Abstract
Background
The global rise in multidrug-resistant bacteria has significantly undermined the efficacy of traditional antibiotics. Multidrug-resistant Streptococcus suis (S. suis), a pathogen capable of infecting pigs and humans, has been identified as a critical threat, causing severe meningitis and rapid mortality. In response, researchers have increasingly focused on herbal compounds as non-traditional antimicrobial agents, which can inhibit bacterial growth while minimizing the risk of resistance development. This study investigates the mechanism through which andrographolide (AP) restores the susceptibility of S. suis to aminoglycoside antibiotics.
Methods
The intracellular ΔpH in S. suis was assessed using the 2’,7’ -bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCCF-AM) probe to evaluate alterations in the proton motive force (PMF) following treatment with AP. Non-targeted metabolomics was employed to confirm changes in the metabolic profile of S. suis upon exposure to AP. Finally, an in vivo infection model was utilized to evaluate the therapeutic efficacy of AP in combination with antibiotics.
Results
Extensive in vitro experiments demonstrated that AP significantly enhances the activity of aminoglycoside antibiotics against diverse pathogens, including S. suis. Further studies revealed that bacterial death results from AP-mediated upregulation of the S. suis PMF, which enhances cellular uptake of tobramycin (TOB). Moreover, AP significantly upregulated pyruvate metabolism in S. suis, accelerated the tricarboxylic acid (TCA) cycle, and increased nicotinamide adenine dinucleotide (NADH) production. This metabolic shift further augmented the PMF. Combining AP with aminoglycoside antibiotics significantly reduced bacterial load and organ lesions in various organs in mice.
Conclusion
AP holds promise as an adjuvant to aminoglycoside antibiotics for combating S. suis-induced infections, offering a theoretical foundation for clinical applications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12917-024-04430-z.
Keywords: Streptococcus suis, Aminoglycoside antibiotics, PMF, Adjuvant
Background
In recent years, we have observed the progressive emergence and spread of multidrug-resistant bacteria, transcending regional boundaries. The emergence of these ‘superbugs’ is strongly associated with the overuse and misuse of antibiotics. Enteric and respiratory pathogens are of particular concern in the livestock sector, accounting for most global animal diseases and exhibiting high morbidity and mortality rates [1]. Streptococcus suis (S. suis) is a Gram-positive, non-flagellated bacterium typically arranged in pairs or short cell chains [2, 3]. S. suis colonizes pigs’ respiratory, nasal, digestive, and gastrointestinal tracts. It is an important zoonotic pathogen responsible for various diseases, including sepsis, arthritis, meningitis, and endocarditis, which lead to substantial economic losses [4, 5]. Severe infections caused by S. suis have limited effective treatment options and monotherapy with antimicrobial agents is frequently inadequate. Moreover, the development of novel drugs poses substantial challenges [6, 7]. Over the past few decades, novel target antibiotics have been approved for clinical use less frequently [8–10]. Studies indicate that combining natural products or synthetic compounds with conventional drugs holds significant potential against combating pathogenic bacterial infections [11, 12]. Our group found that metformin and methyl anthranilate target the S. suis quorum sensing system (QSS) in previous studies. Metformin modulates biofilm formation by inhibiting the production of the AI-2 signaling molecule of S. suis QSS. Meanwhile, methyl anthranilate competitively bound S-adenosine homocysteine nucleosidase with S-adenosylhomocysteine to interfere with AI-2 QSS. The findings suggest that metformin and methyl anthranilate function as S. suis quorum sensing inhibitors, providing a theoretical foundation for restoring bacterial susceptibility to antibiotics [13, 14].
Andrographis paniculata (Burm.f.) Nees, commonly known as “See Happiness” or “Bitter Grass”, is an annual herbaceous medicinal plant of the family Judaeidae. It is thought to have originated in India and Sri Lanka in South Asia and is widely used in traditional medicine for its heat-clearing and detoxifying properties [15]. Recent studies indicate that Andrographis paniculate (A. paniculate) exhibits significant anti-inflammatory, anti-hyperglycemic, and antiviral effects, including activity against HIV [16–18]. The primary bioactive compound in A. paniculate is Andrographolide (AP). Its molecular formula is C20H30O5, whose structure contains a large number of sp3-hybridised carbon atoms, a polycyclic ring without aromatic ring structure and aliphatic side chains, which can effectively bind to multiple biological targets. Studies have shown that AP has anti-inflammatory, antiviral, and anti-tumor activities and various pharmacological effects [19–21]. AP exhibits broad-spectrum antibacterial activity in vitro, showing potent inhibition against common Gram-negative bacteria but weaker effects against Gram-positive bacteria such as Staphylococcus aureus and S. suis [22].
In this study, we explored the synergistic bacteriostatic effect of AP. We found that AP significantly enhances the efficacy of aminoglycoside antibiotics against a broad spectrum of Gram-negative and Gram-positive bacteria, including S. suis, thereby restoring their antibacterial activity. Notably, AP promotes PMF by increasing the ΔpH of S. suis, facilitating the cellular uptake of TOB. Additionally, non-targeted metabolomics revealed that pyruvate metabolism was dramatically upregulated in the presence of AP, leading to an accelerated TCA cycle and increased NADH production [23]. Consequently, the PMF was further intensified, corroborating the observed findings. This study reveals the potential of AP as an antibiotic adjuvant for treating S. suis infection and provides a theoretical basis for effective treatment and prevention.
Materials and methods
Strains and culture conditions
S. suis HA9801,Escherichia coli O157:H7 (E. coli),Bordetella bronchiseptica QD1 (B. bronchiseptica), Streptococcus agalactiae LY016 (S. agalactiae), Glaesserella parasuis HN05 (G. parasuis) were kindly provided by the College of Animal Science and Technology, Henan University of Science and Technology (Luoyang, China). Andrographolide (the concentration is 98%) and antibiotics were obtained from Aladdin (Shanghai, China). S. Suis,S. agalactiae were grown in tryptic soy broth (TSB). E. coli,B. bronchiseptica,G. parasuis were grown in Luria-Bertani broth (LB). All bacteria were propagated at 37 °C and 180 rpm.
Growth kinetics of S. Suis
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of AP were determined using the broth microdilution method [24]. S. suis was inoculated in AP (80–1280 µg/mL) and grown at 180 rpm at 37 ℃. It was used to test the effect of different concentrations of AP on S. suis growth.
