Abstract
The spindle checkpoint coordinates cell cycle progression and chromosome segregation by inhibiting anaphase promoting complex/cyclosome until all kinetochores interact with the spindle properly. During early mitosis, the spindle checkpoint proteins, such as Mad2 and Bub1, accumulate at kinetochores that do not associate with the spindle. Here, we assess the requirement of various kinetochore components for the accumulation of Mad2 and Bub1 on the kinetochore in fission yeast and show that the necessity of the Mis6-complex and the Nuf2-complex is an evolutionarily conserved feature in the loading of Mad2 onto the kinetochore. Furthermore, we demonstrated that Nuf2 is required for maintaining the Mis6-complex on the kinetochore during mitosis. The Mis6-complex physically interacts with Mad2 under the condition that the Mad2-dependent checkpoint is activated. Ectopically expressed N-terminal fragments of Mis6 localize along the mitotic spindle, highlighting the potential binding ability of Mis6 not only to the centromeric chromatin but also to the spindle microtubules. We propose that the Mis6-complex, in collaboration with the Nuf2-complex, monitors the spindle–kinetochore attachment state and acts as a platform for Mad2 to accumulate at unattached kinetochores.
INTRODUCTION
The kinetochore is a multiprotein complex that is formed on centromeric DNA and serves as an interface connecting the spindle and the chromosome during mitosis. From prometaphase to metaphase, the kineotochore acts as a sensor of spindle microtubule (MT) association and regulates the onset of anaphase via the mitotic checkpoint. The mitotic checkpoint detects aberrations in the kinetochore–spindle interaction during early mitosis, transmits inhibitory signals to anaphase promoting complex/cyclosome (APC/C), and thus prevents the metaphase–anaphase transition until all chromosomes have established a bipolar attachment with the spindle (Cleveland et al., 2003). Genes involved in the mitotic checkpoint are evolutionarily conserved, and many of them are implicated in tumorigenesis (Cahill et al., 1998). At the early stage of mitosis, the key components of the mitotic checkpoint, such as Mad2, Bub1, and BubR1, accumulate on kinetochores that are not properly connected with the spindle (Chen et al., 1996; Li and Benezra, 1996; Taylor and McKeon, 1997; Taylor et al., 1998). It has been proposed that Mad2, along with its binding partner Mad1, recognizes and localizes to unattached kinetochores that are not captured by the spindle, whereas Bub1 and BubR1 attach to tension-less kinetochores that are not pulled by the spindle in both directions (Waters et al., 1998; Skoufias et al., 2001). In vertebrate cells, kinetochore components Mis6/CENP-I protein and the Nuf2-complex have been reported to be required for kinetochore accumulation of Mad2 (Martin-Lluesma et al., 2002; DeLuca et al., 2003; Hori et al., 2003; Liu et al., 2003). However, the molecular basis of this accumulation is still largely unknown; particularly, the kinetochore components that accept the checkpoint proteins remain to be identified.
Comprehensive studies in budding yeast revealed that the kinetochore can be dissected into discrete subcomplexes (Cheeseman et al., 2002a,b; De Wulf et al., 2003; Westermann et al., 2003). Based on studies in human and the fission yeast Schizosaccharomyces pombe, at least four well-characterized kinetochore subcomplexes are evolutionarily conserved: the CENP-A–containing nucleosomes, the Mis6-complex, the Mis12-complex, and the Nuf2-complex (Takahashi et al., 2000; Nabetani et al., 2001; Wigge and Kilmartin, 2001; Hayashi et al., 2004; Obuse et al., 2004). CENP-A is a centromere-specific histone H3 variant. Kinetochore localization of SpCENP-A (gene name is cnp1+) requires the Mis6-complex in fission yeast (Takahashi et al., 2000; Hayashi et al., 2004). Fission yeast Mis6, which is homologous to Mis6/LRPR1/CENP-I in vertebrates (Saitoh et al., 1997; Nishihashi et al., 2002; Liu et al., 2003), forms a complex with Mis15, Mis17, and Sim4 (Pidoux et al., 2003; Hayashi et al., 2004). Sim4 seems to be a homologue of vertebrate CENP-H (Pidoux et al., 2003). Another conserved kinetochore protein, Mis12, which is dispensable for CENP-A loading (Takahashi et al., 2000; Goshima et al., 2003), forms a complex with Mis13 and Mis14 (Hayashi et al., 2004; Obuse et al., 2004), but not with Mis6 (Goshima et al., 1999). The Mis6-complex, the Mis12-complex and the CENP-A–containing nucleosomes constitutively localize on the kinetochore.
Nuf2 was originally identified in budding yeast as a component of the spindle pole body (SPB) (Osborne et al., 1994; Wigge et al., 1998), which is equivalent to the centorosome in higher eukaryotes. In addition, Nuf2 was found to be a component of the kinetochore (Wigge and Kilmartin, 2001). Nuf2 forms a protein complex containing Ndc80/Hec1 (Janke et al., 2001; Wigge and Kilmartin, 2001), whose expression is elevated in cancer cell lines (Chen et al., 1997). Unlike three constitutive kinetochore subcomplexes mentioned above, the vertebrate Nuf2-complex localizes on the kinetochore in a cell cycle-dependent manner (Howe et al., 2001; Nabetani et al., 2001); it localizes at the kinetochore during mitosis, and localizes at the centrosome during G1 and S phase (Hori et al., 2003). Fission yeast Nuf2 is required for clustering of the centromeres near the SPB during interphase (Appelgren et al., 2003) and for proper chromosome segregation in mitosis (Nabetani et al., 2001; Appelgren et al., 2003). The Nuf2-complex thus seems to be a mitosis-specific component of the kinetochore. Incorporation of such mitosis-specific components may be essential for the kinetochore to gain its mitotic functions, such as the association with the spindle MTs, and monitoring the MT attachment state.
Interestingly, in the fission yeast mutant cells with defects in any of these four kinetochore complexes, the mitotic phases seem to proceed normally, and fatal unequal chromosome segregation consequently occurs; it implies that the checkpoint does not function properly in these mutant cells (Saitoh et al., 1997; Goshima et al., 1999; Takahashi et al., 2000; Nabetani et al., 2001; Pidoux et al., 2003). Here, we thus investigate the involvement of these kinetochore complexes in the mitotic checkpoint signaling pathway and find that the Mad2-dependant checkpoint pathway is specifically impaired in mis6-302 mutant. We also find that both the Mis6-complex and the Nuf2-complex are vital for the Mad2 accumulation at the kinetochore in fission yeast, suggesting that the mechanism for the kinetochore loading of Mad2 seems to be conserved among fission yeast and vertebrates. We demonstrate that the Mis6 complex physically associates with Mad2 under the condition that the checkpoint is activated, whereas the incorporation of the Nuf2-complex stabilizes the kinetochore association of the Mis6-complex during mitosis. Furthermore, we present evidence to suggest that Mis6 possesses the potential to interact with the spindle MTs. Therefore, the Mis6-complex may act as an acceptor for Mad2 at unattached kinetochores and coordinate Mad2 accumulation and the spindle attachment state.
MATERIALS AND METHODS
General Techniques, Strains, and Plasmids
Fission yeast methods and media have been described in Moreno et al. (1991). YES or YPD was used as rich medium and EMM2 with appropriate supplements as minimal medium. For yeast transformation, immunostaining, and 4,6-diamidino-2-phenylindole (DAPI) staining methods, see references in Saitoh et al. (1997). Strains used in this study are listed in Table 1. To construct a conditional sim4+-shut-off strain, linearized pREP81-sim4+-HA plasmid, which contained LEU2 marker and nmt1-81 (Maundrell, 1993) promoter-driven hemagglutinin (HA)-tagged sim4+ gene (designated as nmt-sim4+), was integrated to the genome of a diploid strain one of whose sim4+ was heterozygously replaced with ura4+ marker. After confirmation that the plasmid was inserted next to nmt1+ gene by genomic Southern hybridization, a haploid strain that lacked native sim4+ gene but had nmt-sim4+ was obtained by selecting a Ura+ Leu+ spore after sporulation. To construct a CFP-tub1+ integrated strain, linearized pREP1-CFP-tub1+ plasmid, which contained nmt1-1 promoter-driven cyan fluorescent protein (CFP)-tagged α-tubulin gene (tub1+), was integrated next to nmt1+ locus.
Table 1.
