Abstract
Background
Polycystic Ovary Syndrome (PCOS) is the most common endocrine disorder, affecting 5–10% of women of reproductive age. Major complications of PCOS include infertility, obesity, endometrial hyperplasia, endometrial cancer, insulin resistance, hyperandrogenism, and cardiovascular issues. This study aims to investigate the polymorphism of the Insulin Resistin (RETN) gene at positions − 420 C\G and + 299 A\G in relation to the susceptibility to PCOS.
Methods
This case-control study included 198 participants (100 diagnosed with PCOS and 98 normal controls). Two single nucleotide polymorphisms of the RETN gene − 420(C/G) (rs1862513) and + 299(G/A) (rs3745367), were analyzed using the PCR-RFLP method. Genomic DNA was extracted from blood samples using a DNA extraction kit. The PCR product was digested with restriction enzymes BbsI and AluI, and the results were analyzed by electrophoresis on an agarose gel. Statistical analysis determined the association of the genotypic and allelic variations with PCOS.
Results
The findings indicate no significant association between the RETN gene polymorphism and PCOS.
Discussion
Our study found that RETN gene polymorphisms do not appear to play a significant role in PCOS susceptibility in the Iranian population. These results suggest that other genetic or environmental factors may contribute more significantly to the development of PCOS. Further research with larger sample sizes and additional genetic markers is necessary to understand the genetic basis of PCOS fully.
Keywords: Polycystic Ovary Syndrome (PCOS), Gene polymorphism, Resistin gene (RETN), PCR-RFLP, Insulin resistance
Introduction
Polycystic Ovary Syndrome (PCOS) is a complex and multifaceted endocrine disorder characterized primarily by chronic anovulation and hyperandrogenism. Affecting 5–10% of women of reproductive age, PCOS manifests with a broad spectrum of symptoms, including reproductive, metabolic, and psychological disturbances, that significantly impact quality of life. The pathophysiology of PCOS is primarily driven by insulin resistance (IR), a condition where cells fail to respond effectively to insulin, leading to hyperinsulinemia. This, in turn, exacerbates hyperandrogenism—a hallmark feature of PCOS characterized by excess levels of male hormones. These hormonal imbalances contribute to symptoms such as menstrual irregularities, infertility, obesity, and metabolic syndrome. Understanding the biochemical mechanisms underlying PCOS, particularly the interaction between IR and androgen production, is crucial for the development of effective diagnostic and therapeutic strategies. The chronic nature of PCOS not only imposes significant health challenges but also results in considerable financial burdens over the lifetime of those affected [1]. The pathophysiology of PCOS is primarily driven by insulin resistance (IR) and hyperandrogenism, both of which are critical to the syndrome’s development. Obesity exacerbates insulin resistance in patients with PCOS, significantly increasing their risk of developing metabolic syndrome, prediabetes, and type 2 diabetes [2].
PCOS is now recognized as the most prevalent endocrine disorder affecting women of reproductive age, with a global prevalence ranging from 6 to 21%, depending on the diagnostic criteria [3, 4]. First described in the mid-18th century, the syndrome was initially associated with pelvic pain and menorrhagia. Throughout this period, environmental factors, such as diet and lifestyle, significantly impacted women’s health. Given that PCOS patients often retain fat and exhibit altered metabolic responses, it is possible that these traits provided survival advantages during periods of famine. These metabolic adaptations may have enabled women with PCOS to continue reproducing under adverse conditions, which would have been crucial for population survival in times of scarcity. From an evolutionary perspective, traits linked to PCOS, including irregular ovulation and metabolic dysfunction, might have been advantageous in specific historical contexts. For example, during food shortages, women with PCOS may have had a reproductive advantage over their peers, as their metabolic systems were more adapted to subsist on limited resources [5, 6]. However, by the early 20th century, new theories emerged, suggesting that PCOS might be associated with inflammation due to infection, vascular congestion caused by pressure, partial ovarian torsion disrupting blood flow, or ovarian dystrophy stemming from nutritional deficiencies [7, 8]. The hallmark features of PCOS encompass a range of clinical manifestations, including infertility, obesity, endometrial hyperplasia, endometrial cancer, insulin resistance, hyperandrogenism, and cardiovascular complications [9, 10].
Understanding the etiology of PCOS is essential for the development of effective diagnostic and therapeutic strategies. The etiology of PCOS is complex and multifactorial, involving both genetic and environmental factors [9]. Genetic predisposition plays a significant role in PCOS development, with various gene polymorphisms being investigated for their potential contributions to the disorder [10]. However, while genetic factors are important, environmental factors that exacerbate insulin resistance (IR) and hormonal imbalances—which may lead to the development of PCOS in genetically predisposed individuals—are also critical. Such factors include obesity, dietary habits, and physical inactivity [11]. Previous studies have emphasized the significance of genetic variations in pathways associated with IR and androgen production, both of which are central to PCOS pathophysiology [12].
