa Cruz Biotechnology) directed against the pmeLUC c-myc tag at a 1:100 dilution in PBS for 1 h at 4°C. At the end of this incubation, cells were incubated with FITC-conjugated anti-mouse antibody at a 1:50 dilution in PBS for 1 h at 4°C. Fluorescence emission was analyzed with a single argon laser cytofluorometer FACS Scan Vantage (Becton Dickinson, Franklin Lakes, NJ).
RESULTS
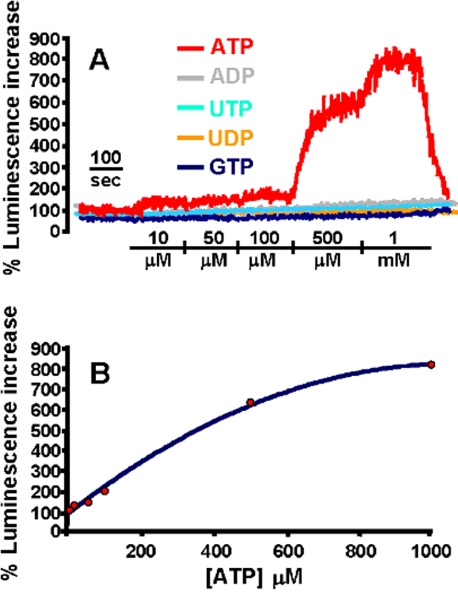
The schematic structure of the novel ATP probe (heretofore referred to as pmeLUC) is shown in Figure 1A. This chimeric protein, thanks to the folate receptor leader sequence, is targeted to the plasma membrane and detects ATP in the extracellular milieu close to the cell surface (Figure 1B). Immunofluorescence and FACS analysis of cells transfected with this construct confirm that pmeLUC is expressed on the plasma membrane (Figure 1, C and D). Cells expressing pmeLUC have a basal level of luminescence emission that depends on the amount of expressed luciferase; however, in the stable HEK293-pmeLUC clones generated by us and used for experiments, basal luminescence emission was comprised within a fairly narrow cps (counts per second) range (i.e., from a lower emission of ∼2500 cps, to a higher of ∼3200 cps). To minimize variations due to minor changes in pmeLUC expression luminescence can be expressed as percent increase over basal, as shown in Figure 2A, where HEK293-pmeLUC cells are challenged with different nucleotides in order to test affinity and selectivity of the probe. Affinity of pmeLUC for ATP is rather low, with a threshold of ∼10 μM; however, subsequent ATP additions elicit further increases in light emission that allow building a calibration curve (Figure 2B). Importantly, pmeLUC is insensitive to all other nucleotides tested (ADP, UTP, UDP, and GTP; Figure 2A). To check for ability of pmeLUC to monitor ATP release triggered by receptor-directed stimuli, we challenged the HEK293-pmeLUC cells with various agonists of G protein-coupled receptors (e.g., carbachol, histamine, bradykinin), obtaining negligible ATP release (unpublished data).
Figure 2.
Response of HEK293-pmeLUC to extracellular nucleotides (A) and ATP calibration curve (B). HEK293-pmeLUC monolayers were placed in the luminometer chamber as described in Materials and Methods and were then perfused with solutions containing increasing concentrations of nucleotides. Basal cps before addition of the nucleotides ranged between 2500 and 3200. Luminescence increase is shown as percent increase over basal. In B, luminescence increase is correlated to the ATP concentration to build a calibration curve.