Strains used in this study
| Name | Genotype |
|---|---|
| SP893 | h-leu1 ura4 Δsim4::ura4+nmt-sim4+-HA::LEU2 mis6+-GFP::LEU2 |
| SP1474 | h-leu1 ura4 Δsim4::ura4+nmt-sim4+-HA::LEU2 lys1+::cnp1+-GFP |
| SP894 | h-leu1 ura4 Δsim4::ura4+nmt-sim4+-HA::LEU2 mis12+-GFP::LEU2 |
| SP895 | h-leu1 ura4 Δsim4::ura4+nmt-sim4+-HA::LEU2 mif2+-GFP::kanR |
| SP357 | h-leu1 ura4 Δsim4::ura4+nmt-sim4+-HA::LEU2 nuf2+-GFP::ura4+ |
| SP132 | h-leu1 ura4 sim4+-GFP::ura4+ |
| SP498 | h-leu1 ura4 sim4+-GFP::ura4+nuf2-1::ura4+ |
| SP498 | h-leu1 ura4 mis12+-GFP::LEU2 nuf2-1::ura4+ |
| SP520 | h-leu1 ura4 bub1+-GFP::ura4+ |
| SP220 | h-leu1 mad2+-GFP::LEU2 |
| SP1714 | h-leu1 ura4 mif2+-CFPcerulean::kanRbub1+-GFP::ura4+ |
| SP1715 | h-leu1 mif2+-CFPcerulean::kanRmad2+-GFP::LEU2 |
| SP1756 | h-leu1 ura4 nmt-CFP-tub1+mad2+-GFP::LEU2 |
| SP896 | h-leu1 ura4 Δsim4::ura4+nmt-sim4-HA::LEU2 bub1+-GFP::ura4+ |
| SP897 | h-leu1 ura4 Δsim4::ura4+nmt-sim4-HA::LEU2 mad2+-GFP::LEU2 |
| SP297 | h-leu1 ura4 mis6-302 bub1+-GFP::ura4+ |
| SP303 | h-leu1 ura4 mis6-302 mad2+-GFP::LEU2 |
| SP309 | h-leu1 mis12-537 bub1+-GFP::ura4+ |
| SP300 | h-leu1 ura4 mis12-537 mad2+-GFP::LEU2 |
| SP636 | h-leu1 ura4 Δcnp1::ura4 lys1+::cnp1-1 bub1+-GFP::ura4+ |
| SP522 | h-leu1 ura4 Δcnp1::ura4 lys1+::cnp1-1 mad2+-GFP::LEU2 |
| SP780 | h-leu1 nuf2-1::ura4+bub1+-GFP:: kanR |
| SP770 | h-leu1 nuf2-1::ura4+mad2+-GFP:: kanR |
| SP682 | h-leu1 ura4 bub1+-GFP::ura4+kanR::ctr4prom::mis12+ |
| SP1757 | h-leu1 mad2+-GFP::LEU2 kanR::ctr4prom::mis12+ |
| SP7 | h-leu1 mis6-302 |
| SP248 | h-leu1 ura4 Δmad2::ura4+ |
| SP313 | h-leu1 ura4 Δmad2::ura4+mis6-302 |
| SP1695 | h-leu1 cdc13+-GFP::hph kanR::ctr4prom::mis6+ |
| SP1693 | h-leu1 cdc13+-GFP::hph kanR::ctr4prom::mis12+ |
| SP1612 | h-leu1 cdc13+-GFP::hph |
| SP864 | h-leu1 cut7-477 mis6-FLAG::kanR mad2+-GFP::LEU2 |
| SP1022 | h-leu1 cut9-665 mis6-FLAG::kanR mad2+-GFP::LEU2 |
| SP37 | h-leu1 |
Strains listed were created as described in Materials and Methods and by crossing. We designate a S. pombe gene for CENP-C (SPBC1861.01c) as mif2+, because of its homology to Saccharomyces cerevisiae MIF2 gene.
For tagging of genomic SpCENP-C gene (SPBC1861.01c), cdc13+, bub1+, or mad2+ with GFP::kanR, GFP::hph, CFPcerulean::kanR, PCR-mediated method (Krawchuk and Wahls, 1999) was used. hph is a hygromycin-resistance marker, and a plasmid containing hph (pFA6a-hphMX6) was kindly provided by Drs. P. Hentges and A. Carr (University of Sussex, Sussex, United Kingdom). bub1+-GFP::ura4+, mad2+-GFP::ura4+, Δbub1, Δmad2, mis6-302, mis12-537, cnp1-1, or nuf2-1 was described previously (Takahashi et al., 1994; Bernard et al., 1998; Kim et al., 1998; Takahashi et al., 2000; Nabetani et al., 2001; Ikui et al., 2002; Toyoda et al., 2002). pGP110 plasmid (Nabeshima et al., 1997) contains nmt1-1 promoter (Maundrell, 1993)-driven green fluorescent protein (GFP). To construct plasmids for expressing truncated Mis6 fused with GFP, a portion of mis6+ gene indicated in Figure 7B was amplified by PCR and ligated into pGP110 vector.
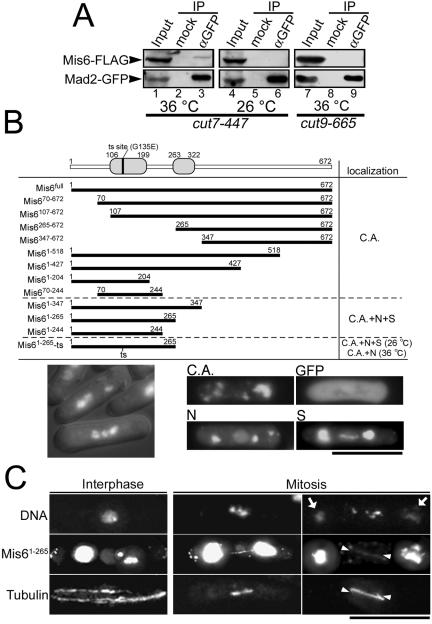
Figure 7.
Mis6 physically interacts with Mad2. (A) Immunoprecipitation experiments were performed using crude extract from cut7-477 or cut9-665 mutant cells harboring integrated mis6+-FLAG and mad2+-GFP genes. The cut7 mutant cells were cultured at either 36°C for 3 h (restrictive condition, lanes 1–3) or 26°C (permissive condition, lanes 4–6). The cut9 mutant cells were cultured at 36°C for 3.5 h (restrictive condition, lanes 7–9). The anti-GFP mAb (Roche Diagnostics) was used for the precipitation of GFP-tagged Mad2 (lanes 3, 6, and 9). In the mock experiment, the buffer was added instead of the antibody (lanes 2, 5, and 8). Twenty percent of the inputs were loaded on lanes 1, 4, and 7. (B) A series of truncated Mis6 were fused with GFP and ectopically expressed under the nmt1-1 promoter, and their subcellular localization was determined. C.A., N, and S stand for cytoplasmic aggregate formation, nuclear localization, and spindle localization, respectively. N-terminal domains that are highly conserved among fission yeast, chicken, and human were indicated by gray boxes in the schematic drawing of Mis6 protein. The mutation site of Mis6-302 temperature-sensitive protein also was indicated by a vertical bar. Representative images of the truncated Mis6 constructs forming cytoplasmic aggregates, localizing in the nuclei or localizing along the mitotic spindle were shown in right bottom panels. An image of ectopically expressed GFP alone, which dispersed throughout the cell, also is presented (GFP). Bottom left, cells expressing Mis61-265 were stained with DAPI. Prometaphase-like cells with hypercondensed chromosomes were frequently observed. (C) The ectopically expressed Mis61-265 localized along the mitotic spindles. Wild-type cells expressing Mis61-265-GFP was immunostained with anti-α-tubulin antibody (TAT1). Chromatin DNA was stained by DAPI. Representative cells in the M phase (middle and right columns) and in the interphase (left column) are presented. GFP fluorescence of the cytoplasmic aggregates was so intense that it leaked into the DAPI channel in some samples (arrows). The position of astral MTs in the mitotic cells was indicated by arrowheads.
Immunoprecipitation
Cell extract was prepared by mixing cells with glass beads vigorously in extraction buffer (50 mM HEPES-Na, 125 mM NaCl, 10% glycerol, 0.5 mM dithiothreitol [DTT] NP-40, 2 mM phenylmethylsulfonyl fluoride, and proteinase inhibitor cocktail; Nacalai Tesque, Kyoto, Japan). The extract was clarified by centrifugation at 18,000 × g for 10 min and mixed with appropriate monoclonal antibody (mAb) and Dynabeads M-280-coupled anti-mouse IgG antibody (Dynal Biotech, Oslo, Norway). After incubation at 4°C, the beads were washed in extraction buffer three times and boiled in SDS-PAGE sample solution.