Resistin, a hormone predominantly secreted by adipose tissue, has been the subject of significant research in the context of obesity-related insulin resistance (IR) and metabolic disorders [13, 14]. The RETN gene, located on chromosome 19p13.2, is of particular interest due to its implications in metabolic and reproductive health. In conditions such as polycystic ovary syndrome (PCOS), where IR commonly disrupts glucose homeostasis and lipid metabolism, the role of genes like RETN becomes especially critical. Notably, elevated resistin levels have been observed in patients with PCOS, suggesting a potential link between RETN gene polymorphisms and the pathophysiology of the condition [15]. Investigating the complex interactions between resistin, the RETN gene, insulin resistance, and metabolic dysfunctions is essential for unraveling the underlying mechanisms of PCOS and for developing targeted therapeutic interventions.
This study focuses on the polymorphisms of the RETN gene at positions − 420 C\G and + 299 A\G, examining their possible association with susceptibility to PCOS in the Iranian population. By exploring genetic variations in RETN, this research seeks to deepen the understanding of the genetic determinants contributing to PCOS. Insights gained from this study could inform future genetic screening strategies and pave the way for personalized treatment approaches for PCOS patients.
Ultimately, this investigation aligns with broader efforts to leverage genetic insights to improve the management of PCOS, shedding light on the complex relationship between genetic factors and susceptibility to this syndrome within an Iranian cohort [16].
Method and materials
Study population and patient selection
The study population consisted of 198 Iranian women, aged 18 to 35, including 100 patients diagnosed with PCOS and 98 normal female controls. These participants were recruited from Motahari Clinic, affiliated with Shiraz University of Medical Sciences, Shiraz, Iran. Shiraz, located in the southern region of Iran, represents a diverse population from different ethnic backgrounds. The study period extended from May 2011 to February 2012. All participants provided written informed consent, and the study protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences (302.3.421). All experiments were conducted in accordance with the ethical standards of the Declaration of Helsinki and relevant guidelines and regulations. The diagnosis of PCOS followed the Rotterdam criteria, ensuring standardized and internationally recognized diagnostic guidelines [17].
Blood samples (7–8 ml) containing EDTA anticoagulant were collected from each participant after an overnight fast, at some point within 2–6 days of the menstrual cycle and was stored appropriately.
At a follow-up visit, participants with ultrasound-confirmed polycystic ovaries and normal thyroid and prolactin test results were enrolled. A comprehensive questionnaire was completed for each participant, collecting data on age, marital status, height, weight, menstrual history, infertility status, ultrasound findings, and hormone test results. All participants were examined for signs of hyperandrogenism, metabolic syndrome, and central obesity. Those showing any of these signs were referred for further evaluation. Married participants also underwent vaginal examinations. Informed consent was obtained for all additional procedures.
Inclusion and exclusion criteria
PCOS was diagnosed when at least two of the following three criteria were met: [1] clinical and/or biochemical signs of hyperandrogenism [2], chronic anovulation (fewer than six menstrual cycles in a year), and [3] polycystic ovaries. These criteria align with the guidelines established by the ESHRE/ASRM PCOS Consensus Workshop in 2003. Patients diagnosed by a physician were included in the study. Exclusion criteria were as follows: androgen-secreting tumors, Cushing’s syndrome, 21-hydroxylase enzyme deficiency, hyperprolactinemia, thyroid dysfunction, or prior hormonal therapy. Additionally, women with severe symptoms of androgen excess, such as frontal baldness or deepening of the voice, and those who had used hormonal contraceptives or insulin-sensitizing drugs in the three months prior to the study, were excluded.
Control group selection
The control group consisted of 98 women matched for age, weight, and body mass index (BMI) with the PCOS group. These women had regular menstrual cycles, no signs of hyperandrogenism, and no history of infertility. Control participants were required to have had at least one uncomplicated pregnancy with vaginal delivery. Women with a history of cesarean section or pelvic surgery were excluded to avoid potential effects of ovarian manipulation. Ultrasound examinations confirmed normal ovarian function. Additionally, participants who had experienced pregnancy, childbirth, or breastfeeding within the last year were excluded.