The fractional inhibitory concentration indices (FIC) index determination
FIC was determined regarding the procedure of Li [25]. Briefly, 100 µL of TSB was added to each well of a 96-well plate, followed by the addition of equal volumes of antibiotics (including TOB, Amikacin (AMK), streptomycin sulfate (SM), Kanamycin (KAN), Spectinomycin (SPT), Gentamicin (GEN), and Neomycin sulfate), mixed with AP. The antibiotics and AP were subjected to horizontal and vertical two-fold dilutions. Subsequently, 100 µL of bacterial solution (106 CFU/mL) was added to each well. The plates were incubated overnight at 37 °C, and absorbance at 600 nm was measured using a microplate reader. The calculation was done by dividing the MIC of the two drugs when used together by the MIC when used alone. FIC was the sum of the two drugs, and synergism was defined by a FIC index ≤ 0.5.
Bactericidal curve of S. suis
Based on the MIC measurements for TOB and SM, the drug concentrations used in this study were as follows: 10 µg/mL TOB, 250 µg/mL AP, 10 µg/mL TOB + 250 µg/mL AP, 320 µg/mL SM, 250 µg/mL AP, 320 µg/mL SM + 250 µg/mL AP. S. suis was diluted to 106 CFU/mL, and the corresponding antimicrobial drugs at configured concentrations were added. A blank control group, devoid of drugs, was included for comparison. The mixed bacterial solution was incubated at 37 °C and 180 rpm. Samples were collected every two hours, plated on TSB agar, and incubated overnight at 37 °C. Bactericidal curves for the different antimicrobial treatments were constructed based on colony counts.
Analysis of resistance development
To evaluate whether AP (31.25 µg/mL) mitigates the development of bacterial resistance to aminoglycoside antibiotics, S. suis was inoculated in TSB with sub-inhibitory concentrations of antibiotics, with or without AP, and subjected to sequential passages. The drug’s MIC was determined every five generations of culture. The procedure was performed in triplicate over a period of thirty consecutive days [26].
Biofilm inhibition assay
Reference to the method of Gao, with slight modifications, was followed for monitoring biofilm formation and removal of S. suis [14]. Freshly cultured S. suis was diluted to 106 CFU/mL and divided into four experimental groups (S. suis; S. suis + AP; S. suis + aminoglycoside antibiotic; and S. suis + AP + aminoglycoside antibiotic) (aminoglycoside antibiotic including TOB, AMK, SM, KAN, SPT, GEN, and Neomycin sulfate). The cultures were then incubated for 24 h at 37 °C. Once biofilm was formed, planktonic bacteria were gently removed using sterile phosphate-buffered saline (PBS). The biofilm was then fixed with 95% ethanol for 20 min and stained with 1% crystal violet for 10 min. After staining, the biofilm was rinsed twice with sterile PBS, and the crystal violet dye was solubilized with ethanol. The absorbance was detected at 595 nm.
Scanning electron microscope (SEM) of biofilm
Place a cell crawler sheet at the bottom of a 24-well plate, add 1 mL of S. suis at a concentration of 106 CFU/mL to each well, and incubate at 37 °C for 48 h. Planktonic bacteria were gently washed away with sterile PBS, and the biofilm was treated with 2.5% (w/v) glutaraldehyde for 5–8 h. PBS removed excess glutaraldehyde, and the biofilm was fixed using 1% osmium tetroxide. The membranes were dehydrated in 25%, 40%, 55%, 75%, 90%, and 100% ethanol. Gold was sputtered with a sputter coater (10 mA, 3 min) and observed by scanning electron microscopy (JSM-5610LV, Japan).
Effect of pH on combined bacterial inhibition
The pH of the TSB was adjusted by adding HCl or NaOH to a range from 5.0 to 8.0. The bacteria was adjusted to 105 CFU/mL, the combination drug was added, and the bacterial suspension was incubated at 37 °C and 180 rpm. The bacteria were incubated for 6 h and 12 h, after which 10 µL of gradient dilutions were plated, and the colonies were counted.
Determination of ΔpH by BCECF-AM
The ΔpH of AP-treated S. suis was assessed using the pH-sensitive fluorescent probe BCECF-AM (Yi Sheng Biotechnology Co., LTD). The bacteria were cultured to the logarithmic phase, the bacterial concentration was adjusted to 106 CFU/mL, and the culture was centrifuged at 8000 rpm for 5 min to pellet the cells. Bacteria were washed and resuspended in HEPES buffer (Beyotime) to stabilize the internal and external bacterial pH. Glucose (as a positive control) or a sub-inhibitory concentration of AP, and a final concentration of 10 µM BCECF-AM were then added. The fluorescence was monitored using a fluorescence spectrometer with excitation/emission wavelengths set at 500 nm/522 nm [27].
Swimming motility assay
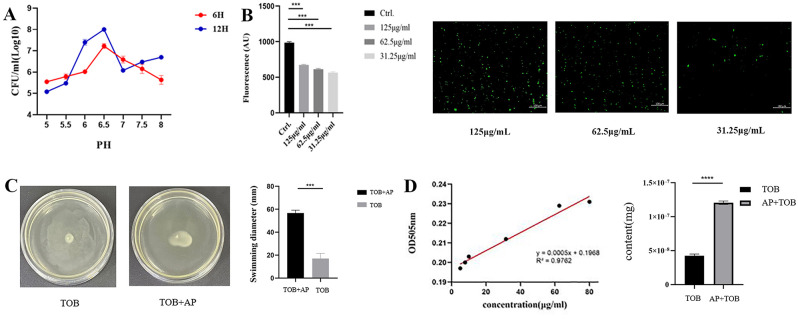
Bacterial motility plates were prepared with 0.3% LB agar. Three groups were established: 20 µg/mL TOB, 250 µg/mL AP, and 20 µg/mL TOB + 250 µg/mL AP. E. coli was inoculated into the different media groups and incubated at 37 °C. A 3 µL drop was placed in the center of each agar motility plate, and the plates were incubated. Bacterial growth was monitored, and then the diameter of the motility zone was measured using calipers [28].
Determination of intracellular TOB content
TOB was detected using Liang’s modified method [29]. Saline, 5 µg /mL TOB, 5 µg /mL TOB + 125 µg/mL AP were added to freshly cultured S. suis and incubated for 8 h at 37℃. The bacteria suspension was centrifuged, washed, and resuspended. The organisms were then placed on ice and subjected to ultrasonic disruption. The thoroughly broken bacterial suspension was centrifuged, and the supernatant was taken. Next, 1 mL of BR buffer (made by mixing 0.04 mol/L phosphoric acid, boric acid, and acetic acid) pH 6.5, 3 mL of Congo red solution (Aladdin) with a final concentration of 1 × 10− 4 mol/L and a certain amount of TOB standard or sample solution were sequentially added to a 10 mL cuvette, diluted to the scale with water. The absorbance value is detected at the maximum fading wavelength of 505 nm after standing for 10 min.