Living Cell Analysis
S. pombe living cells were cultured in EMM2 and observed by Leica ASMDW live cell imaging system (Leica, Wetzlar, Germany) equipped with a 100× objective lens (numerical aperture 1.4) and a temperature-controlling unit. Cells were cultured at 33°C, and a series of time-lapse images were taken by 0.5-min intervals unless otherwise mentioned. Images were collected every 0.5 μm at z-axis and processed by nonblind deconvolution methods to generate a two-dimensional projection of the three-dimensional images.
H1 Kinase Assay
Two micrograms of total cell extract was mixed with 5 μg of histone H1 (Roche Diagnostics, Basel, Switzerland) and 0.1 mM radiolabeled ATP for each reaction. Reaction was performed in HB buffer containing 25 mM Tris-Cl, pH 7.5, 30 mM NaCl, 60 mM β-glycerophosphate-Na2, 15 mM p-nitrophenylphosphate, 5 mM EGTA, 15 mM MgCl2, 0.1 mM Na2VO3, 1 mM DTT, 0.1% NP-40, and proteinase inhibitors. After 20-min incubation at 22°C, samples were boiled in SDS-PAGE solution and separated on SDS-PAGE. The amount of radioactivity incorporated to histone H1 was measured with Typhoon (Amersham Bioscience, Piscataway, NJ).
RESULTS
The Kinetochore Accumulation of Mad2 Requires Mis6-Sim4 and Nuf2 but Not SpCENP-A or Mis12
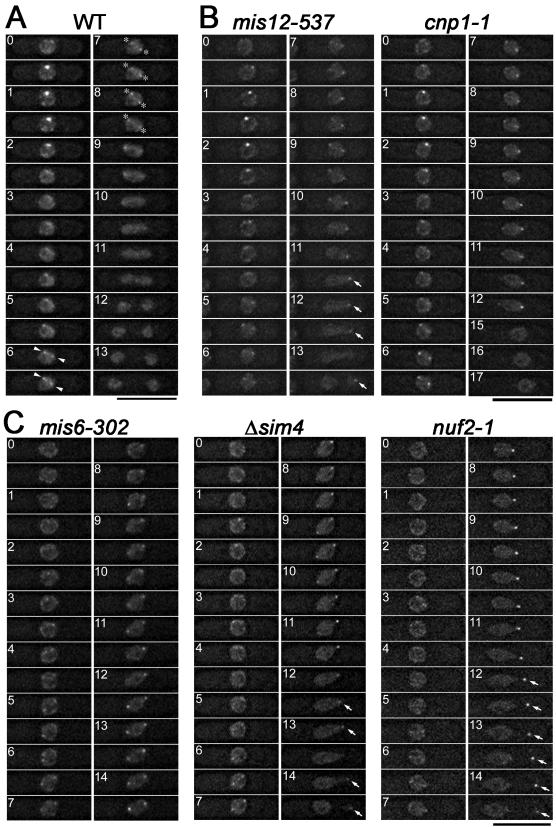
Mitotically arrested cells were rarely seen in the fission yeast kinetochore-defective mutants mis6-302, mis12-537, cnp1-1, nuf2-1 (Saitoh et al., 1997; Goshima et al., 1999; Takahashi et al., 2000; Nabetani et al., 2001) and sim4+-shut-off (our unpublished data; see Figure 2, legend). It suggests that these mutations may impair the spindle checkpoint signaling pathway and that the kinetochore aberrance may not be properly detected in these mutants. As the first attempt to investigate how the checkpoint signaling is impaired in these kinetochore-defective mutants, we examined the mitotic behavior of Mad2 in them. It has been reported that some specific alleles of nuf2 or sim4 mutants (nuf2-2, nuf2-3, and sim4-193) gave a high proportion of mitotic-arrested cells at the restrictive conditions (Nabetani et al., 2001; Pidoux et al., 2003). The mutated proteins expressed from these alleles might retain some residual activities because the nuf2 or sim4 gene-disruptant cells did not show such a mitotic-delay phenotype (Nabetani et al., 2001; our unpublished data). In the following experiments, we thus used the nuf2-1 mutant that showed the same phenotype as the null mutant did at the restrictive temperature (Nabetani et al., 2001), and the conditional sim4+-shut-off mutant. To analyze the dynamic behavior of Mad2, we performed live cell analyses wherein time-lapse images of Mad2-GFP in live cells were taken under a three-dimensional deconvolution microscope.
Figure 2.
The proper kinetochore accumulation of Mad2 requires Mis6-Sim4 and Nuf2, but not Mis12 or SpCENP-A. (A) Fission yeast wild-type cells expressing Mad2-GFP were observed by time-lapse three-dimensional deconvolution microscopy. Images were taken every 0.5 min. Dot-like GFP signals occurring at the early stage of mitosis (time = 0.5–3.5 min) represent the accumulation of Mad2 at the kinetochore. Arrowheads and asterisks indicate the transient accumulation of Mad2 on the spindle and the spindle poles, respectively. (B and C) Mitotic behavior of Mad2 in the indicated mutant cells that were cultured at their restrictive conditions (6–8 h at 36°C for mis6-302 and mis12-537, 8–10 h at 33°C for cnp1-1, 1.5–4 h at 36°C for nuf2-1, and 12–16 h at 33°C with 2 μM thiamine for sim4+-shut-off (Δsim4)) is shown. The kinetochore accumulation of Mad2 was observed in the mutants in B, but it was greatly diminished or not observed in the mutants in C. In the sim4+-shut-off cells, sim4+ gene expression is placed under the control of thiamine-repressible nmt1-81 promoter. Sim4 protein was virtually undetectable, and >80% of cells showed unequal nuclear division phenotype 14 h after the addition of thiamine. Movies of the cells shown in this figure are provided as supplemental materials (Movies S1–S6).
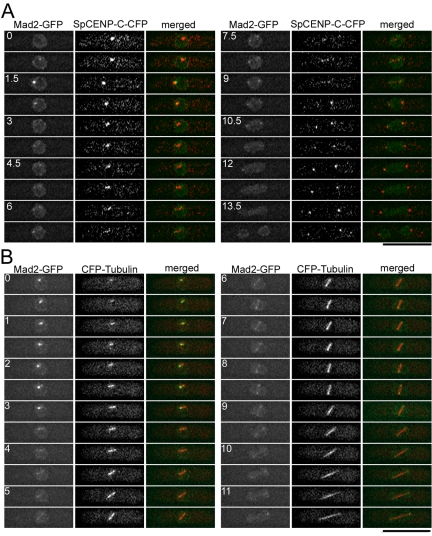
The localization of Mad2 in fixed fission yeast cells has been reported previously (Ikui et al., 2002). However, the dynamic behavior of Mad2 in live cells has not yet been documented. Thus, we performed double labeling experiments in which Mad2-GFP was visualized in combination with either SpCENP-C-CFP (a kinetochore maker, Figure 1A) or CFP-tubulin (a spindle maker, Figure 1B) in living wild-type cells. SpCENP-C (encoded by SPBC1861.01c gene), which is homologous to budding yeast Mif2 and vertebrate CENP-C, constitutively binds to the central core region of the centromere (Supplemental Figure S1) (Holland et al., 2005). Images were taken every 45 s (Figure 1A) or 30 s (Figure 1B). Mad2-GFP localized in the nucleus and at the nuclear periphery during interphase (Figure 1A, time = 0 min). On entry into mitosis, bright dot(s) of Mad2-GFP occurred in the midnuclear region (Figure 1A, time = 0.75–2.25 min and Figure 1B, time = 0–3.5 min). These dots colocalized with a subset of kinetochores (Figure 1A), suggesting that Mad2 accumulated on unattached kinetochores during the early stage of mitosis. Then, the fluorescence intensity of these dots was gradually decreased. Shortly after these bright dots disappeared, Mad2-GFP transiently localized along the spindle and/or on the spindle poles (Figure 1B, time = 5.5–9.5 min). During this period of mitosis, kinetochores, which no longer colocalized with Mad2-GFP, moved back and forth between two dots of Mad2 signals at the spindle poles, implying that the anaphase had not yet started (Figure 1A, time = 6–9 min) (Funabiki et al., 1993). Taking these results together, in wild-type fission yeast cells, Mad2 transiently accumulates on unattached kinetochores at early mitosis, and then, presumably when the kinetochore is captured by the spindle, leaves the attached kinetochore and relocates to the spindles and/or the SPBs before the onset of anaphase. It could be clearly determined whether Mad2-GFP localized on kinetochores or the SPBs in the absence of the kinetochore/spindle markers, if the localization of GFP signals was recorded throughout mitosis (Figure 2A); two dots of Mad2-GFP occurring at the nuclear periphery just before nuclear division indicates that Mad2 localizes on the SPBs, whereas bright dot(s) occurring in the midnuclear region roughly 10 min before nuclear division indicates that Mad2 accumulates on kinetochores. We noticed that the presence of CFP-Tub1 perturbed the Mad2 behavior itself and/or the cell cycle progression in some stages of mitosis; Mad2 localization on the kinetochore and on the spindle/SPBs persisted significantly longer in cells expressing CFP-Tub1 than cells without it (compare Figure 1B with Figures 1A and 2A). Therefore, to exclude such experimental artifacts caused by tagging kinetochore/spindle components with fluorescence proteins, live cell analyses of Mad2 behavior in kinetochore-defective mutants were performed in the absence of the kinetochore/spindle markers.