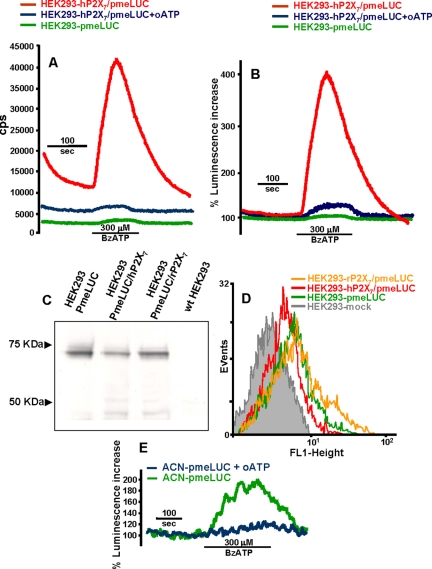
Early experiments from our and other laboratories had previously shown that supernatants from cells expressing the P2X7 receptor contained a high level of ATP, which was not due to an accelerated rate of cell lysis (Baricordi et al., 1999; Adinolfi et al., 2003; Solini et al., 2004). This suggested to us that the P2X7R might be one of the pathways mediating ATP translocation across the plasma membrane. To test this hypothesis, we generated several HEK293 clones stably transfected with the human or rat P2X7R (HEK293-hP2X7 or HEK293-rP2X7, respectively). These clones were then transfected with pmeLUC (HEK293-P2X7 /pmeLUC). As shown in Figure 3A, the HEK293-hP2X7/pmeLUC cells exhibit a severalfold higher level of basal fluorescence compared with HEK293-pmeLUC (12250 ± 1500 vs. 2300 ± 850 cps, respectively, n = 8). Addition of BzATP, a potent P2X7 agonist, triggers a large luminescence increase in the HEK293-hP2X7/pmeLUC or HEK293-rP2X7/pmeLUC (only HEK293-hP2X7/pmeLUC shown), but not in the HEK293-pmeLUC. The luminescence increase triggered by BzATP is fully blocked by pretreatment with oxidized ATP (oATP), a powerful and irreversible blocker of the P2X7R (Murgia et al., 1993). To rule out a possible inhibitory effect of oATP on the luciferase itself, we also performed a calibration in the presence and absence of this inhibitor, showing that the ATP-dependent luminescence increase is not affected (unpublished data but see also Figure 4). Calibration of BzATP triggered luminescence increase yields a peak ATP concentration of ∼250 μM.
Figure 3.
ATP release occurs through the P2X7 receptor. (A and B) HEK293 cells cotransfected with hP2X7 and pmeLUC (HEK293-hP2X7/pmeLUC) were placed in the luminometer chamber and perfused with a BzATP-containing solution, with (blue trace) or without (red trace) prior treatment with 300 μM oATP for 2 h. HEK293-pmeLUC (green trace) are shown as a control. Luminescence increase is expressed as cps in A and percent increase over basal in B. In C, total pmeLUC protein expressed in HEK293-pmeLUC, HEK293-hP2X7/pmeLUC, and HEK293-rP2X7/pmeLUC is determined by Western blotting. wtHEK293 are shown as a control. In D, plasma membrane-expressed pmeLUC is measured by FACS analysis in the three cell populations. ATP release from human neuroblastoma ACN cells incubated in the absence (green trace) or presence (blue trace) of 300 μM oATP is shown in E.
Figure 4.
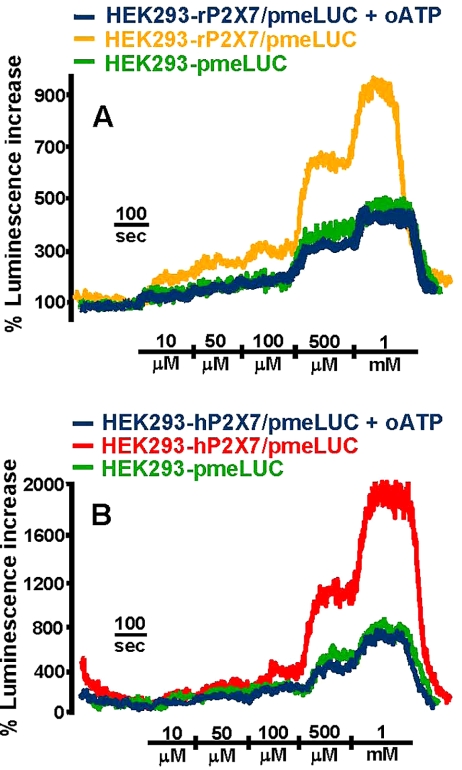
Extracellular ATP triggers release of ATP from P2X7-expressing cells. HEK293 cells transfected with the rat (A) or human (B) P2X7R were placed in the luminometer chamber and perfused with increasing ATP concentrations. HEK293-rP2X7/pmeLUC yellow trace; HEK293-rP2X7/pmeLUC preincubated with 300 μM oATP, blue trace; HEK293-pmeLUC, green trace. HEK293-hP2X7/pmeLUC, red trace; HEK293-hP2X7/pmeLUC preincubated with 300 μM oATP, blue trace; HEK293-pmeLUC, green trace.