Liquid chromatograph mass spectrometer metabolomics
S. suis was cultured overnight at 37 °C with or without the addition of sub-MIC concentration AP. The precipitate was collected by centrifugation of 3 mL of fresh bacterial solution and quenched in liquid nitrogen. Add 500 µL of the extraction solution (acetonitrile: methanol: water = 2:2:1) and sonicate until the solution becomes clear. Centrifuge at 12,000 rpm for 10 min, remove the supernatant, filter using a 0.22 μm filter, and place in the refrigerator at -80 °C for use. S. suis non-targeted metabolite profiles were analyzed by a Thermo Fisher UPLC system (Thermo Fisher, SanJose, CA, USA) in combination with an LTQ XL mass spectrometer. Refer to Ma for specific program Settings [30]. Metabolomics subordinate data are output as RAW files, and we parse their files using specific MSDIAL software. Firstly, the substances corresponding to each peak were compared with the online database. Then, we got raw data containing the substance name, peak time, and peak area. Finally, some data with match coefficients less than 70 were deleted. The rest of the data was on the MetaboAnalyst website for data analysis.
Determination of NADH content in S. suis
Single colonies of S. suis were cultured overnight, adjusted to an OD600 = 0.6, centrifuged to collect the bacterial pellet, and resuspended in PBS (pH = 7.4). The bacterial suspension was treated with a final concentration of 125 µg/mL AP, or 5 µg/mL TOB + 125 µg/mL AP, and incubated for 1–2 h. Subsequently, the bacteria were centrifuged and washed. The NADH levels were determined separately by the Coenzyme I NAD(H) content assay kit.
Cytotoxicity test
RAW264.7 was cultured in RPMI-1640 (PM150110, Procell, China) containing 10% bovine serum (16170060, Thermo Fisher, USA). Each well was inoculated with 1 × 104 cells into a 96-well plate and incubated for 24 h at 37 °C in an incubator containing 5% CO2. 10 µL of AP (125–500 µg/mL) was added to each well, and incubation was continued for a specified period. Following this, the instructions of the Cell Proliferation-Toxicity Assay Kit (Severn Innovation Beijing Biotechnology Co., Ltd.) was followed.
In vivo drug dose screening
In vivo, drug dose screening experiments were performed as described with minor changes. We tested the protective effect of different concentrations of AP on S. suis-infected mice [31]. The sixteen 4–6 weeks of age female Balb/c mice were selected, and each was intraperitoneally injected with 100 µL of S. suis at a concentration of 108 CFU/mL. The mice were randomly divided into four groups (Group 1: PBS. Group 2: 5 mg/kg AP. Group 3: 10 mg/kg AP. Group 4: 20 mg/kg AP), and the first treatment was performed two hours after the injection of the organism, followed by two treatments per day. After three days, the mental condition of the mice was observed, and the organs of the mice were collected for observation.
Mouse anti-infection assay
We selected 20 female Balb/c mice (Purchased from Henan Skibbes Bio-technology Co.) from 4 to 6 weeks of age and divided them into 4 groups, and each mouse was injected with 100 µL of S. suis (108 CFU/mL). The mice were randomly divided into four groups (Group 1: PBS. Group 2: 20 mg/kg AP. Group 3: 10 mg/kg TOB. Group 4: 20 mg/kg AP + 10 mg/kg TOB), and the first treatment was performed two hours after the injection of the organism, followed by two treatments per day. After 3 days, all mice were deeply anesthetized by intravenous pentobarbital (at a dose of 70 mg/kg). After deep anesthesia, non-dead mice were euthanized by cervical dislocation. Some heart, liver, spleen, lung, kidney, and brain tissues were taken, well-ground, and coated on the TSB agar with a particular gradient of diluted PBS. Counting the number of colonies on the plates, made it possible to calculate the bacterial load in each mouse organ. Lung, liver, spleen, and brain tissue portions were fixed in 4% paraformaldehyde. The above tissues were embedded in paraffin and cut into 4 μm thick sections. Sections were stained with hematoxylin and eosin and observed with a light microscope, followed by scanning and imaging with a section scanner [27, 31].
Statistical methods
The experimental data were statistically analyzed using GraphPad Prism 9.5, and P values were calculated using unpaired t-tests between groups or one-way ANOVA between multiple groups. *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Antibacterial activity of AP
The MIC of AP against S. suis HA9801 was 250 µg/mL by micro broth dilution method. To evaluate the effect of different concentrations of AP on the growth of S. suis, a growth kinetics test was performed. AP at concentrations of 1/2 MIC, 1/4 MIC, and 1/8 MIC did not significantly inhibit bacteria growth compared to the control (Figure S1). This suggests that sub-MIC of AP do not affect bacterial growth. To test the resistance of S. suis to clinically relevant antibiotics, 30 aminoglycoside antibiotics, tetracycline antibiotics, and fluoroquinolone antibiotics were selected for testing (Table 1). Interestingly, the bacteria developed significant resistance to aminoglycoside antibiotics when compared to the clinical and laboratory standards institute (CLSI) criteria.
Table 1.