Figure 1.
Mad2 accumulates on kinetochores in early mitosis and relocates to the spindle and the SPBs before the onset of anaphase. (A) Fission yeast cells expressing Mad2-GFP along with SpCENP-C-CFP, a kinetochore marker, were observed by time-lapse three-dimensional deconvolution microscopy. For CFP-tagging of SpCENP-C, a modified version of CFP, named “cerulean,” was used (Rizzo et al., 2004). These images were taken every 0.75 min. In merged images in the right column, Mad2-GFP signal was pseudocolored green and SpCENP-C-CFP signal was pseudocolored red. Dot-like Mad2-GFP signals occurring at the early stage of mitosis (time = 0.75–2.25 min) colocalized with a subset of kinetochores. Two Mad2-GFP dots occurring at the later stage (time = 6–9 min) did not colocalize with kinetochores, which moved along short linear path between these two dots. (B) Time-lapse images of a cell expressing Mad2-GFP and CFP-α-tubulin, a spindle marker, were shown. Images were taken every 0.5 min. In merged images, Mad2-GFP signal was colored green and CFP-α-tubulin was colored red. Under the imaging condition in this experiment, CFP-α-tubulin signals on cytoplasmic astral MTs were too weak to be detected. Two Mad2-GFP dots occurring at the later stage of mitosis (time = 5.5–9.5 min) located at the both poles of the spindle. Mad2-GFP also localized along the spindle at this stage. The number indicates the duration in minutes. Bar, 10 μm.
Figure 2B shows the time-lapse images of Mad2-GFP in a cnp1-1 mutant cell and a mis12-537 mutant cell. These mutant cells were incubated at their restrictive temperature (33°C for cnp1-1 and 36°C for mis12-537). These mutant kinetochore proteins seemed to be inactivated under these conditions, because, on the basis of nuclear staining by Mad2-GFP, these mutant cells showed unequal nuclear division (time = 13.5 min in mis12-537 and 17 min in cnp1-1), which was seen in mis12 and cnp1 null mutants (Goshima et al., 1999; Takahashi et al., 2000). Mad2 localized at the kinetochore during the early stage of mitosis (time = 0.5–3.5 min) in both mutants. Mad2 also accumulated on the kinetochore in mis12+-shut-off cells. These results suggest that SpCENP-A and Mis12 are dispensable for the Mad2 accumulation.
Mitotic behavior of Mad2 in mis6-302, nuf2-1, and sim4+-shut-off cells is shown in Figure 2C. In sharp contrast to wild-type cells, the kinetochore accumulation of Mad2 at early M phase was reproducibly diminished in these mutant cells, although Mad2 localized on the spindle and the poles at the later stage. To quantify the amount of Mad2 accumulating at the kinetochore, the maximum fluorescence intensity of Mad2-GFP dots at the early stage of mitosis was measured in the Sim4-depleted cells. Conditional sim4+-shut-off strain cells cultured in medium lacking thiamine were used as a Sim4-expressing control. Given that the average of the intensity in the control cells is 100% (n = 5, SD = 18.9), that in Sim4-depleted cells was reduced to 22.6% (n = 7, SD = 6.7). These observations suggest that two kinetochore subcomplexes, the Mis6–Sim4-complex and the Nuf2-complex, play pivotal roles in the Mad2 accumulation on the kinetochore during early mitosis.
Mad2 often remained on the SPB at the late stage of anaphase in the mutant cells, which showed unequal nuclear division (indicated by arrows), whereas Mad2 delocalized from the SPBs before the onset of nuclear division in wild-type cells. The significance of this abnormal SPB localization remains unclear. This SPB localization was prominent particularly on the SPB in a smaller nucleus, which seemed to loose chromosomes. Mad2 might respond to not only unattached kinetochores but also the loss of the chromosomes in the late stage of mitosis.
The Bub1 Accumulation on the Kinetochore Does Not Require Mis6, Sim4, Mis12, Nuf2, or CENP-A
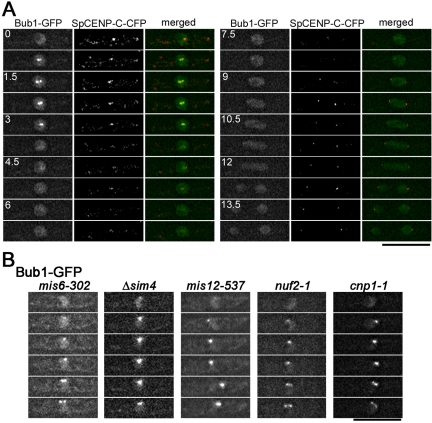
As shown in Figure 3A, Bub1, another mitotic checkpoint protein, also accumulated on a subset of kinetochores during early mitosis, consistent with previous report (Toyoda et al., 2002). In fission yeast, it was suggested that Bub1 recognized tension-less kinetochores, whereas Mad2 detected unattached kinetochores (Garcia et al., 2002). We performed live cell analyses to examine whether Bub1-GFP accumulates on the kinetochore in mis6-302, mis12-537, nuf2-1 mutant, or sim4+-shut-off cells. As shown in Figure 3B, in all the mutant cells tested, Bub1-GFP accumulated at the kinetochore and was observed as bright dots during early mitosis. These observations suggest that none of Mis6, Sim4, Mis12, SpCENP-A, and Nuf2 is essential for the accumulation of Bub1.
Figure 3.
The kinetochore accumulation of Bub1 in kinetochore mutants. (A) Time-lapse images of a cell expressing Bub1-GFP and SpCENP-C-CFP were shown. Images were taken every 0.75 min. Bub1-GFP localized in nuclei during interphase (time = 0 min). During early M phase (time 0.75–3 min), bright dot-like signals of Bub1-GFP were observed, and these signals colocalized with a subset of kinetochores. In the merged images, Bub1-GFP and SpCENP-C-CFP are shown in green and red, respectively. (B) The mitotic behavior of Bub1 was examined in various kinetochore-defective mutants at their restrictive conditions. Time-lapse images of the first six frames (2.5 min) of each mutant cell in mitosis are shown. The dot-like signals represent the accumulation of Mad2 on kinetochores. Bub1 localizes on the nuclear chromatin during interphase, and accumulates on kinetochores during early mitosis in mis6-302, sim4+-shut-off (Δsim4), mis12-537, nuf2-1, and cnp1-1 mutant cells.
mis6-302 Mutation Impairs the Spindle Checkpoint Response
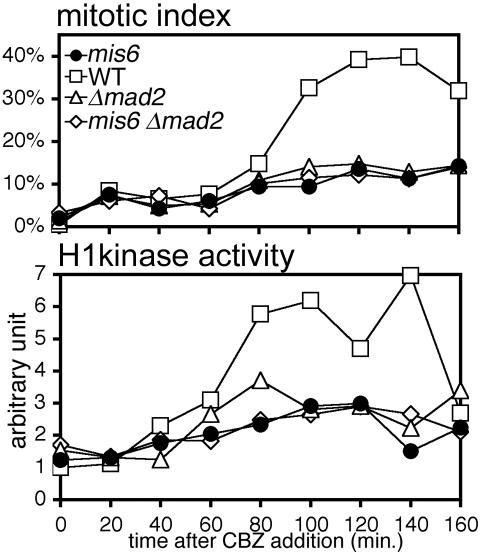
Because our observations suggested that the Mis6-complex plays an essential role in the kinetochore accumulation of Mad2, we then examined whether the mis6-302 mutant indeed have a defect in the Mad2-related checkpoint. For this purpose, we treated cells with a MT-destabilizing drug, carbendazim (CBZ) and measured the mitotic index by counting the number of cells with hypercondensed chromosomes (Figure 4, top). Cells were cultured at 30°C, a semi-permissive temperature for mis6-302 mutant. As reported previously (Millband and Hardwick, 2002), wild-type cells were arrested at prometaphase-like stage in the presence of CBZ (Figure 4, top, open square), and the mitotic index rose to 32–40% at 100–160 min after the addition of the drug. This arrest partly depended on Mad2 function because the mitotic index remained <15% in the mad2-disrupted strain (Δmad2, open triangle). Similar to the result of Δmad2, the mitotic index did not increase in the mis6-302 mutant strain (closed circle). Consistent with this cytological observation, H1 kinase activity in the mis6-302 mutant did not increase in the presence of CBZ (Figure 4, bottom). These results indicated that the mitotic checkpoint did not function properly in mis6-302 mutant cells. The combination of mis6-302 mutation with Δmad2 did not result in synergistic reduction of the mitotic index, indicating that the deficiency in the checkpoint response in mis6-302 mutant is largely due to the dysfunction of Mad2.