Differences in basal levels of luminescence emission are neutralized by expressing luminescence as percentage increase over basal (Figure 3B); however, the higher basal luminescence of HEK293-hP2X7/pmeLUC might reflect a real increased level of pericellular ATP compared with HEK293-pmeLUC, rather than a higher expression of pmeLUC. If this were the case, reporting luminescence as percent increase over basal would mask such a difference in the extracellular ATP concentration. To clarify this issue, we measured total pmeLUC protein by western blotting (Figure 3C). Blots show that, although there is some variability in protein expression in the different stable transfectants, HEK293-hP2X7/pmeLUC or HEK293-rP2X7/pmeLUC have if anything a lower content of luciferase than that of HEK-pmeLUC, and this cannot account for the higher basal luminescence emission in the P2X7-transfected clones. To measure quantitatively surface pmeLUC expression, we analyzed the different clones by FACS. As shown in Figure 3D, pmeLUC expression profile of HEK293-hP2X7/pmeLUC, HEK293-rP2X7/pmeLUC, and HEK293-pmeLUC closely overlaps. Therefore, in keeping with previous findings (Baricordi et al., 1999; Solini et al., 2004), these data suggest that cells expressing the P2X7R maintain a higher pericellular ATP concentration. Reduction of basal luminescence by oATP pretreatment (5800 ± 1300 cps, n = 8) also supports this interpretation. Finally, we tested the effect of BzATP on the human ACN neuroblastoma, a cell line expressing the native P2X7R (Raffaghello, Pistoia, Di Virgilio, unpublished observations). As shown in Figure 3E, also in this case BzATP induces a large ATP release that is fully blocked by oATP. Basal luminescence levels in this cell line were 3500 ± 350 and 1500 ± 260 cps (n = 5), before and after treatment with oATP, respectively, further supporting the finding that cells expressing a functional P2X7R maintain a higher ATP concentration in the pericellular space.
As an independent proof that HEK293-P2X7 release a larger amount of ATP than mock-transfected HEK293 (HEK293-mock), we measured extracellular ATP in the supernatants using soluble luciferase. Quiescent HEK293-mock maintained an average extracellular ATP concentration of 80 ± 20 nM (n = 12), compared with 220 ± 34 nM (n = 10) for HEK-hP2X7. Addition of BzATP had no effect on the HEK293-mock, but increased extracellular ATP to ∼400 ± 55 nM (n = 10) in the HEK293-hP2X7 supernatants.
Like BzATP, ATP itself should trigger ATP release in the HEK293-P2X7/pmeLUC cells, because, albeit at high concentrations, ATP is the only known physiological activator of P2X7 (Di Virgilio et al., 2001; North, 2002). If so, then ATP addition to HEK293-P2X7/pmeLUC should trigger an extra increase in luminescence compared with HEK293-pmeLUC. The extra increase in luminescence should be due to ATP release via the P2X7R. Figure 4 shows that this is the case, whether the transfected receptor is the human or rat P2X7R. Interestingly, in the HEK293 cells transfected with the rat receptor (Figure 4A) the extra increase in luminescence emission (expressed as percent increase over basal) is detectable already at the lowest ATP concentration used (10–50 μM), whereas in cells transfected with the human ortholog (Figure 4B) a luminescence increase over control cells is detectable only at ATP concentrations higher than 100 μM. This is in keeping with the known lower affinity for ATP of the human receptor (Surprenant et al., 1996; Rassendren et al., 1997). If the extra luminescence observed in the HEK293-P2X7/pmeLUC cells is due to ATP release via P2X7, then it should be abolished by pretreatment with oATP. This prediction is fulfilled, because in the oATP-treated cells the luminescence increase matches exactly that of HEK293-pmeLUC (Figure 4, A and B).