Diagnostic procedures
Participants underwent pelvic or transvaginal ultrasound as part of the diagnostic evaluation. Hormonal tests were conducted between days 2 to 4 of the menstrual cycle. For women with amenorrhea, progesterone was administered before testing. Tests included thyroid function and prolactin levels. If PCOS was diagnosed without evidence of other endocrine disorders, participants were included in the study.
Genotype analysis
Genomic DNA was extracted from the blood samples using a commercial kit (Gentebio, Korea). A PCR-RFLP method was performed to genotype the RETN gene regions containing the − 420 C/G and + 299 A/G polymorphisms [16, 18]. The table related to gene primers is mentioned in Table 1. The PCR products were then digested with BbsI and AluI restriction enzymes, respectively (Cinnagen, Iran). The digested products were run on 2% or 3% gels. The RETN 62 (G→A) digested products were run on 10% acrylamide gels. The gels were then stained with ethidium bromide and visualized under UV light.
Table 1.
Primer sequences, PCR conditions and RFLP patterns
| Forward primer Reverse primer | Annealing temperature | RFLP analysis | Genotype: RFLP fragments size (bp) | ||
|---|---|---|---|---|---|
| Restriction enzyme | Incubation temperature | ||||
| RETN − 420 C/G |
5’-TGTCATTCTCACCCAGAGAC-3’ 5’-TGGGCTCAGCTAACCAAATC − 3’ |
52 °C | BbsI | 37 °C |
CC: 533 CG: 309 + 204 GG: 533 + 309 + 204 |
| RETN + 299 A/G |
5’-CAGCGCTCACCAAATCTCATCC − 3’ 5’-TCCAGGACCCTGTCTTGAGTTGG-3’ |
58 °C | AluI | 37 °C |
AA: 173 AG: 82 + 91 + 173 GG: 82 + 91 |
*PCR conditions were optimized for each set of primers to ensure specific amplification of the target gene regions. The restriction enzymes used in the RFLP analysis were BbsI and AluI, which digest the PCR products into specific fragment sizes for genotyping the polymorphisms. RETN: Resistin Gene, PCR: Polymerase Chain Reaction, RFLP: Restriction Fragment Length Polymorphism, bp: Base Pair, °C: Degrees Celsius, Incubation temperature is the temperature at which the enzymes were incubated during the PCR-RFLP analysis
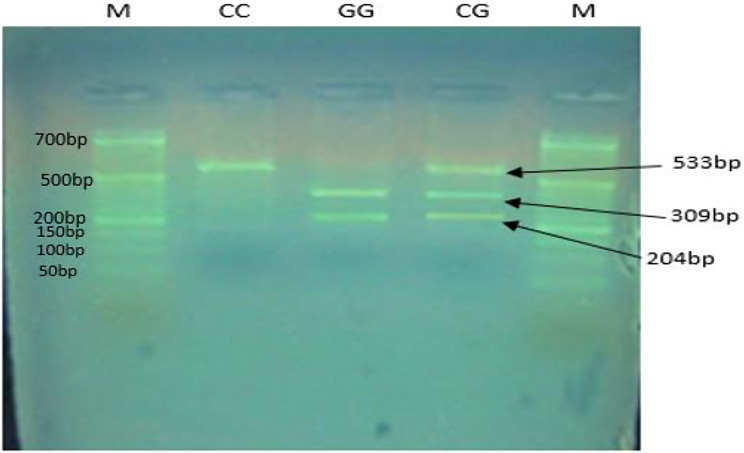
As shown in Fig. 1, the PCR-RFLP analysis of the − 420 C/G polymorphism in the RETN gene was performed following digestion with the BbsI enzyme. Figure 2 illustrates the PCR-RFLP analysis of the + 299 A/G polymorphism in the RETN gene, using the AluI enzyme.
Fig. 1.
PCR-RFLP analysis of the − 420 C/G polymorphism in the RETN gene after digestion with the BbsI enzyme. The electrophoresis was performed on an agarose gel. The bands correspond to the following genotypes: CC genotype: 533 bp. CG genotype: 533 bp, 309 bp, 204 bp. GG genotype: 309 bp, 204 bp. Marker (M): 50 bp ladder
Fig. 2.