MICs of 30 antimicrobial agents for S. suis
| Number | Name | Type | MIC(µg/mL) |
|---|---|---|---|
| 1 | Tobramycin | Aminoglycoside antibiotics | 10 |
| 2 | Amikacin sulfate | 40 | |
| 3 | Neomycin sulfate | 40 | |
| 4 | Gentamicin | 2.5 | |
| 5 | Streptomycin sulfate | 320 | |
| 6 | Kanamycin | 20 | |
| 7 | Spectinomycin | 40 | |
| 8 | Levofloxacin hydrochloride | Quinolone antibiotics | <0.625 |
| 9 | Enrofloxacin | <0.625 | |
| 10 | Norfloxacin | 5 | |
| 11 | Ciprofloxacin | 10 | |
| 12 | Timicoxin | Macrolide antibiotics | 5 |
| 13 | Tylosin | <0.625 | |
| 14 | Penicillin | <0.625 | |
| 15 | Sulfadiazine | Sulfonamide antibiotics | 320 |
| 16 | Sulfamethoxazole | 320 | |
| 17 | Sulfadimethoxypyrimidine | 320 | |
| 18 | Sodium sulfamethazine | 3200 | |
| 19 | Trimethoprim | 2.5 | |
| 20 | Sulfaquinoxaline Sodium | 160 | |
| 21 | Doxycycline hydrochloride | Tetracycline antibiotics | 5 |
| 22 | Aureomycin | 50 | |
| 23 | Oxytetracycline | 25 | |
| 24 | Ceftriaxone sodium | β-lactams | <0.625 |
| 25 | Amoxicillin | <0.625 | |
| 26 | Flufenicol | Acyl alcohol antibiotics | 2.5 |
| 27 | Lincomycin | Lincomycin antibiotics | <0.625 |
| 28 | Clindamycin hydrochloride | <0.625 | |
| 29 | Apramycin sulfate | Aminocyclic alcohol antibiotics | 40 |
| 30 | Vancomycin | Polypeptides | <0.625 |
AP enhances the antimicrobial activity of aminoglycoside antibiotics
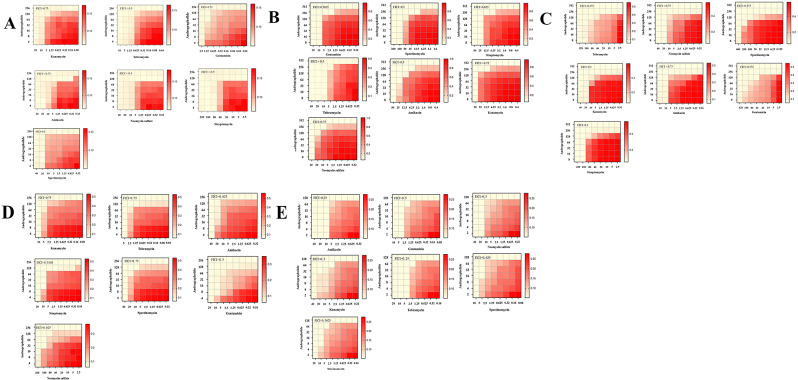
Given the high resistance rates of organisms to aminoglycoside antibiotics, a graded inhibition index was employed to assess the efficacy of these antibiotics in combination with AP. AP exhibited strong synergistic effects with aminoglycoside antibiotics against S. suis (Fig. 1A). Notably, the combination of AP and SM demonstrated the highest synergistic effect (FIC values < 0.25), reducing of the MIC for SM from 320 µg/mL to 40 µg/mL. We further tested the potentiating effect of AP on other aminoglycoside antibiotics (including TOB, AMK, SM, KAN, SPT, GEN, and Neomycin sulfate). As expected, the antimicrobial activity of all aminoglycoside antibiotics was significantly enhanced in the presence of AP.
Fig. 1.
Effects of AP on the antimicrobial activity of aminoglycoside antibiotics against the bacterium. A) Interaction between andrographolide and aminoglycoside antibiotics against S. suis. B) Interaction between andrographolide and aminoglycoside antibiotics against E coil. C) Interaction between andrographolide and aminoglycoside antibiotics of against B. bronchiseptica. D) Interaction between andrographolide and aminoglycoside antibiotics against S. agalactia. E) Interaction between andrographolide and aminoglycoside antibiotics against G. parasuis
In light of these findings, we assessed whether this synergistic effect is also observed with other classes of antibiotics. We tested the combination of oxytetracycline, ciprofloxacin, and apramycin sulfate with AP, but found no synergistic effect with these antibiotic classes (Figure S2). These results suggest that the synergistic effect may be exclusive to aminoglycoside antibiotics. Given that AP demonstrated synergistic effects with S. suis in combination with aminoglycoside antibiotics, we next explored whether similar synergistic effects were observed in other drug-resistant bacterial strains. Four clinical isolates of pathogenic bacteria, E. coli, B. bronchiseptica, S. agalactiae, and G. parasuis, were selected for this study. The MIC of seven aminoglycoside antibiotics was first tested, followed by the testing of FIC values using the checkerboard method. Interestingly, the combination of AP and aminoglycoside antibiotics exhibited synergistic effects in all four tested bacteria. Specifically, E. coli exhibited FIC ranging from 0.5 to 0.75 (Fig. 1B), B. bronchiseptica had FIC ranging from 0.25 to 0.5 (Fig. 1C), S. agalactiae had FIC ranging from 0.5 to 0.75 (Fig. 1D), and G. parasuis had FIC ranging from 0.25 to 0.75 Fig. 1E). In B. bronchiseptica, the MIC of TOB decreased from 320 µg/mL to 80 µg/mL in the presence of AP at 62.5 µg/mL. In contrast, the MIC of aminoglycoside antibiotics against E. coli ranged from 20 to 200 µg/mL. In the presence of AP, it decreased to 2.5–12.5 µg/mL (Table S2). In summary, the synergistic effect of AP with aminoglycoside antibiotics has been demonstrated in multiple drug-resistant pathogenic bacteria, highlighting AP’s potential to reverse bacterial resistance.
Based on the FIC values, four combination drug concentrations were selected for screening: 1/2 MIC TOB + 1/2 MIC AP, 1/2MIC TOB + 1/4 MIC AP, 1/4 MIC TOB + 1/2 MIC AP, 1/4 MIC TOB + 1/4 MIC AP. The optimal concentration was also tested in the SM group. The optimal concentrations were 1/2MIC TOB + 1/2 MIC AP (5 µg/mL TOB + 125 µg/mL AP) and 1/2 MIC SM + 1/4 MIC AP (160 µg/mL SM + 62.5 µg/mL AP) as shown in Fig. 2A. In addition, the synergistic antimicrobial activity between AP and TOB/ SM was further confirmed through a antibacterial curve assay. After 24 h, the combination of the optimal concentrations significantly inhibited the growth of S. suis (Fig. 2B). Notably, we evaluated the effect of AP on the development of resistance induced by aminoglycoside antibiotics. As shown in Fig. 2C, AP significantly reduced the occurrence of aminoglycoside-induced antibiotic resistance over 30 consecutive generations in sub-MIC environments, both with and without AP, compared to TOB/SM alone.
Fig. 2.
Effects of AP on the antimicrobial activity of aminoglycoside antibiotics against S. suis. A) Selection of the optimal combined concentration. B) Antibacterial curve of S. suis in the presence of AP (125–62.5 µg/mL), TOB(5 µg/mL), and SM(160 µg/mL). C) Resistance development during serial passaging of bacterial strains in the presence of SM/TOB alone or SM/TOB coupled with AP
Combined use of AP and aminoglycoside antibiotics has bactericidal activity and inhibits the biofilm of S. suis
Bacteria exposed to sub-MIC of antibiotic pressure are generally not killed. Consequently, we tested whether combining AP with aminoglycoside antibiotics bactericidal effect, enhancing its therapeutic efficacy. To test this hypothesis, bactericidal curve experiments were conducted on S. suis. Notably, direct synergistic bactericidal effects were observed. Specifically, 10 µg/mL TOB or 250 µg/mL AP exhibited minimal antibacterial activity (between 12 and 24 h). In contrast, the combination of TOB and AP (10 µg/mL + 250 µg/mL) displayed excellent bactericidal activity, resulting in an approximately 10-fold reduction in bacterial load compared to the negative control (no dosing) (Fig. 3A, Figure S3).
Fig. 3.