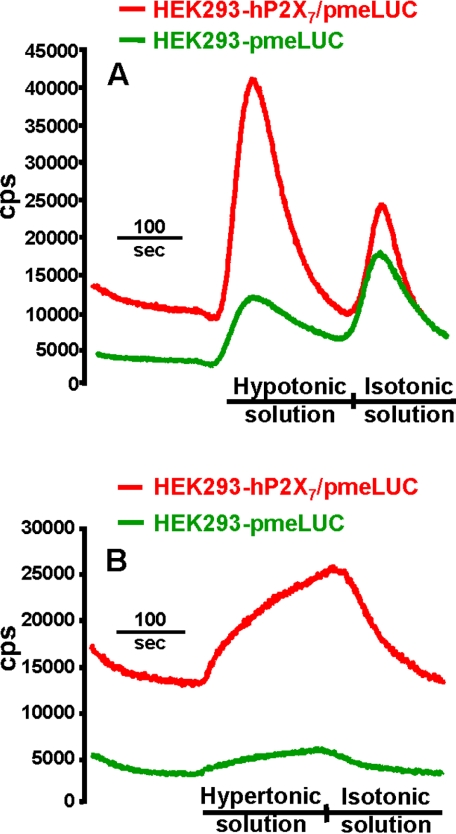
One of the most potent stimuli for ATP secretion is plasma membrane stretching. To test ability of pmeLUC to detect stretch-induced ATP release, we exposed HEK293-P2X7/pmeLUC and HEK293-pmeLUC to a change in tonicity of the perfusion buffer. Cell monolayers were initially perfused with the usual isotonic solution used in all the experiments and then switched to a hypotonic buffer. The tonicity shift causes a clearly detectable release of ATP from both clones (Figure 5A). However, ATP release is severalfold higher in the P2X7-transfected cells. These cells, as shown in the previous experiments, also maintain a higher basal pericellular ATP level with respect to HEK293-pmeLUC (14200 ± 2300 vs. 3150 ± 970 cps, n = 7). Interestingly, a burst of ATP release is triggered both by a shift from iso- to hypotonicity and from hypo- to isotonicity, as well as from a shift from iso- to hypertonicity (Figure 5B). These findings suggest that, although stretch-induced ATP release can occur independently of the P2X7R, it is strongly potentiated by expression of this receptor.
Figure 5.
Expression of the P2X7R enhances ATP release triggered by membrane stretching. (A) HEK293-hP2X7/pmeLUC (red trace) and HEK293-pmeLUC (green trace) were placed in the luminometer chamber and perfused with isotonic buffer followed by a hypotonic solution obtained by diluting the standard saline buffer with distilled water (1–4; final tonicity 78 mOsm/l), followed again by isotonic buffer. (B) HEK293-hP2X7/pmeLUC (red trace) and HEK293-pmeLUC (green trace) were perfused with isotonic buffer followed by a hypertonic solution obtained by dissolving 25 ml of sucrose 1 M solution into 75 ml of standard saline (final osmolarity, 560 mOsm/l).