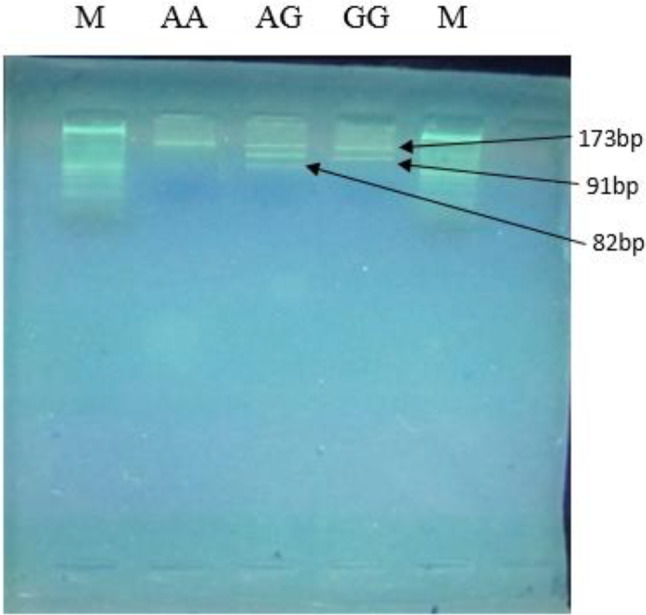
PCR-RFLP analysis of the + 299 A/G polymorphism in the RETN gene after digestion with the AluI enzyme. The electrophoresis was performed on an agarose gel. The bands correspond to the following genotypes: AA genotype: 173 bp. AG genotype: 173 bp, 91 bp, 82 bp. GG genotype: 91 bp, 82 bp. Marker (M): 50 bp ladder
Statistical analysis
In this study, statistical differences were analyzed using SPSS version 16. For the statistical analysis of genomic data, the chi-square test was used. For the A/G + 299 locus, a confidence interval of 95% was applied, and for the C/G -420 locus, a confidence interval of 85% was used. The Minor Allele Frequency (MAF) for the RETN gene polymorphisms was calculated. MAF was calculated for both the PCOS and control groups for the − 420 C/G polymorphism to assess the allelic distribution. P-values less than 0.05 were considered statistically significant.
Results
The demographic characteristics of the study population are presented in Table 2, showing the comparison between the PCOS and control groups. Significant differences were observed in variables such as BMI, hirsutism, menstrual irregularity, and infertility history, all with p-values less than 0.05, indicating statistically significant differences between the two groups. The results of the genotype analysis are shown in Table 3. The results indicated no significant differences in the RETN gene polymorphisms (-420 C/G and + 299 A/G) between the PCOS and control groups. However, it was observed that the frequency of the CG genotype and the G allele in the − 420 C/G polymorphism increased slightly in the control group, while the frequency of the GG genotype decreased. For the + 299 A/G polymorphism, the AG genotype frequency slightly increased, and the AA genotype frequency slightly decreased in the control group, despite these differences being statistically insignificant. In our initial analysis, an 85% confidence interval was used for the − 420 C/G polymorphism to account for the smaller sample size and lower frequency of the polymorphism, which might have reduced the statistical power to detect significant associations. However, upon further review, we reanalyzed the data using a 95% CI for both polymorphisms to ensure consistency and robustness. No significant associations were found using the 95% CI. The MAF for the G allele of the RETN − 420 C/G polymorphism was 0.545 in the PCOS group and 0.535 in the control group. Similarly, the MAF for the G allele of the RETN + 299 A/G polymorphism was 0.495 in the PCOS group and 0.505 in the control group.
Table 2.
Demographic characteristics of the Study Population
| Characteristic | PCOS Group (n = 100) | Control Group (n = 98) |
|---|---|---|
| Age (years, mean ± SD) | 27 ± 5 | 28 ± 4 |
| BMI (kg/m², mean ± SD) | 28 ± 4 | 24 ± 3 |
| Marital Status (%) | Married: 65% | Married: 70% |
| Hirsutism (n, %) | 75 (75%) | 0 (0%) |
| Menstrual Irregularity (%) | 85% | 0% |
| Infertility History (%) | 40% | 0% |
| Polycystic Ovaries on Ultrasound (%) | 100% | 0% |
| Hyperandrogenism Symptoms (%) | 100% | 0% |
| Pregnancy History (at least 1 pregnancy, %) | 30% | 70% |
*Data are presented as mean ± SD for continuous variables (e.g., Age, BMI) and percentages for categorical variables (e.g., Marital Status, Hirsutism, etc.). BMI: Body Mass Index, SD: Standard Deviation, PCOS: Polycystic Ovary Syndrome
Table 3.