Figure 4.
The Mad2-dependent spindle checkpoint is defective in the mis6-302 mutant. Cells with one of the following genetic backgrounds were cultured in YES medium in the presence of 50 μg/ml CBZ at 30°C: WT, Δmad2, mis6-302, or mis6-302 Δmad2 double mutant. The frequency (percentage) of cells containing hypercondensed chromosomes was determined by DAPI staining in 20-min intervals (top). Histone H1 kinase activity in the total cell lysate was also measured (bottom).
We next measured the frequency of prometaphase cells in Mis6-depleted and Mis12-depleted cells by counting the number of cells in which GFP-tagged Cdc13 (a mitotic cyclin) localized on the mitotic apparatus (Minoda et al., 2005). For the depletion of Mis6 or Mis12, we constructed conditional shut-off strains in which the promoter of either of mis6+ or mis12+ genes was replaced with a copper-repressible ctr4+ promoter (Zhou and Thiele, 2001); 9.9% of Mis6-depleted cells were in prometaphase, whereas 62% of binuclear cells showed unequal nuclear division. This prometaphase frequency was comparable to that in the wild-type control (10.1%), indicating that the mitotic checkpoint did not significantly respond to deficient kinetochores lacking Mis6. In contrast to the depletion of Mis6, the frequency of prometaphase cells was slightly elevated to 18.4% in Mis12-depleted cells, whereas 78% of the binuclear cells showed unequal nuclear division phenotype. Thus, the depletion of Mis12 from the kinetochore seemed to cause transient mitotic delay, although mitosis eventually proceeded with chromosomes segregated unequally.
The Kinetochore Is Not Fully Disorganized in the Sim4-depleted Cells
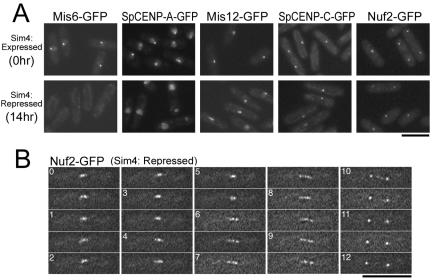
According to the results described above, two distinct kinetochore subcomplexes, the Mis6-complex and the Nuf2-complex, are essential for the kinetochore accumulation of Mad2. One simple explanation for the relationship between these two complexes is that elimination of the Mis6-complex, which is a constitutive kinetochore component, might fully disorganize the kinetochore and Nuf2 would not be loaded on the kinetochore. Indeed, it has been reported that the kinetochore localization of SpCENP-A was severely impaired in mis6-302 and sim4-193 temperature-sensitive mutant cells (Takahashi et al., 2000; Pidoux et al., 2003). To assess how the depletion of the Mis6-complex influences the localization of kinetochore proteins, we examined whether a variety of kinetochore proteins—Mis6, SpCENP-A, Mis12, SpCENP-C, and Nuf2—localized on the kinetochore in Sim4-depleted cells. For depletion of Sim4, sim4+-shut-off cells were cultured for 14 h in the presence of thiamine.
GFP-fused kinetochore proteins were observed as a single dot in wild-type cells, because all three kinetochores cluster adjacent to the SPB during interphase (Funabiki et al., 1993). As shown in Figure 5A, GFP-fused Mis6, SpCENP-A, Mis12, SpCENP-C, and Nuf2 localized on the kinetochore and were observed as a single bright dot in Sim4-expressing control cells during interphase (top). When Sim4 protein was depleted from cells, the kinetochore localization of Mis6, the binding partner of Sim4, and SpCENP-A was greatly impaired (Figure 5A, bottom). These results were consistent with the previous results using the temperature-sensitive sim4 mutant (Pidoux et al., 2003). In contrast, Mis12, SpCENP-C, and Nuf2 were still observed as a bright dot in Sim4-depleted cells, indicating that the depletion of Sim4 did not affect the kinetochore localization of Mis12 and SpCENP-C. Because Nuf2 is reported to localize on the centrosome during interphase in other organisms, the dot-like signal of Nuf2-GFP may represent its localization on the SPB but not on the kinetochore. To test whether Nuf2 localizes on the kinetochore in mitosis in the absence of Sim4, we performed live cell analysis of Nuf2-GFP in Sim4-depleted cells. The mitotic behavior of Nuf2-GFP in Sim4-depleted cells was identical to that in wild-type cells reported previously (Figure 5B) (Nabetani et al., 2001; Wigge and Kilmartin, 2001), indicating that Sim4 depletion did not impair the kinetochore localization of Nuf2 during mitosis. These results suggest that the Sim4 depletion does not fully disorganize the kinetochore, and a number of kinetochore proteins, including Mis12, SpCENP-C, and Nuf2, are likely to remain on the kinetochore without the Mis6-complex and the SpCENP-A–containing nucleosomes.
Figure 5.
The depletion of Mis6-Sim4 does not impair the kinetochore localization of SpCENP-C, Mis12, or Nuf2. (A) The sim4+-shut-off cells carrying either the integrated Mis6-GFP, SpCENP-A-GFP, Mis12-GFP, SpCENP-C-GFP, or Nuf2-GFP gene were cultured in EMM2 at 33°C for 0 (Sim4 was expressed) and 14 h (Sim4 was repressed) after the addition of thiamine. The photographs of GFP fluorescence were taken after fixation with methanol pre-chilled at –80° C. (B) The time-lapse serial images of Nuf2-GFP in Sim4-depleted cells are shown.
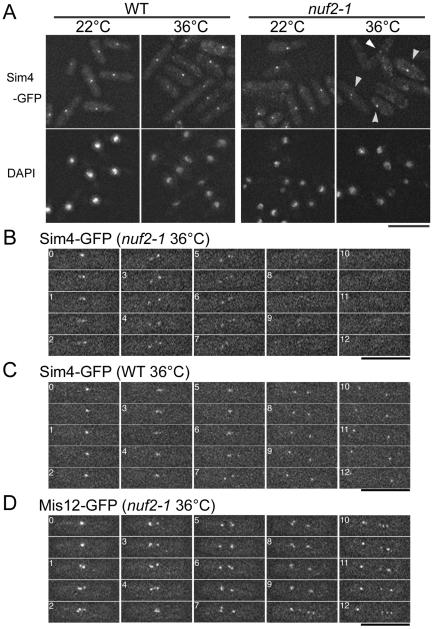
nuf2-1 Mutation Destabilizes the Association of Mis6 to the Kinetochore during Mitosis
We next examined whether the localization of the Mis6-complex was affected by nuf2-1 mutation. nuf2-1 mutant cells expressing Sim4-GFP at the native level were cultured at the restrictive temperature (36°C), and the localization of Sim4-GFP was determined. Sim4-GFP was observed as a bright dot in approximately two-thirds of the mutant cells at 36°C (Figure 6A), indicating that the kinetochore localization of the Mis6-complex was not significantly affected by Nuf2 inactivation during interphase. However, we realized that dot-like signals of Sim4-GFP were diminished or eliminated in the remaining one-third of cells, particularly in mitotic cells. To test the possibility of the mitotic localization of the Mis6–Sim4-complex being specifically impaired in nuf2-1 mutant cells, we performed live cell analysis of Sim4-GFP in nuf2-1 mutant cells (Figure 6B). In interphase (time = 0 min), Sim4-GFP was seen as a single dot. Several minutes after the mutant cells entered mitosis (time = 0.5–9 min), Sim4-GFP fluorescence was still visible on kinetochores. As mitosis proceeded, however, the Sim4-GFP fluorescence gradually decreased to an undetectable level. This disappearance of Sim4-GFP in nuf2-1 mutant was reproducible in all of seven examples tested and was not due to photobleaching; Sim4-GFP was visible throughout mitosis in wild-type cells at the same condition (Figure 6C). In contrast to Sim4-GFP, Mis12-GFP localized on the kinetochore throughout mitosis in nuf2-1 mutant cells (Figure 6D). These observations indicate that, during the passage of mitosis, Nuf2 is crucial for stable kinetochore association of Mis6-Sim4, but not of Mis12.
Figure 6.