DISCUSSION
ATP is now accepted as an ubiquitous extracellular messenger (Burnstock, 2004; Di Virgilio et al., 2004; Wang et al., 2004). Responses elicited by this nucleotide, depending on the concentration and the given P2R subtype expressed by the target cell, range from chemotaxis (Oshimi et al., 1999) to cell adhesion (Freyer et al., 1988), cytokine release (Perregaux and Gabel, 1994) to neurotransmitter secretion (Illes and Norenberg, 1993), activation of apoptosis (Zanovello et al., 1990) to stimulation of cell proliferation (Neary et al., 2003). Although it is generally agreed that many disease conditions (trauma, inflammation, ischemia) may lead to an increase in the extracellular ATP concentration as a consequence of mere cell lysis, the pathways that support nonlytic ATP release are less clear. Furthermore, there is an on-going debate on the actual concentration that ATP may reach in the extracellular space (Ferrari et al., 1997; Beigi et al., 1999; Newman, 2001). Most measurements are performed by using the standard luciferin/luciferase assay in off- or on-line settings. All off-line techniques measure ATP in the cell-free supernatants and therefore after ATP has diffused and equilibrated into the incubation medium. On-line measurements give a more accurate estimate of the actual ATP levels reached close to the site of release, but still involve manipulations that may seriously affect the measurements. A protein A-luciferase chimera was engineered by Dubyak and coworkers to detect local ATP release at the membrane level (Beigi et al., 1999). Use of this probe involves coating of the cell surface with IgG to allow binding of the protein A-luciferase chimera. The cell-attached probe yielded an ATP release from thrombin-stimulated platelets 10- to 15-fold higher than those recorded by soluble luciferase under similar experimental conditions. Biosensor methods based on the opening of P2X channels expressed by a sensor cell placed in the vicinity of a target cell releasing ATP have also been used (Brown and Dale, 2002; Hayashi et al., 2004).
The technique that we have introduced has some advantages over methods so far available: 1) pmeLUC is expressed as a plasma membrane protein, thus exposed to the very environment in which we aim to measure ATP; 2) pmeLUC can now be engineered to be targeted to virtually any plasma membrane region, thus allowing the measurement of extracellular ATP at discrete plasma membrane sites; 3) genetic manipulation may allow to measure with this technique ATP levels in vivo. Of course there are drawbacks: in the first place the need for transfection, which poses a limit to the cell types that may be investigated by this mean; second, the low affinity of pmeLUC that allows measurement only above the 5–10 μM ATP level.
HEK293-pmeLUC transfectants did not appreciably release ATP in response to most stimuli applied. However it is well known that HEK293 cells express few endogenous receptors for extracellular ligands, which incidentally also makes these cells a good model for transfection studies. Expression of the P2X7R on the contrary endows these cells with the ability to release large amounts of ATP in response to BzATP or ATP itself. The kinetic of BzATP-stimulated release is transient, reaching a peak within 2 min from the addition and then rapidly declining to near basal level. This kinetic may be surprising because it is well known that the P2X7 is a nondesensitizing receptor; thus one would expect that so far the receptor stays open ATP should efflux. However, it is also likely that ATP release is self-limiting because of local restrictions to the availability of the nucleotide in the cytoplasmic region faced by the receptor. Because the P2X7 pore is nonselective, we expect that virtually all cytoplasmic solutes below 900 Da and not restricted in their movement through the cytoplasm should also efflux via P2X7. Studies reporting that glutamate permeates across the P2X7R show that this might well be the case (Duan et al., 2003).
Opening of the P2X7 pore allows to reach a periplasmalemmal ATP concentration in the hundred micromolar range, sufficient to activate even the low-affinity P2X7R. The high concentration reached by ATP in the environment sensed by pmeLUC, especially if compared with the much lower levels detected by standard luciferase assay, at first sight might be surprising. However, it does not substantially differ from the levels (70–80 μM) measured in the pericellular space by single-cell analysis of mechanically stimulated astrocytoma cells bathed in a luciferin/luciferase-containing medium (Newman, 2001). Local release of such a high amount of ATP supports the hypothesis that once an initial event triggers ATP release to a level sufficient to activate P2X7, neighbor cells expressing this receptor (mainly inflammatory cells) may function as amplification devices by sustaining a process of ATP-induced ATP release. A similar mechanism was recently proposed for the propagation of interastrocyte Ca2+ waves (Anderson et al., 2004). In the setting of inflammation this might be instrumental for spreading activation and recruitment of inflammatory cells.
Increasing attention is paid to those signals (danger or alert signals) that alert the immune system during the early phases of tissue damage or pathogen invasion (Matzinger, 2002; la Sala et al., 2003; Skoberne et al., 2004). Intracellular nucleotides are considered likely candidates to this role for their ubiquitous distribution, high intracellular concentration, negligible extracellular levels under quiescent conditions, presence of specific receptors, and ability to modulate dendritic cell differentiation. The additional feature described here, unveiling a nonlytic and self sustaining release mechanism, makes ATP an even more appealing danger signal.