AP assisted aminoglycoside antibiotics for biofilm formation against S. suis. A) Bactericidal curve of S. suis in the presence of andrographolide, TOB and SM. B) Clearance of S. suis mature biofilm by TOB (5 µg/mL) in combination with AP (125 µg/mL), and by SM (160 µg/mL) and AP (62.5 µg/mL). C) TOB (5 µg/mL), SM (160 µg/mL), and AP (125–62.5 µg/mL) for biofilm formation against S. suis. D) SEM observations of biofilm formation in AP or TOB against S. suis. Scale bar = 1 μm. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Furthermore, biofilm inhibition and biofilm removal assays were conducted with and without AP to explore whether combining AP with sub-MIC of aminoglycoside antibiotics can synergistically inhibit biofilm formation. As shown in Fig. 3B and C, the combination of 125 µg/mL of AP with 5 µg/mL TOB significantly reduced the pre-formed biofilm of S. suis. The 62.5 µg/mL AP combination with 160 µg/mL SM significantly inhibited S. suis biofilm formation. The results of AP in combination with several other aminoglycoside antibiotics against S. suis biofilm are presented in Figure S3. SEM observed the formation of biofilms in the presence of AP or TOB. As shown in Fig. 3D, for S. suis in the untreated group, the biofilm exhibits a dense 3D structure with compact bacterial connectivity. In contrast, bacteria dispersed and formed minimal biofilm in the AP and TOB co-treatment groups. In summary, we unexpectedly found that the combination of aminoglycoside antibiotics and AP exhibits potent bactericidal activity and effectively inhibits biofilm in various states of S. suis.
AP stimulates PMF to promote cellular uptake of TOB
The PMF is essential for maintaining physiological processes associated with bacteria, as is reflected in the formation of pH gradients across the inner membrane [27]. Therefore, the pH of the TSB medium was adjusted to a range of 5–8 before evaluating the binding inhibitory effect. The optimal combined concentration was used for co-culture with S. suis for 6 h and 12 h, with the weakest inhibition observed at a pH of 6.5 (Fig. 4A). Since PMF correlates with ΔpH, BCECF-AM was used to measure this key parameter. The results showed that AP caused a dose-dependent increase in fluorescence in S. suis, indicating a significant rise in intracellular pH (Fig. 4B). These results suggest that AP affects the PMF primarily by altering the bacterial intracellular pH gradient. It is well known that PMF regulates bacterial flagella and influences motility [32]. Therefore 62.5 µg/mL of AP was added to the TOB incubation with E. coil, and bacterial motility was observed on a motility plate. The diameter of bacterial motility was measured using calipers (Fig. 4C). When treated with AP, the swimming diameter of E. coli increased, indicating enhanced motility due to the promotion of PMF. The negative control (PMF dispersant) and blank control are plotted in Figure S4.
Fig. 4.
The effect of AP on bacterial PMF. A) Antibacterial activity of the combination of AP and TOB in different pH values medium. B) Detection of the intracellular pH of S. suis by monitoring the fluorescence intensity of BCECF-AM. C) The effect of AP detection on bacterial swimming activity. D) The standard curve of TOB and intracellular TOB content of S. suis. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Reports have shown that the uptake of aminoglycoside antibiotics is associated with PMF [33]. This study examined intracellular TOB content in S. suis after co-treatment with AP and TOB was examined. Congo red discoloration method showed that the intracellular antibiotic content of S. suis was significantly higher when treated with both AP and TOB compared to TOB alone, with a 2.8-fold increase (Fig. 4D).
Non-targeted metabolomics analysis
To further explore the underlying mechanism of AP’s function, we analyzed cellular metabolites in the presence or absence of AP, collating and evaluating all detected compounds. A fold change (FC) threshold greater than 2 was applied; 105 up-regulated and 117 down-regulated compounds were identified in the positive ion mode, while 16 up-regulated and 12 down-regulated compounds were identified in the negative ion mode following AP treatment (Fig. 5A). The number of down-regulated metabolites exceeded the number of up-regulated metabolites in both models. Criteria such as variable importance in projection (VIP) > 1 (calculated via orthogonal partial least squares discriminant analysis), p < 0.001 (t-test), and FC > 2 identified 51 and 4 differential metabolites in the positive and negative ion modes, respectively (Fig. 5B). These differential metabolites predominantly comprise ketones, amines, and organic heterocyclic compounds. Additionally, based on P-values, the top 25 most significant metabolic pathways were selected. In-depth pathway analysis revealed that metabolic pathways, including pyruvaldehyde degradation, pyruvate metabolism, pyrimidine metabolism, glycolysis, and amino sugar metabolism, were significantly affected (Fig. 5C). Further analysis demonstrated a significant pyruvate metabolism in the presence of AP. These results indicated a substantial up-regulation of pyruvate metabolism in the presence of both AP and TOB. The drug combination may lead to enhanced pyruvate metabolism, accelerated TCA cycle, and more NADH production in S. suis. As shown in Fig. 5D, the NADH content increased within 1 h following the addition of AP and TOB. Notably, NADH strongly correlates with PMF production, which is consistent with the previously presented results.
Fig. 5.