Sim4 delocalizes from the kinetochore during mitosis in nuf2-1 mutant cells. (A) Wild-type cells (left) or nuf2-1 mutant cells (right) with sim4+-GFP gene integrated were cultured at either 36°C for 4 h or 22°C (permissive condition) and fixed with methanol pre-chilled at –80° C. Three-dimensional serial images were taken and projected into a single plane after deconvolution. Arrowheads indicate dot-like signals of Sim4 observed in nuf2-1 mutant cells at the restrictive condition. (B–D) Time-lapse images of nuf2-1 mutant cells with Sim4-GFP (B) or Mis12-GFP (D) or of wild-type cells with Sim4-GFP (C) are shown. The cells were incubated at 36°C for 1.5–2 h.
Mis6 Physically Interacts with Mad2
To elucidate how the Mis6-complex is involved in the Mad2 accumulation on unattached kinetochores, we examined the physical interaction between Mad2 and the Mis6-complex in vivo. Immunoprecipitation was performed using cell extracts from cut7-477 mutant cells that expressed both FLAG-epitope–tagged Mis6 and Mad2-GFP at their native levels. Cut7, a kinesin, plays an essential role in the formation of the bipolar spindle (Hagan and Yanagida, 1990). Using a temperature-sensitive cut7-477 allele results in the inactivation of Cut7 and induction of mitotic cell cycle arrest in a mitotic checkpoint-dependent manner (Kim et al., 1998). As shown in Figure 7A, Mis6 was coimmunoprecipitated with Mad2 when the extract was prepared from cut7-477 mutant cells cultured at the restrictive temperature (lane 3). In contrast, coprecipitation of Mis6 with Mad2 was not detectable when the extract was prepared from the cells asynchronously growing at the permissive temperature (lane 6) or cut9-665 mutant cells that were arrested at metaphase due to the inactivation of APC/C but not to the failure in bipolar spindle attachment to kinetochores (Yamada et al., 1997) (lane 9). These results indicate that Mis6 physically interacts with Mad2 and that this interaction occurs only when the mitotic checkpoint is activated.
An Evolutionarily Conserved N-Terminal Region of Mis6 Interacts with the Mitotic Spindle
Sequence comparison analysis revealed that the N-terminal region of Mis6 is evolutionarily conserved. Two domains, in particular, indicated by gray boxes in Figure 7B (amino acids 106–199 and 263–322), are highly conserved among fission yeast, chicken, and human. In one of these domains, residue 135 is glycine in wild-type mis6+; it is mutated to glutamate (G135E) in mis6-302 mutant allele (Saitoh et al., 1997). Thus, this N-terminal conserved region seems to be important for Mis6 function. We determined the subcellular localization of a series of truncated Mis6 proteins (Figure 7B) and found that some of GFP-fused Mis6 N-terminal fragments (Mis61-347, Mis61-265, and Mis61-244) localized in the nuclei and along the mitotic spindle. When ectopically expressed, all the GFP-fused truncated Mis6 fragments formed aggregates in the cytoplasm. Beside this cytoplasmic aggregate (C.A.), the N-terminal fragments mentioned above localized in the nuclei (N) during interphase and along the spindle MTs (S) in M phase. Mis61-265 with temperature-sensitive G135E mutation did not localize along the spindle MTs at the restrictive temperature, implying that this spindle localization has physiological relevance to Mis6 function. In Figure 7C, microtubules were immunostained in the cells expressing Mis61-265-GFP. Mis61-265 localized along the pole-to-pole in M phase, but it did not localize on cytoplasmic MTs during interphase (left column). Mis61-265 did not localize along the cytoplasmic astral MTs even in M phase (right column, arrowheads). These results suggest that Mis6 has an ability to interact with the spindle MTs through its conserved N-terminal region. The N-terminal fragment containing longer C-terminal potion (such as Mis61-427), as well as the full-length Mis6, did not localize on the spindle or in the nucleus, implicating that the C-terminal region might regulate Mis6 localization and prevent it from localizing on the spindle and/or in the nucleus.
Prometaphase-like cells with hypercondensed chromosomes were frequently observed when Mis61-265 was overexpressed (Figure 7B, bottom left), indicating that the ectopic expression of Mis61-265 caused mitotic delay. Mis61-265 without GFP fusion also caused this mitotic delay; however, Mis61-265 with the temperature-sensitive mutation did not cause this delay at the restrictive temperature (our unpublished data). In these prometaphase-like cells, the spindle seemed to be longer compared with the metaphase spindle in wild-type cells (Figure 7C, right column). Ectopically expressed Mis61-265 may affect the architecture of the bipolar spindle.
DISCUSSION
The kinetochore is a huge multiprotein complex consisting of a number of distinct subcomplexes that are formed on the CENP-A–containing nucleosomes (CENP-A-H4-H2A-H2B) (Cleveland et al., 2003). In fission yeast, the Mis6-complex (Mis6-Sim4-Mis15-Mis17), the Mis12-complex (Mis12-Mis13-Mis14), and the Nuf2-complex (Nuf2-Hec1-Spc24-Spc25) have been proposed to represent different kinetochore subcomplexes (Wigge and Kilmartin, 2001; Appelgren et al., 2003; Hayashi et al., 2004; Obuse et al., 2004). In this report, we showed that inactivation of the component in either the Mis6-complex or the Nuf2-complex impairs the kinetochore accumulation of the mitotic checkpoint protein Mad2 but not Bub1 in fission yeast. In contrast, mutations in either Mis12 or SpCENP-A did not severely affect the Mad2 or the Bub1 localization. The present study is consistent with previous reports that Mis6/CENP-I and Nuf2-Hec1 are required for the kinetochore accumulation of Mad2 in higher eukaryotes (Martin-Lluesma et al., 2002; DeLuca et al., 2003; Hori et al., 2003; Liu et al., 2003). Therefore, the importance of both Mis6/CENP-I and Nuf2-Hec1 in a mechanism of the Mad2 accumulation on the kinetochore seemed to be evolutionarily conserved. To adjust the MT-driven dynamic behavior of chromosomes with the progression of mitosis, at least two aspects of kinetochore states, i.e., the interkinetochore tension and the outer-kinetochore attachment to MTs are thought to be monitored by the BubR1-Bub1–dependent and the Mad2-Mad1–dependent checkpoint pathways, respectively (Waters et al., 1998; Skoufias et al., 2001). The mis6 mutation causes a synthetic defect in cell growth with elevated chromosome missegregation when combined with Δbub1 but not with Δmad2 (our unpublished data), suggesting that the Mad2-dependent spindle attachment checkpoint response is specifically impaired in cells defective in the Mis6-complex. Noteworthy, in CENP-I–depleted HeLa cells, the treatment with MT-depolymerizing drug delayed mitotic progression in Mad2-dependent manner despite the mislocalization of Mad2 (Liu et al., 2003), whereas fission yeast mis6-302 mutation substantially impaired the checkpoint response to the drug treatment. The kinetochore targeting of Mad2 seems to be crucial for the checkpoint activation in fission yeast; additional checkpoint components existing only in higher eukaryotes might alleviate the requirement of Mad2 targeting.
Our findings not only confirm the results of previous vertebrate studies but also provide further insight into the molecular mechanism of the Mad2 accumulation on the kinetochore. We demonstrated that Mis6 physically interacts with Mad2 under the condition that the Mad2-dependent checkpoint is activated. This suggests that the Mis6-complex acts as a platform for the Mad2 accumulation at the kinetochore. Recently, fission yeast Mad2 was reported to bind to the central core domain of the centromere (cnt and imr), but not to the franking heterochromatic region (otr) (Vanoosthuyse et al., 2004). This is consistent with the present study, because Mis6 binds specifically to the central core region (Saitoh et al., 1997). We showed that the Nuf2-complex also was required for the kinetochore accumulation of Mad2. Although it remains to be clarified whether fission yeast Nuf2-complex localizes on the kinetochore in a cell-cycle dependent manner, it is likely that fission yeast Nuf2 is incorporated to the kinetochore specifically during mitosis based on the results of the homologues in other organism (Howe et al., 2001; Nabetani et al., 2001; Hori et al., 2003). Given that the fission yeast Nuf2-complex is a mitosis-specific kinetochore component, it would be explained why Mad2 accumulates at unattached kinetochores only during mitosis, but not in interphase; incorporation of the Nuf2-complex may cause the structural change in the kinetochore that allows the Mis6-complex to associate with Mad2. We found that Sim4 delocalized from the kinetochore during passage of mitosis in nuf2-1 mutant cells, indicating that Nuf2 is essential for the stable association of the Mis6-complex on the kinetochore during mitosis. Fission yeast Mis6 has been shown to be required for the loading of SpCENP-A to the centromeric chromatin (Takahashi et al., 2000) and thus has been assumed to function near the chromatin and not at the kinetochore surface that the spindle MTs attach to. In contrast, it has been suggested that Nuf2 localizes at the kinetochore periphery, the outer plate, close to the spindle MTs (Wigge and Kilmartin, 2001; Deluca et al., 2005). Therefore, Nuf2-complex may bring the Mis6-complex to the kinetochore periphery where it can physically interact with Mad2 during early mitosis. Alternatively, Mad2 may simultaneously interact with both the Mis6- and the Nuf2-complex. Mad2 was shown to form a complex with Mad1, which plays an important role in Mad2 localization (Chen et al., 1998, 1999; Jin et al., 1998; Ikui et al., 2002). Two-hybrid assay revealed that Mad1 can physically bind to human Hec1 (Martin-Lluesma et al., 2002), which forms a complex with Nuf2. Thus, ternary subcomplex interaction among Mis6-Sim4, Nuf2-Hec1, and Mad1-Mad2 may be established upon entry into mitosis, because Mad1-Mad2 forms a link between the other two complexes; this inter-subcomplex interaction might be crucial for Mad2 to recognize the kinetochore that has not properly attached to the spindle MTs. Consistently, it was recently shown that CENP-H, a putative homologue of Sim4, was coimmunoprecipitated with both the Nuf2-complex and Mad2 in a chicken cell (Mikami et al., 2005). The Mis6-complex and the Nuf2-complex may be involved in different aspects of Mad2 accumulation, such as “recruitment” and “retention,” onto the kinetochore.