In conclusion, we have described a novel technique for measuring the peri-plasmalemmal ATP concentration and identified a novel pathway for ATP release.
Acknowledgments
This work was supported by grants from the Ministry of Education and Scientific Research (Cofin, FIRB), the Italian Association for Cancer Research (AIRC), Telethon of Italy, and institutional funds from the University of Ferrara.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–03–0222) on June 8, 2005.
Abbreviations used: PmeLUC, plasma membrane luciferase; P2X7R, P2X7 receptor; BzATP, benzoyl ATP; oATP, oxidized ATP; DTT, dithiothreitol; HEK293-hP2X7, HEK293 cells transfected with the human P2X7R; HEK293-rP2X7, HEK293 cells transfected with the rat P2X7R; HEK293-P2X7/pmeLUC, HEK293 cells transfected with both P2X7R and pmeLUC; HEK293-mock, mock-transfected HEK293 cells.
References
- Adinolfi, E., Kim, M., Young, M. T., Di Virgilio, F., and Surprenant, A. (2003). Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J. Biol. Chem. 278, 37344–37351. [DOI] [PubMed] [Google Scholar]
- Anderson, C. M., Bergher, J. P., and Swanson, R. A. (2004). ATP-induced ATP release from astrocytes. J. Neurochem. 88, 246–256. [DOI] [PubMed] [Google Scholar]
- Baricordi, O. R., Melchiorri, L., Adinolfi, E., Falzoni, S., Chiozzi, P., Buell, G., and Di Virgilio, F. (1999). Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J. Biol. Chem. 274, 33206–33208. [DOI] [PubMed] [Google Scholar]
- Beigi, R., Kobatake, E., Aizawa, M., and Dubyak, G. R. (1999). Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. Cell Physiol. 276, C267–C278. [DOI] [PubMed] [Google Scholar]
- Bodin, P., and Burnstock, G. (2001). Purinergic signalling: ATP release. Neurochem. Res. 26, 959–969. [DOI] [PubMed] [Google Scholar]
- Brini, M., Pinton, P., King, M. P., Davidson, M., Schon, E. A., and Rizzuto, R. (1999). A calcium signaling defect in the pathogenesis of a mitochondrial DNA inherited oxidative phosphorylation deficiency. Nat. Med. 5, 951–954. [DOI] [PubMed] [Google Scholar]
- Brown, P., and Dale, N. (2002). Spike-independent release of ATP from Xenopus spinal neurons evoked by activation of glutamate receptors. J. Physiol. 540, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock, G. (2004). Introduction: P2 receptors. Curr. Top. Med. Chem. 4, 793–803. [DOI] [PubMed] [Google Scholar]
- Di Virgilio, F., Chiozzi, P., Ferrari, D., Falzoni, S., Sanz, J. M., Morelli, A., Torboli, M., Bolognesi, G., and Baricordi, O. R. (2001). Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood 97, 587–600. [DOI] [PubMed] [Google Scholar]
- Di Virgilio, F., Baricordi, O. R., Romagnoli, R., and Baraldi, P. G. (2004). Leukocyte P2 receptors: a novel target for anti-inflammatory and anti-tumour therapy. Curr. Drug Targets 5, 85–99. [DOI] [PubMed] [Google Scholar]
- Duan, S., Anderson, C. M., Keung, E. C., Chen, Y., Chen, Y., and Swanson, R. A. (2003). P2X7 Receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 23, 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari, D., Chiozzi, P., Falzoni, S., Hanau, S., and Di Virgilio, F. (1997). Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 185, 579–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer, D. R., Boxer, L. A., Axtell, R. A., and Todd, R. F., III. (1988). Stimulation of human neutrophil adhesive properties by adenine nucleotides. J. Immunol. 141, 580–586. [PubMed] [Google Scholar]
- Hayashi, S., Hazama, A., Dutta, A. K., Sabirov, R. Z., and Okada, Y. (2004). Detecting ATP release by a biosensor method. Sci. STKE 258, 114. [DOI] [PubMed] [Google Scholar]