Non-targeted metabolomics analysis. A) Volcano plot of differentially detected metabolites after AP addition at ESI (+) (left) and ESI (-) (right). B) Venn of differentially detected metabolites after AP addition at ESI (+) (left) and ESI (-) (right). C) Overview of pathway enrichment of differentially detected metabolites after AP addition at ESI (+) (left) and ESI (-) (right). D) NADH content in TOB (5 µg/mL) alone compared to AP (125 µg/mL) and TOB (5 µg/mL) co-operation
Safety and efficacy of AP in combination with aminoglycoside antibiotics in vivo
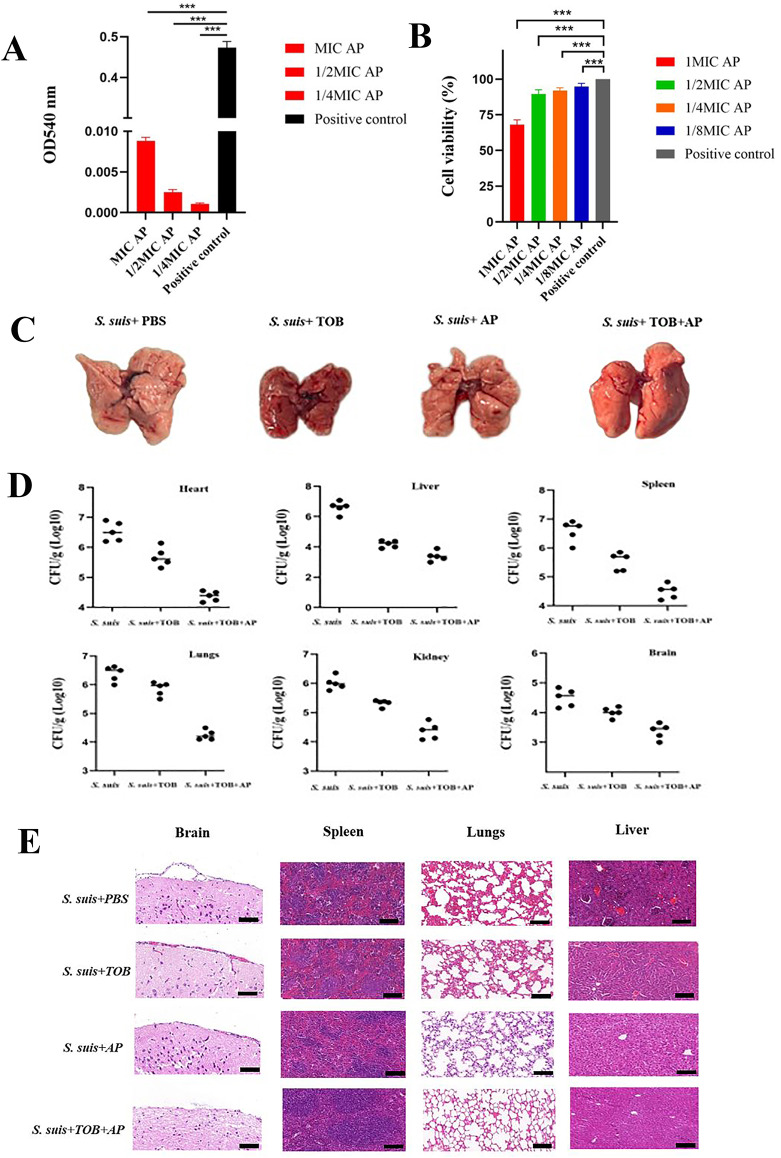
A primary consideration in combination therapies is ensuring in vivo safety. AP exhibited minimal hemolytic activity on erythrocytes, even at concentrations as high as 250 µg/mL (Fig. 6A). We next evaluated the cytotoxicity of AP on RAW264.7. We found that 250 µg/mL AP caused only minimal toxicity compared to the control, with no cytotoxicity observed at concentrations below 62.5 µg/mL (Fig. 6B). AP was administered at varying concentrations (0, 5, 10, 20 mg/kg) to identify the optimal therapeutic dose. Histological analysis of tissues following AP administration revealed that the brain and lung were the primary sites of S. suis infestation. Notably, a significant therapeutic effect was observed at 20 mg/kg (Figure S5).
Fig. 6.
In vivo antimicrobial efficacy of combination therapy. A) Hemolysis of andrographolide at sub-MIC concentrations. B) RAW264.7 cell survival rate of andrographolide at sub-MIC concentrations. C) Ocular lung organ damage in mice by S. suis under TOB and AP treatment. D) Tissue load of mice under TOB and AP treatment. E) Histopathological sections of TOB and AP in a mouse model of S. suis infection. Scale bar = 50 μm. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
The efficacy of the combination therapy was subsequently evaluated by injecting S. suis into the peritoneal cavities of mice for group trials. The combination therapy alleviated pneumonia induced by S. suis in the lung, resulting in a moist and fully expanded lung. In contrast, the PBS-treated group exhibited lung atrophy and whitening. Treatment with TOB and AP significantly reduced ocular and lung organ damage caused by S. suis infection in mice (Fig. 6C). The combination of AP + TOB significantly reduced the bacterial load in all organs of the mice compared to the administration of each drug individually (Fig. 6D). The histological cross-sectional analysis demonstrated that the combination therapy exerted a significantly more potent effect than the single-drug treatments. Pathological analysis of the brain sections revealed a significant reduction in meningitis caused by S. suis infection in the combination therapy group compared to the control group; liver sections showed well-preserved hepatic architecture with reduced inflammatory infiltration in the combination therapy group; lung sections revealed intact alveolar structures, with no thickening of the alveolar walls and a reduction in inflammatory factors in the combination therapy group (Fig. 6E). These findings indicate that the combination of AP and TOB is safe and effective against S. suis.
Discussion
The emergence of multidrug-resistant bacteria severely compromises the clinical efficacy of antibiotics [1], creating an urgent need to identify novel antimicrobial targets. The current study demonstrated that AP, in combination with aminoglycoside antibiotics, exhibited significant synergistic antimicrobial activity across a broad spectrum of pathogens, with this synergy being independent of bacterial species. Mechanistic studies revealed that bacterial death is mediated by AP-induced upregulation of S. suis PMF, which facilitates enhanced cellular uptake of TOB. Furthermore, in the presence of AP, S. suis pyruvate metabolism is significantly upregulated, leading to an acceleration of the TCA cycle and increased NADH production, which subsequently promotes PMF.
It was found that AP had a strong inhibitory effect on S. aureus DNA synthesis, which in turn inhibited S. aureus RNA and protein synthesis [34]. AP also significantly attenuates the inflammatory response by inhibiting cyclooxygenase-2 NF-κB activation, and modulating the expression of cytokines (interleukin-6 and tumor necrosis factor-α) in macrophages [35]. In summary, AP holds promise as an antibiotic adjuvant, and its combination with antibiotics for treating S. suis may represent a novel therapeutic approach. The electrochemical gradient generated by proton production through the electron transport chain in bacteria is called the PMF [36]. ΔpH was measured using BCECF-AM, and fluorescence increased with increasing AP dose, indicating a substantial rise in intracellular pH. This phenomenon suggests that AP modulates PMF by influencing intracellular pH. It has been shown that PMF is critical for bacterial flagellar function. To further confirm this, we examined the motility of E. coli by measuring the diameter of bacterial movement in the presence of AP. Following previous findings, AP treatment promoted E. coli swimming motility, suggesting bacterial mobility is closely linked to PMF. Previous studies have shown that Pixantrone significantly reduces E. coli internal pH, leading to PMF disruption and sensitizing a broad range of Gram-negative bacteria to rifampicin. Our findings are consistent with these reports [27]. Collins et al. found that allicin demonstrated synergistic effects when combined with aminoglycoside antibiotics. This compound disrupts ΔpH in PMF, downregulates the expression of genes associated with cell motility, and exhibits bactericidal activity against a variety of pathogens, including Mycobacterium nucleatum and carbapenem-resistant Enterobacteriaceae [37]. Aminoglycoside antibiotics depend on PMF for cellular uptake in bacteria [38]. This is consistent with the present study, where the addition of AP resulted in increased intracellular TOB content compared to cells treated with TOB alone, attributed to the promotion of PMF by AP.