An important question with regard to the regulation of the Mad2 localization is the absence of Mad2 accumulation on kinetochores that attached to the spindle. At present, this question remains to be answered. However, we demonstrated that ectopically expressed Mis6 N-terminal fragments localize along the mitotic spindle, but not along the interphase MTs, highlighting the potential binding ability of Mis6 not only to the centromeric chromatin but also to a subset of the spindle MTs, presumably MTs attaching to kinetochores. In addition, Mis6 also interacts with Mad2. We currently speculate that Mis6 might interact with the spindle MTs that attach onto kinetochores and that this Mis6–MT interaction might prevent further association of Mad2 in a competitive manner. Therefore, Mis6 may be a sensor of the spindle attachment for the Mad2-related checkpoint. Consistent with this hypothesis, mis6-302 mutant cells were shown to override the Mad2-dependent checkpoint response to MT depolymerization.
In fission yeast, compromising Mad2 function does not cause fetal chromosome missegregation. This suggests that the Mis6-complex acts not solely as a component of the mitotic checkpoint. Localization of SpCENP-A also is impaired in any of the mis6, mis15, mis17 and sim4 mutant (Takahashi et al., 2000; Pidoux et al., 2003; Hayashi et al., 2004), indicating that the Mis6-complex plays dual roles in both SpCENP-A incorporation into the centromere nucleosomes and the Mad2 accumulation on the surface of unattached kinetochores. It has been proposed that CENP-A may be correctly incorporated at mitosis only when proper kinetochore-spindle attachments produce tension at functional kinetochores (Mellone and Allshire, 2003; Pidoux et al., 2003). The Mis6-complex could be a good candidate for a molecular interface that potentially transmits the positional information regarding the spindle attachment site to the SpCENP-A loading pathway.
Supplementary Material
Acknowledgments
We thank Drs. A. M. Carr, K. Gull, Y. Hiraoka, J.-P. Javerzat, T. Matsumoto, and M. Yanagida for providing materials used in this study. We thank F. Masuda, S. Soejima, M. Kobayashi, Y. Hiraga, and M. Kondo for technical assistance. This work was supported by the Grant-in-Aid for Scientific Research on Priority Areas “Genome”, “Cancer,” “Nuclear Dynamics” (to K. T.) and “Cell Cycle Control” (to K. T. and S. S.) from Ministry of Education, Culture, Sports, Science and Technology; the Grant–in-Aid for Scientific Research (B) (to K. T.) and for Young Scientists (B) (to S. S.) from Japan Society for the Promotion of Science; grants from The Novartis Foundation, Toray Science Foundation, and Uehara Memorial Foundation (to K. T.); and Nissan Science Foundation and Nakajima Foundation (to S. S.).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–01–0014) on June 1, 2005.
Abbreviations used: APC/C, anaphase promoting complex/cyclosome; CBZ, carbentazim; MT, microtubule; SPB, spindle pole body.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Appelgren, H., Kniola, B., and Ekwall, K. (2003). Distinct centromere domain structures with separate functions demonstrated in live fission yeast cells. J. Cell Sci. 116, 4035–4042. [DOI] [PubMed] [Google Scholar]
- Bernard, P., Hardwick, K., and Javerzat, J. P. (1998). Fission yeast bub1 is a mitotic centromere protein essential for the spindle checkpoint and the preservation of correct ploidy through mitosis. J. Cell Biol. 143, 1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill, D. P., Lengauer, C., Yu, J., Riggins, G. J., Willson, J. K., Markowitz, S. D., Kinzler, K. W., and Vogelstein, B. (1998). Mutations of mitotic checkpoint genes in human cancers. Nature 392, 300–303. [DOI] [PubMed] [Google Scholar]
- Cheeseman, I. M., Anderson, S., Jwa, M., Green, E. M., Kang, J., Yates, J. R., 3rd, Chan, C. S., Drubin, D. G., and Barnes, G. (2002a). Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111, 163–172. [DOI] [PubMed] [Google Scholar]
- Cheeseman, I. M., Drubin, D. G., and Barnes, G. (2002b). Simple centromere, complex kinetochore: linking spindle microtubules and centromeric DNA in budding yeast. J. Cell Biol. 157, 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. H., Brady, D. M., Smith, D., Murray, A. W., and Hardwick, K. G. (1999). The spindle checkpoint of budding yeast depends on a tight complex between the Mad1 and Mad2 proteins. Mol. Biol. Cell 10, 2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. H., Shevchenko, A., Mann, M., and Murray, A. W. (1998). Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J. Cell Biol. 143, 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, R. H., Waters, J. C., Salmon, E. D., and Murray, A. W. (1996). Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science 274, 242–246. [DOI] [PubMed] [Google Scholar]
- Chen, Y., Riley, D. J., Chen, P. L., and Lee, W. H. (1997). HEC, a novel nuclear protein rich in leucine heptad repeats specifically involved in mitosis. Mol. Cell. Biol. 17, 6049–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland, D. W., Mao, Y., and Sullivan, K. F. (2003). Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 112, 407–421. [DOI] [PubMed] [Google Scholar]
- De Wulf, P., McAinsh, A. D., and Sorger, P. K. (2003). Hierarchical assembly of the budding yeast kinetochore from multiple subcomplexes. Genes Dev. 17, 2902–2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca, J. G., Dong, Y., Hergert, P., Strauss, J., Hickey, J. M., Salmon, E. D., and McEwen, B. F. (2005). Hec1 and Nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol. Biol. Cell, 16, 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca, J. G., Howell, B. J., Canman, J. C., Hickey, J. M., Fang, G., and Salmon, E. D. (2003). Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr. Biol. 13, 2103–2109. [DOI] [PubMed] [Google Scholar]
- Funabiki, H., Hagan, I., Uzawa, S., and Yanagida, M. (1993). Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol. 121, 961–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, M. A., Koonrugsa, N., and Toda, T. (2002). Spindle-kinetochore attachment requires the combined action of Kin I-like Klp5/6 and Alp14/Dis1-MAPs in fission yeast. EMBO J. 21, 6015–6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., Kiyomitsu, T., Yoda, K., and Yanagida, M. (2003). Human centromere chromatin protein hMis12, essential for equal segregation, is independent of CENP-A loading pathway. J. Cell Biol. 160, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima, G., Saitoh, S., and Yanagida, M. (1999). Proper metaphase spindle length is determined by centromere proteins Mis12 and Mis6 required for faithful chromosome segregation. Genes Dev. 13, 1664–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan, I., and Yanagida, M. (1990). Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature 347, 563–566. [DOI] [PubMed] [Google Scholar]
- Hayashi, T., Fujita, Y., Iwasaki, O., Adachi, Y., Takahashi, K., and Yanagida, M. (2004). Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell 118, 715–729. [DOI] [PubMed] [Google Scholar]
- Holland, S., Ioannou, D., Haines, S., and Brown, W. R. (2005). Comparison of Dam tagging and chromatin immunoprecipitation as tools for the identification of the binding sites for S. pombe CENP-C. Chromosome Res. 13, 73–83. [DOI] [PubMed] [Google Scholar]
- Hori, T., Haraguchi, T., Hiraoka, Y., Kimura, H., and Fukagawa, T. (2003). Dynamic behavior of Nuf2-Hec1 complex that localizes to the centrosome and centromere and is essential for mitotic progression in vertebrate cells. J. Cell Sci. 116, 3347–3362. [DOI] [PubMed] [Google Scholar]
- Howe, M., McDonald, K. L., Albertson, D. G., and Meyer, B. J. (2001). HIM-10 is required for kinetochore structure and function on Caenorhabditis elegans holocentric chromosomes. J. Cell Biol. 153, 1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikui, A. E., Furuya, K., Yanagida, M., and Matsumoto, T. (2002). Control of localization of a spindle checkpoint protein, Mad2, in fission yeast. J. Cell Sci. 115, 1603–1610. [DOI] [PubMed] [Google Scholar]