- Illes, P., and Norenberg, W. (1993). Neuronal ATP receptors and their mechanism of action. Trends Pharmacol. Sci. 14, 50–54. [DOI] [PubMed] [Google Scholar]
- Jouaville, L. S., Pinton, P., Bastianutto, C., Rutter, G. A., and Rizzuto, R. (1999). Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. USA 96, 13807–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- la Sala, A., Ferrari, D., Di Virgilio, F., Idzko, M., Norgauer, J., and Girolomoni, G. (2003). Alerting and tuning the immune response by extracellular nucleotides. J. Leukoc. Biol. 73, 339–343. [DOI] [PubMed] [Google Scholar]
- Lazarowski, E., Boucher, R. C., and Harden, T. K. (2003). Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 64, 785–795. [DOI] [PubMed] [Google Scholar]
- Matzinger, P. (2002). The danger model: a renewed sense of self. Science 296, 301–305. [DOI] [PubMed] [Google Scholar]
- Mezghrani, A., Fassio, A., Benham, A., Simmen, T., Braakman, I., and Sitia, R. (2001). Manipulation of oxidative protein folding and PDI redox state in mammalian cells. EMBO J. 20, 6288–6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia, M., Hanau, S., Pizzo, P., Rippa, M., and Di Virgilio, F. (1993). Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J. Biol. Chem. 268, 8199–8203. [PubMed] [Google Scholar]
- Neary, J. T., Kang, Y., Willoughby, K. A., and Ellis, E. F. (2003). Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J. Neurosci. 23, 2348–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman, E. A. (2001). Propagation of Intercellular Calcium Waves in Retinal Astrocytes and Muller Cells. J. Neurosci. 21, 2215–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North, R. A. (2002). Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067. [DOI] [PubMed] [Google Scholar]
- Oshimi, Y., Miyazaki, S., and Oda, S. (1999). ATP-induced Ca2+ response mediated by P2U and P2Y purinoceptors in human macrophages: signalling from dying cells to macrophages. Immunology 98, 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perregaux, D., and Gabel, C. A. (1994). Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203. [PubMed] [Google Scholar]
- Rassendren, F., Buell, G. N., Virginio, C., Collo, G., North, R. A., and Surprenant, A. (1997). The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 272, 5482–5486. [DOI] [PubMed] [Google Scholar]
- Skoberne, M., Beignon, A. S., and Bhardwaj, N. (2004). Danger signals: a time and space continuum. Trends. Mol. Med. 10, 251–257. [DOI] [PubMed] [Google Scholar]
- Solini, A., Chiozzi, P., Morelli, A., Adinolfi, E., Rizzo, R., Baricordi, O. R., and Di Virgilio, F. (2004). Enhanced P2X7 activity in human fibroblasts from diabetic patients: a possible pathogenetic mechanism for vascular damage in diabetes. Arterioscler. Thromb. Vasc. Biol. 24, 1240–1245. [DOI] [PubMed] [Google Scholar]
- Surprenant, A., Rassendren, F., Kawashima, E., North, R. A., and Buell, G. (1996). The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272, 735–738. [DOI] [PubMed] [Google Scholar]
- Wang, X., Arcuino, G., Takano, T., Lin, J., Peng, W. G., Wan, P., Li, P., Xu, Q., Liu, Q. S., Goldman, S. A., and Nedergaard, M. (2004). P2X7 receptor inhibition improves recovery after spinal cord injury. Nat. Med. 10, 821–827. [DOI] [PubMed] [Google Scholar]
- Zanovello, P., Bronte, V., Rosato, A., Pizzo, P., and Di Virgilio, F. (1990). Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. J. Immunol. 145, 1545–1550. [PubMed] [Google Scholar]
- Zimmermann, H. (2000). Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch. Pharmacol. 362, 299–309. [DOI] [PubMed] [Google Scholar]