Given the efficient synergistic effect between AP and TOB, which may involve additional mechanisms, metabolite differences in the presence of AP were analyzed using HPLC-MS and in the presence of AP revealed 105 upregulated and 117 downregulated metabolites in the positive ionic mode, and 16 upregulated and 12 downregulated metabolites in the negative ion mode. Pathway analysis revealed that AP significantly upregulates pyruvate metabolism, promoting the TCA cycle, increasing NADH production, and enhancing PMF (Fig. 7). Kyle R. Allison’s team demonstrated that specific metabolites can eradicate persistent E. coli and S. aureus. Aminoglycoside antibiotics combined with specific metabolites can effectively treat E. coli and S. aureus biofilms, improving the treatment of chronic infections in mouse models of urinary tract infection [39]. Su found that exogenous fructose activates the TCA cycle, producing NADH, which enhances PMF, leading to elevated intracellular levels of KAN and subsequent bacterial killing [40]. These findings align with recent reports highlighting changes in the TCA cycle as a response to antibiotic resistance.
Fig. 7.
AP could effectively promote pyruvate metabolism and TCA cycle, and stimulate PMF in bacteria, causing an increase in intracellular antibiotic concentration
Although many experiments were conducted in this study to demonstrate the synergistic antimicrobial potential of AP in combination with aminoglycoside antibiotics, only a preliminary mechanistic understanding has yet to be achieved. Future work requires further in-depth investigations into the individual synergistic pathway.
Conclusion
In this study, the presence of AP elevates the intracellular pH and stimulates PMF, resulting in increased TOB uptake, which restores S. suis sensitivity to TOB. Non-targeted metabolomics analysis confirmed that the pyruvate cycle is accelerated in the presence of AP, leading to increased NADH production. These findings suggest that AP could be an antimicrobial adjuvant when co-incubated with aminoglycoside antibiotics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- AP
Andrographolide
- ΔpH
Transmembrane Proton Gradient
- PMF
Proton Motive Force
- NADH
Nicotinamide Adenine Dinucleotide
- QSS
Quorum Sensing System
- TOB
Tobramycin
- SM
Streptomycin Sulfate
- ANK
Amikacin
- SPT
Spectinomycin
- GEN
Gentamicin
- KAN
Kanamycin
- TSB
Tryptic Soy Broth
- TSA
Soybean Casein Digest Agar
- LB
Luria-Bertani
- MIC
Minimum Inhibitory Concentration
- MBC
Minimum Bactericidal Concentration
- FIC
Fractional Inhibitory Concentration
- CFU
Colony-Forming Units
- PBS
Phosphate-Buffered Saline
- SEM
Scanning Electron Microscope
- BR buffer
Britton-Robinson Buffer Solution
- RAW264.7
Mouse Mononuclear Macrophages Cells 264.7
- RPMI-1640
Roswell Park Memorial Institute-1640
- CLSI
Clinical and Laboratory Standards Institute
- FC
Fold Change
- VIP
Variable Importance in Projection
- TCA
Tricarboxylic acid cycle
Author contributions
BQ.Xue. and HL. Li. wrote the main manuscript text and SJ. Gao. conducted data analysis. YY. Quan and YX. Wang prepared figures. Y. Wang. and Y. Li provided supervision and support.
Funding
This work was supported by the National Natural Science Foundation of China (32172852) and Excellent Youth Foundation of Henan Scientific Committee (222300420005), He’nan Provincial Science and Technology Research Project (232102110095) and Program for Innovative Research Team (in Science and Technology) in University of Henan Province (24IRTSTHN033).
Data availability
The datasets generated during and analyzed during the study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The animal experiments were approved by the Animal Care and Use Committee of Henan University of Science and Technology (approval number: SKKUIACUC-20-04-14-3).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Li Yi, Email: lilili123168@163.com.
Yang Wang, Email: wangyocean@163.com.
References
- 1.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infec. 2012;18:268–81. [DOI] [PubMed] [Google Scholar]
- 2.Weinert LA, Chaudhuri RR, Wang J, Peters SE, Corander J, Jombart T, Baig A, Howell KJ, Vehkala M, Valimaki N, et al. Genomic signatures of human and animal disease in the zoonotic pathogen Streptococcus suis. Nat Commun. 2015;6:6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yi L, Li JP, Fan QY, Mao CL, Jin MY, Liu YC, Sun LY, Grenier D, Wang Y. The otc gene of Streptococcus suis plays an important role in biofilm formation, adhesion, and virulence in a murine model. Vet Microbiol. 2020;251:108925. [DOI] [PubMed] [Google Scholar]
- 4.Haas B, Grenier D. Understanding the virulence of Streptococcus suis: a veterinary, medical, and economic challenge. Med et maladies Infectieuses. 2018;48:159–66. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Fan Q, Wang Y, Yi L, Wang Y. Rethinking the control of Streptococcus suis infection: biofilm formation. Vet Microbiol. 2024;290:110005. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Liu B, Li J, Gong S, Dong X, Mao C, Yi L. LuxS/AI-2 system is involved in fluoroquinolones susceptibility in Streptococcus suis through overexpression of efflux pump SatAB. Vet Microbiol. 2019;233:154–8. [DOI] [PubMed] [Google Scholar]
- 7.Liu B, Yi L, Li J, Wang Y, Mao C, Wang Y. Autoinducer-2 influences tetracycline resistance in Streptococcus suis by regulating the tet(M) gene via transposon Tn916. Res Vet Sci. 2020;128:269–74. [DOI] [PubMed] [Google Scholar]
- 8.Lewis K. The Science of Antibiotic Discovery. Cell. 2020;181:29–45. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Ding SY, Shen JZ, Zhu K. Nonribosomal antibacterial peptides that target multidrug-resistant bacteria. Nat Prod Rep. 2019;36:573–92. [DOI] [PubMed] [Google Scholar]
- 10.Zuo J, Quan Y, Li J, Li Y, Song D, Li X, Wang Y, Yi L, Wang Y. Tackling Antibiotic Resistance: exploring 5-Fluorouracil as a Promising Antimicrobial Strategy for the treatment of Streptococcus suis infection. Animals. 2024;14:1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Li RC, Xiao X, Wang ZQ. Antibiotic adjuvants: an alternative approach to overcome multi-drug resistant gram-negative bacteria. Crit Rev Microbiol. 2019;45:301–14. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Tong ZW, Shi JR, Li RC, Upton M, Wang ZQ. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics. 2021;11:4910–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuo J, Shen YM, Wang HK, Gao SJ, Yuan S, Song D, Wang YX, Wang Y. Effects of metformin on LuxS/AI-2 quorum sensing system and biofilm formation. Microb Pathog. 2023;181. [DOI] [PubMed]
- 14.Gao S, Shen Y, Yuan S, Quan Y, Li X, Wang Y, et al. Methyl anthranilate deteriorates biofilm structure of Streptococcus suis and antagonizes the capsular polysaccharide defence effect. Int J Antimicrob Agents. 2023;62:106996. [DOI] [PubMed]
- 15.Raina AP, Gupta V, Sivaraj N, Dutta MJGR, Evolution C. Andrographis paniculata (burm. f.) wall. Ex Nees (kalmegh), a traditional hepatoprotective drug from India. Genet Resour Crop Evol. 2013;60:1181–9.