- Janke, C., Ortiz, J., Lechner, J., Shevchenko, A., Magiera, M. M., Schramm, C., and Schiebel, E. (2001). The budding yeast proteins Spc24p and Spc25p interact with Ndc80p and Nuf2p at the kinetochore and are important for kinetochore clustering and checkpoint control. EMBO J. 20, 777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, D. Y., Spencer, F., and Jeang, K. T. (1998). Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93, 81–91. [DOI] [PubMed] [Google Scholar]
- Kim, S. H., Lin, D. P., Matsumoto, S., Kitazono, A., and Matsumoto, T. (1998). Fission yeast Slp 1, an effector of the Mad2-dependent spindle checkpoint. Science 279, 1045–1047. [DOI] [PubMed] [Google Scholar]
- Krawchuk, M. D., and Wahls, W. P. (1999). High-efficiency gene targeting in Schizosaccharomyces pombe using a modular, PCR-based approach with long tracts of flanking homology. Yeast 15, 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., and Benezra, R. (1996). Identification of a human mitotic checkpoint gene: hsMAD2. Science 274, 246–248. [DOI] [PubMed] [Google Scholar]
- Liu, S. T., Hittle, J. C., Jablonski, S. A., Campbell, M. S., Yoda, K., and Yen, T. J. (2003). Human CENP-I specifies localization of CENP-F, MAD1 and MAD2 to kinetochores and is essential for mitosis. Nat. Cell Biol. 5, 341–345. [DOI] [PubMed] [Google Scholar]
- Martin-Lluesma, S., Stucke, V. M., and Nigg, E. A. (2002). Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science 297, 2267–2270. [DOI] [PubMed] [Google Scholar]
- Maundrell, K. (1993). Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127–130. [DOI] [PubMed] [Google Scholar]
- Mellone, B. G., and Allshire, R. C. (2003). Stretching it: putting the CEN(P-A) in centromere. Curr. Opin. Genet. Dev. 13, 191–198. [DOI] [PubMed] [Google Scholar]
- Meluh, P. B., and Koshland, D. (1995). Evidence that the MIF2 gene of Saccharomyces cerevisiae encodes a centromere protein with homology to the mammalian centromere protein CENP-C. Mol. Biol. Cell 6, 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami, Y., Hori, T., Kimura, H., and Fukagawa, T. (2005). The functional region of CENP-H interacts with the Nuf2 complex that localizes to centromere during mitosis. Mol. Cell. Biol. 25, 1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millband, D. N., and Hardwick, K. G. (2002). Fission yeast Mad3p is required for Mad2p to inhibit the anaphase-promoting complex and localizes to kinetochores in a Bub1p-, Bub3p-, and Mph1p-dependent manner. Mol. Cell. Biol. 22, 2728–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoda, A., Saitoh, S., Takahashi, K., and Toda, T. (2005). BAF53/Arp4 homolog Alp5 in fission yeast is required for histone H4 acetylation, kinetochore-spindle attachment, and gene silencing at centromere. Mol. Biol. Cell 16, 316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, S., Klar, A., and Nurse, P. (1991). Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823. [DOI] [PubMed] [Google Scholar]
- Nabeshima, K., Saitoh, S., and Yanagida, M. (1997). Use of green fluorescent protein for intracellular protein localization in living fission yeast cells. Methods Enzymol. 283, 459–471. [DOI] [PubMed] [Google Scholar]
- Nabetani, A., Koujin, T., Tsutsumi, C., Haraguchi, T., and Hiraoka, Y. (2001). A conserved protein, Nuf2, is implicated in connecting the centromere to the spindle during chromosome segregation: a link between the kinetochore function and the spindle checkpoint. Chromosoma 110, 322–334. [DOI] [PubMed] [Google Scholar]
- Nishihashi, A., Haraguchi, T., Hiraoka, Y., Ikemura, T., Regnier, V., Dodson, H., Earnshaw, W. C., and Fukagawa, T. (2002). CENP-I is essential for centromere function in vertebrate cells. Dev. Cell 2, 463–476. [DOI] [PubMed] [Google Scholar]
- Obuse, C., Iwasaki, O., Kiyomitsu, T., Goshima, G., Toyoda, Y., and Yanagida, M. (2004). A conserved Mis12 centromere complex is linked to heterochromatic HP1 and outer kinetochore protein Zwint-1. Nat. Cell Biol. 6, 1135–1141. [DOI] [PubMed] [Google Scholar]
- Osborne, M. A., Schlenstedt, G., Jinks, T., and Silver, P. A. (1994). Nuf2, a spindle pole body-associated protein required for nuclear division in yeast. J. Cell Biol. 125, 853–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux, A. L., Richardson, W., and Allshire, R. C. (2003). Sim 4, a novel fission yeast kinetochore protein required for centromeric silencing and chromosome segregation. J. Cell Biol. 161, 295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, M. A., Springer, G. H., Granada, B., and Piston, D. W. (2004). An improved cyan fluorescent protein variant useful for FRET. Nat. Biotechnol. 22, 445–449. [DOI] [PubMed] [Google Scholar]
- Saitoh, H., Tomkiel, J., Cooke, C. A., Ratrie, H., 3rd, Maurer, M., Rothfield, N. F., and Earnshaw, W. C. (1992). CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell 70, 115–125. [DOI] [PubMed] [Google Scholar]
- Saitoh, S., Takahashi, K., and Yanagida, M. (1997). Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell 90, 131–143. [DOI] [PubMed] [Google Scholar]
- Skoufias, D. A., Andreassen, P. R., Lacroix, F. B., Wilson, L., and Margolis, R. L. (2001). Mammalian mad2 and bub1/bubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl. Acad. Sci. USA 98, 4492–4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., Chen, E. S., and Yanagida, M. (2000). Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science 288, 2215–2219. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., Murakami, S., Chikashige, Y., Funabiki, H., Niwa, O., and Yanagida, M. (1992). A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol. Biol. Cell 3, 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., Yamada, H., and Yanagida, M. (1994). Fission yeast minichromosome loss mutants mis cause lethal aneuploidy and replication abnormality. Mol. Biol. Cell 5, 1145–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S. S., Ha, E., and McKeon, F. (1998). The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J. Cell Biol. 142, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S. S., and McKeon, F. (1997). Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell 89, 727–735. [DOI] [PubMed] [Google Scholar]
- Toyoda, Y., Furuya, K., Goshima, G., Nagao, K., Takahashi, K., and Yanagida, M. (2002). Requirement of chromatid cohesion proteins rad21/scc1 and mis4/scc2 for normal spindle-kinetochore interaction in fission yeast. Curr. Biol. 12, 347–358. [DOI] [PubMed] [Google Scholar]
- Vanoosthuyse, V., Valsdottir, R., Javerzat, J. P., and Hardwick, K. G. (2004). Kinetochore targeting of fission yeast Mad and Bub proteins is essential for spindle checkpoint function but not for all chromosome segregation roles of Bub1p. Mol. Cell. Biol. 24, 9786–9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, J. C., Chen, R. H., Murray, A. W., and Salmon, E. D. (1998). Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141, 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann, S., Cheeseman, I. M., Anderson, S., Yates, J. R., 3rd, Drubin, D. G., and Barnes, G. (2003). Architecture of the budding yeast kinetochore reveals a conserved molecular core. J. Cell Biol. 163, 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge, P. A., Jensen, O. N., Holmes, S., Soues, S., Mann, M., and Kilmartin, J. V. (1998). Analysis of the Saccharomyces spindle pole by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry. J. Cell Biol. 141, 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge, P. A., and Kilmartin, J. V. (2001). The Ndc80p complex from Saccharomyces cerevisiae contains conserved centromere components and has a function in chromosome segregation. J. Cell Biol. 152, 349–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, H., Kumada, K., and Yanagida, M. (1997). Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J. Cell Sci. 110, 1793–1804. [DOI] [PubMed] [Google Scholar]
- Zhou, H., and Thiele, D. J. (2001). Identification of a novel high affinity copper transport complex in the fission yeast Schizosaccharomyces pombe. J. Biol. Chem. 276, 20529–20535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.