Genotype and allelic frequency of the RETN gene polymorphisms in PCOS and controls
| PCOS (n = 100) | Control (n = 98) | Odds Ratio | P-value | |
|---|---|---|---|---|
| RETN − 420 C/G | ||||
| Genotypes, n (%) | ||||
| CC | 20 (19.8%) | 20 (20.4%) | 0.97 (95% CI: 0.48 to 1.97) | 0.854 |
| CG | 51 (50.4%) | 53 (54%) | 0.89 (95% CI: 0.51 to 1.55) | |
| GG | 29 (28.7%) | 25 (25.5%) | 1.18 (95% CI: 0.61 to 2.28) | |
| Alleles, n (%) | ||||
| G | 109 (54.5%) | 103 (52.5%) | 0.69 | |
| C | 91 (45.5%) | 93 (47.4%) | ||
| RETN + 299 A/G | ||||
| Genotypes, n (%) | ||||
| AA | 13 (12.8%) | 12 (12.2%) | 1.07 (95% CI: 0.46 to 2.48) | 0.958 |
| AG | 75 (74.2%) | 73 (74.4%) | 1.03 (95% CI: 0.54 to 1.95) | |
| GG | 12 (11.8%) | 13 (13.2%) | 0.89 (95% CI: 0.39 to 2.06) | |
| Alleles, n (%) | ||||
| A | 101 (50.5%) | 97 (49.4%) | 0.69 | |
| G | 99 (49.5%) | 99 (50.5%) |
*The chi-square test was applied to compare the distribution of genotypes and alleles between the PCOS and control groups. P-values less than 0.05 were considered statistically significant. PCOS: Polycystic Ovary Syndrome, RETN: Resistin Gene, Genotypes refer to the genetic makeup of an organism with respect to a trait, n: Number of participants, Alleles: Different forms of the same gene, P-value: The statistical measure indicating the significance of results
Based on Table 4, the frequency of RETN gene haplotypes at positions − 420 and + 299 shows the following results between patient and control groups: The CA haplotype was observed in 51.06% of patients and 48.94% of controls. The CG haplotype was present in 50.37% of patients and 49.63% of controls. The GA haplotype was found in 48.68% of patients and 51.32% of controls. The GG haplotype was seen in 48.28% of patients and 51.72% of controls. These differences were not statistically significant.
Table 4.
Haplotype frequencies of RETN Gene at positions − 420 and + 299 in patient and control groups
| + 299 & -420 haplotypes, n (%) | PCOS | Control | X2 | P Value |
|---|---|---|---|---|
| CA | 24(51.06%) | 23(48.94%) | 0.0529 | 0.94 |
| CG | 68(5.37%) | 67(49.63%) | 0.0639 | 0.88 |
| GA | 74(48.68%) | 78(51.32%) | 0.0645 | 0.88 |
| GG | 28(48.28%) | 30(51.72%) | 0.5780 | 0.53 |
*The chi-square test was used to evaluate the association between the RETN haplotypes and PCOS status in both the patient and control groups. P-values less than 0.05 were considered statistically significant. RETN: Resistin Gene, PCOS: Polycystic Ovary Syndrome, X²: Chi-square test value, Haplotypes: Combinations of alleles at multiple loci that are transmitted together
Discussion
This study investigated the polymorphisms of the RETN gene at positions − 420 C/G and + 299 A/G in relation to the susceptibility to PCOS in an Iranian population. Our findings indicate no significant association between these RETN gene polymorphisms and PCOS. The analysis revealed that the frequencies of the genotypes and alleles at both positions did not differ significantly between the PCOS patients and the control group, suggesting that these polymorphisms in the RETN gene do not play a significant role in PCOS susceptibility in this population [19]. These results align with studies conducted in other populations, such as those from European and Asian, which similarly found no significant associations between RETN gene variants and PCOS.
In our study, no significant association was found between the RETN gene polymorphisms (-420 C/G and + 299 A/G) and susceptibility to PCOS in the Iranian population. This finding is consistent with a study conducted in Pakistan, where no significant correlation between RETN gene polymorphisms and PCOS was observed. Similarly, research on Iraqi adolescents diagnosed with PCOS also failed to find any major association between RETN gene variants and the syndrome. These results suggest that in Middle Eastern populations, the RETN gene may not be a major factor in PCOS susceptibility. However, in South Asian populations, such as in India, studies have reported conflicting findings. For instance, one study found a potential link between RETN gene polymorphisms and insulin resistance in women with PCOS, highlighting the role that genetic diversity and environmental factors may play in influencing PCOS risk. In contrast, a Turkish study focusing on metabolic syndrome in PCOS patients did not observe a significant association with RETN gene polymorphisms, reinforcing the idea that the genetic background of populations plays a critical role in determining the impact of such polymorphisms. These regional variations highlight the complexity of PCOS etiology and the potential influence of both genetic and environmental factors. While the RETN gene is a well-known contributor to insulin resistance, its role in PCOS may be more nuanced and population-specific [12, 14, 17, 18].