- 16.Chang RS, Ding L, Chen GQ, Pan QC, Smith KM. Dehydroandrographolide Succinic Acid Monoester as an Inhibitor against the Human Immunodeficiency Virus. Proceedings of the Society for Experimental Biology and Medicine. 1991;197:59–66. [DOI] [PubMed]
- 17.Chao WW, Kuo YH, Hsieh SL, Lin BF. Inhibitory effects of Ethyl acetate extract of Andrographis paniculata on NF-κB trans-activation activity and LPS-Induced Acute inflammation in mice. Evidence-based Complement Altern Medicine: eCAM. 2011:254531. [DOI] [PMC free article] [PubMed]
- 18.Satia MC, Damani RR, Goyal RK. Beneficial effects of Clonidine in Streptozotocin-induced diabetes and DOCA‐hypertensive rats. J Pharm Pharmacol. 2011;49:1030–5. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Singh B, Bajpai V. Andrographis paniculata (Burm.f.) Nees: traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J Ethnopharmacology: Interdisciplinary J Devoted Bioscientific Res Indigenous Drugs. 2021;275. [DOI] [PubMed]
- 20.Tran QTN, Tan WSD, Wong WSF, Chai CLL. Polypharmacology of andrographolide: beyond one molecule one target. Nat Prod Rep. 2021;38:682–92. [DOI] [PubMed] [Google Scholar]
- 21.Dong G, Li J, Chen L, Bi W, Zhang X, Liu H, Zhi X, Zhou T, Cao J. Effects of sub-minimum inhibitory concentrations of ciprofloxacin on biofilm formation and virulence factors of Escherichia coli. Brazilian J Infect Dis. 2019;23:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao-Xiang S, Yuan-Yuan Y, Jing S, Ke-Qiao LU, Meng-Xiang Z, Tian-Ming W. Chang-Zhong WJCJoM: mechanisms of the combination effects of Andrographolide and Fluconazole against fluconazole-resistant. Candida albicans. 2014;26:7. [Google Scholar]
- 23.Lozoya OA, Martinez-Reyes I, Wang TY, Grenet D, Bushel P, Li JY, Chandel N, Woychik RP, Santos JH. Mitochondrial nicotinamide adenine dinucleotide reduced (NADH) oxidation links the tricarboxylic acid (TCA) cycle with methionine metabolism and nuclear DNA methylation. Plos Biol. 2018;16. [DOI] [PMC free article] [PubMed]
- 24.Ahmar Rauf M, Oves M, Ur Rehman F, Rauf Khan A, Husain N. Bougainvillea flower extract mediated zinc oxide’s nanomaterials for antimicrobial and anticancer activity. Biomed Pharmacother. 2019;116:108983. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Fan Q, Zuo J, Xue B, Zhang X, Wei Y, Sun L, Grenier D, Yi L, Hou X, et al. Paeoniflorin combined with norfloxacin ameliorates drug-resistant Streptococcus suis infection. J Antimicrob Chemother. 2022;77:3275–82. [DOI] [PubMed] [Google Scholar]
- 26.Crabbé A, Ostyn L, Staelens S, Rigauts C, Risseeuw M, Dhaenens M, Daled S, Van Acker H, Deforce D, Van Calenbergh S, et al. Host metabolites stimulate the bacterial proton motive force to enhance the activity of aminoglycoside antibiotics. PLoS Pathog. 2019;15:e1007697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.She P, Li Z, Li Y, Liu S, Li L, Yang Y, Zhou L, Wu Y. Pixantrone sensitizes Gram-negative pathogens to Rifampin. Microbiol Spectr. 2022;10:e0211422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harding CM, Tracy EN, Carruthers MD, Rather PN, Actis LA, Munson RS. Acinetobacter baumannii strain M2 produces type IV Pili which play a role in Natural Transformation and Twitching Motility but not surface-Associated Motility. Mbio. 2013;4:16–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang YD, Chen CQ, Li BN. Fading reaction of Congo Red by tobramycin and its analytical application. J XI Univ Sci Technol. 2013;33:6. [Google Scholar]
- 30.Ma YK, Shi QS, He QX, Chen G. Metabolomic insights into the inhibition mechanism of methyl N-methylanthranilate: a novel quorum sensing inhibitor and antibiofilm agent against Pseudomonas aeruginosa. Int J Food Microbiol. 2021;358. [DOI] [PubMed]
- 31.Zhang G, Jiang C, Xie N, Xu Y, Liu L, Liu N. Treatment with andrographolide sulfonate provides additional benefits to imipenem in a mouse model of Klebsiella pneumoniae pneumonia. Biomed Pharmacother. 2019:117. [DOI] [PubMed]
- 32.Paul K, Erhardt M, Hirano T, Blair DF, Hughes KT. Energy source of flagellar type III secretion. Nature. 2008;451:489–92. [DOI] [PubMed] [Google Scholar]
- 33.Hancock R, Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Diseases: Official Publication Eur Soc Clin Microbiol. 1988;7:713–20. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee M, Parai D, Chattopadhyay S, Mukherjee S. Andrographolide. Antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiol. 2017;62:237–44. [DOI] [PubMed] [Google Scholar]
- 35.Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, Yang T, Kim H, Drake D, Liu X, et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature. 2014;507:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farha MA, Verschoor CP, Bowdish D, Brown ED. Collapsing the proton motive force to identify synergistic combinations against Staphylococcus aureus. Chem Biol. 2013;20:1168–78. [DOI] [PubMed] [Google Scholar]
- 37.Stokes JM, Yang K, Swanson K, Jin W, Collins JJ. A Deep Learning Approach to Antibiotic Discovery. Cell. 2020;180:688–e702613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sidders A, Radlinski L, Rowe S, Conlon BP. Stimulating Aminoglycoside Uptake to kill Staphylococcus aureus Persisters. Methods Mol Biol. 2021;2357:223–36. [DOI] [PubMed] [Google Scholar]
- 39.Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su YB, Peng B, Han Y, Li H, Peng XX. Fructose restores susceptibility of Multidrug-Resistant Edwardsiella tarda to Kanamycin. J Proteome Res. 2015;14:1612–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analyzed during the study are available from the corresponding author on reasonable request.