This discrepancy highlights the complexity of PCOS etiology and the potential influence of ethnic and genetic diversity on disease susceptibility [20]. The calculated MAFs in this study for both polymorphisms are consistent with findings from other genetic studies of PCOS, where no significant differences in allele frequencies were observed between PCOS patients and controls. These results suggest that the RETN gene polymorphisms may not play a major role in PCOS susceptibility, aligning with findings in Middle Eastern populations.
It is known that abnormal insulin signaling and metabolic dysfunction in insulin-responsive tissues are known to contribute to IR in women with PCOS, leading to various metabolic and reproductive abnormalities. Studies have shown that women with PCOS face IR and overexposure to androgens, resulting in increased risks of metabolic syndrome, prediabetes, and type 2 diabetes [21].
In our study, the absence of a significant association between RETN gene polymorphisms and PCOS aligns with previous research in diverse populations. For instance, a study conducted by Urbanek et al. [22] found no association between variations in the RETN gene promoter and PCOS, further supporting the lack of a direct genetic link. Moreover, research by Ou et al. [23] examined the RTN4 indel polymorphisms and highlighted their association with tumorigenesis in the Chinese Han population, yet did not establish a connection to reproductive disorders like PCOS. These findings emphasize the complexity of PCOS etiology and suggest that while RETN gene polymorphisms may contribute to other health conditions, their role in PCOS susceptibility remains unclear. Further studies involving larger and more diverse populations are necessary to fully elucidate the genetic factors underlying PCOS [24, 25]. In the study conducted by Dr. Han Zhao and colleagues, it has been shown that abnormal insulin signaling and metabolic dysfunction in insulin-responsive tissues cause women with PCOS to develop IR. IR is highly prevalent in PCOS and has a major detrimental effect on health [26]. It has been demonstrated in the research that Women with PCOS face IR and overexposure of androgen, leading to a number of metabolic and reproductive abnormalities [27]. An Iranian study revealed that women with PCOS had significantly higher serum concentrations of glucose, insulin, and chemerin, indicating a strong association between metabolic dysfunction and PCOS [28].
In light of the current findings, future research should focus on conducting studies with larger sample sizes to increase the statistical power and validate the results in diverse populations. Expanding the sample size may help uncover subtle genetic associations that were not detected in this study due to limited participant numbers. Additionally, investigating other genetic markers and their interactions with environmental factors could offer a more comprehensive understanding of the genetic basis of PCOS [25]. One significant limitation of this study is the exclusive focus on the RETN gene without considering other potential genetic and biochemical factors [26]. PCOS is a multifactorial disorder, and multiple genes and their interactions with environmental factors likely contribute to its development [8].
Given the conflicting results across different populations, future research should focus on larger and more ethnically diverse cohorts to validate the findings and explore potential genetic variations that may not have been detected due to limited sample sizes. Studies like those conducted in South Asian populations (e.g., Nambiar et al.; Dadachanji et al.) suggest that genetic factors related to insulin resistance may vary across ethnicities. Expanding the investigation to other genetic markers and their interactions with environmental factors could provide a more comprehensive understanding of the genetic basis of PCOS. Additionally, longitudinal studies that track the progression of PCOS in diverse populations would be valuable in clarifying the role of genetic polymorphisms in the long-term outcomes of the disorder.
Future studies should adopt a more holistic approach by examining a broader range of genetic markers and considering the influence of biochemical and environmental variables. Longitudinal studies that follow patients over time would also be essential to elucidate the progression of PCOS and the influence of genetic factors in its long-term outcomes [27, 29].
In conclusion, while our study did not find a significant association between RETN gene polymorphisms and PCOS, it underscores the need for continued research with larger and more diverse populations. The findings of this study highlight the complexity and variability in the genetic associations of PCOS, particularly concerning RETN gene polymorphisms. Exploring the physiological mechanisms by which these polymorphisms influence metabolic and reproductive traits across different populations remains crucial. Given that genetic susceptibility can be heavily influenced by ethnic, cultural, and environmental factors, future research should focus on larger and more diverse cohorts, incorporating a broader range of genetic markers and their interactions with lifestyle and environmental influences. Such efforts are essential to unravel the complex genetic architecture of PCOS and to develop effective diagnostic and therapeutic strategies tailored to diverse populations.
Conclusion
In conclusion, our study investigated the polymorphisms of the RETN gene at positions − 420 C/G and + 299 A/G and their association with susceptibility to PCOS in an Iranian population. The results indicated no significant association between these polymorphisms and PCOS, suggesting that RETN gene variants do not play a major role in the susceptibility to this condition in the studied population. These findings align with similar studies in other populations that also found no significant link between RETN gene polymorphisms and PCOS.
However, the complexity of PCOS etiology, influenced by both genetic and environmental factors, necessitates further research. Future studies should include larger sample sizes and consider additional genetic markers to provide a more comprehensive understanding of the genetic basis of PCOS. This study is one of the first represents the first investigation of RETN gene variants in an Iranian population and suggests that different populations may have distinct genetic profiles related to PCOS. Further cross-population studies and longitudinal research are needed to develop targeted diagnostic and therapeutic strategies.
In summary, while our study did not find a significant association between RETN gene polymorphisms and PCOS, it highlights the need for ongoing research to unravel the genetic architecture of PCOS and to develop effective diagnostic and therapeutic strategies.
Acknowledgements
None.
Abbreviations
- PCOS
Polycystic Ovary Syndrome
- RETN
Resistin
- IR
Insulin resistance
Author contributions
MBG, AA, FD, MAH, BGF, and ZM contributed in material preparation, data collection and analysis. ZM and MBG writing the first draft of the manuscript. All authors contributed to the conception and design of the study. They commented on the manuscript, read, and approved the final manuscript.
Funding
Shiraz University of Medical Sciences financially supported the present article (Grant number: 3847).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Additionally, information on the RETN gene polymorphisms (-420 C/G and +299 A/G) analyzed in this study can be accessed through the NCBI SNP database at the following links: rs1862513: https://www.ncbi.nlm.nih.gov/snp/rs1862513rs3745367: https://www.ncbi.nlm.nih.gov/snp/rs3745367.
Declarations
Ethics approval and consent to participate
All participants provided informed consent, and the study was approved by the Ethics Committee of Shiraz University of Medical Sciences (approval code: 302.3.421). All experiments were conducted in accordance with the ethical standards of the Declaration of Helsinki and relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Behrouz Gharesi-Fard, Email: gharesifb@sums.ac.ir.
Ziba Majidi, Email: majidi.ziba@gmail.com.
References
- 1.El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly cystic ovarian syndrome: an updated overview. Front Physiol. 2016;7:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yau TT, Ng NY, Cheung L, Ma RC. Polycystic ovary syndrome: a common reproductive syndrome with long-term metabolic consequences. Hong Kong Med J. 2017;23(6):622. [DOI] [PubMed] [Google Scholar]
- 3.Manu TS, Victoria, Pranav Kumar P. Pathophysiology of polycystic ovarian syndrome. In: Zhengchao W, editor. Polycystic ovary syndrome. Rijeka: IntechOpen; 2022. Ch. 1. [Google Scholar]
- 4.Witchel SF, Oberfield SE, Peña AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. 2019;3(8):1545–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azziz R, Dumesic DA, Goodarzi MO. Polycystic ovary syndrome. Fertil Steril. 2011;95(5):1544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suturina L, Belkova N, Igumnov I, Lazareva L, Danusevich I, Nadeliaeva I, et al. Polycystic ovary syndrome and gut microbiota: phenotype matters. Life. 2022;13(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saeed S, Aslam T, Javed E, Choudhary M, Lateef M, Bajwa RM. Polycystic ovary syndrome (PCOS): a concerning Hormonal Condition and its bodily impact on women. BioScientific Rev. 2022;4(4):1–20. [Google Scholar]
- 8.Zhao Y, Pang J, Fang X, Yan Z, Yang H, Deng Q, et al. Causal relationships between modifiable risk factors and polycystic ovary syndrome: a comprehensive mendelian randomization study. Front Endocrinol. 2024;15:1348368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrivastava S, Conigliaro RL. Polycystic ovarian syndrome. Med Clin North Am. 2023;107(2):227–34. [DOI] [PubMed] [Google Scholar]
- 10.Barkade GD, Bhongal SA, Dani PK, Gund SR. A systematic review: polycystic ovarian syndrome (PCOS). 2022.
- 11.Bhimwal T, Puneet, Priyadarshani A. Understanding polycystic ovary syndrome in light of associated key genes. Egypt J Med Hum Genet. 2023;24(1):38. [Google Scholar]
- 12.Al-Awadi AM, Saldhana FL, Bauyrzhanova Z, Nemr R, Mahmood NA, Almawi WY. Relation of resistin gene variants to resistin plasma levels and altered susceptibility to polycystic ovary syndrome: a case control study. Am J Reprod Immunol. 2023;90(1):e13731. [DOI] [PubMed] [Google Scholar]
- 13.Bakry M, Abd El-Hameed E, El-Aziz N, Reda A. MZ Hamada M. Resistin; a physiological overview. Zagazig Veterinary J. 2021;49(1):78–91. [Google Scholar]
- 14.Nambiar V, Vijesh VV, Lakshmanan P, Sukumaran S, Suganthi R. Association of adiponectin and resistin gene polymorphisms in South Indian women with polycystic ovary syndrome. Eur J Obstet Gynecol Reproductive Biology. 2016;200:82–8. [DOI] [PubMed] [Google Scholar]
- 15.Baba T, Endo T, Sata F, Nagasawa K, Honnma H, Kitajima Y, et al. The contributions of resistin and adiponectin gene single nucleotide polymorphisms to the genetic risk for polycystic ovary syndrome in a Japanese population. Gynecol Endocrinol. 2009;25(8):498–503. [DOI] [PubMed] [Google Scholar]
- 16.Layacha SY, Biswas DA. Women with polycystic ovary syndrome: a review of susceptibility to type 2 diabetes. Cureus. 2023;15(1). [DOI] [PMC free article] [PubMed]
- 17.Jabok SKA, Al Ibrahimi NA. Polycystic ovarian syndrome in some sample of Iraqi adolescents: implementation of Consensus guidelines. Kufa Med J. 2023;19(1):7–17. [Google Scholar]
- 18.Nawaz Y, Ghazanvi S, Rasheed N, Jahan S, Ullah MI. Association of Serum Resistin Level and Resistin (RETN) Gene (-420 C > G) polymorphism in Pakistani women with polycystic ovarian syndrome. Turkish J Endocrinol Metabolism. 2020;24(1).
- 19.Fathy P, Cheraghi E, Miresmaeili SM. Association between single nucleotide polymorphisms (rs1484215 and rs6495096) in CYP11A1 gene in Iranian women with polycystic ovary syndrome. J Reprod Infertility. 2023;24(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dadachanji R, Shaikh N, Mukherjee S. Genetic variants associated with hyperandrogenemia in PCOS pathophysiology. Genet Res Int. 2018;2018(1):7624932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyrmi JS, Arffman RK, Pujol-Gualdo N, Kurra V, Morin-Papunen L, Sliz E, et al. Leveraging northern European population history: novel low-frequency variants for polycystic ovary syndrome. Hum Reprod. 2022;37(2):352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbanek M, Du Y, Silander K, Collins FS, Steppan CM, Strauss JF III, et al. Variation in resistin gene promoter not associated with polycystic ovary syndrome. Diabetes. 2003;52(1):214–7. [DOI] [PubMed] [Google Scholar]
- 23.Ou X, Peng S, Han X, Zhou S, Fan L, Gao X. Association of RTN4 indel polymorphisms with the risk of tumorigenesis in the Chinese Han population. Cell Mol Biol. 2023;69(2):74–8. [DOI] [PubMed] [Google Scholar]
- 24.Khan MJ, Ullah A, Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet. 2019;12:249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma D, Verma K, Musharraf A. An epidemiological study to assess the risk factors and symptoms of PCOS. Asian J Curr Res. 2024;9(2):189–202. [Google Scholar]
- 26.Kaur I, Kishore K, Suri V, Sahni N, Rana SV, Singh A. Determinants of polycystic ovary syndrome: a matched case–control study. J Hum Nutr Dietetics. 2024;37(2):583–92. [DOI] [PubMed] [Google Scholar]
- 27.Dapas M, Diamanti-Kandarakis E, Dunaif A, Franks S, Kandaraki E, Laven J, et al. RF10| PMON217 replication of PCOS Reproductive and metabolic subtypes in diverse cohorts–towards a Rationale Approach to PCOS classification. J Endocr Soc. 2022;6(Supplement1):A711–A. [Google Scholar]
- 28.Haghighi S, Yaghmaei P, Hashemi F, Saadati N, Tehrani FR, Hedayati M. The association between serum chemerin concentration and polycystic ovarian syndrome. Tehran Univ Med J. 2012;70(5).
- 29.Majidi Z, Emamgholipour S, Omidifar A, Fard SR, Poustchi H, Shanaki M. The CTRP5 circulating levels and the ratio of CTRP1 to CTRP5 in plasma are significant predictors for Carotid Intima-Media thickness (cIMT) value in patients with type 2 diabetes: a preliminary study. 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Additionally, information on the RETN gene polymorphisms (-420 C/G and +299 A/G) analyzed in this study can be accessed through the NCBI SNP database at the following links: rs1862513: https://www.ncbi.nlm.nih.gov/snp/rs1862513rs3745367: https://www.ncbi.nlm.nih.gov/snp/rs3745367.