Abstract
Selective cargo capture into ER-derived vesicles is driven by the Sec24p subunit of the COPII coat, which contains at least three independent cargo-binding sites. One of these, the “A-site,” interacts with a NPF motif found on the SNARE, Sed5p. We have characterized the Sec24p-Sed5p interaction through mutation of the putative ER export motifs of Sed5p and the cargo-binding A-site of Sec24p. Mutational analysis of Sed5p suggests that the NPF motif is the dominant ER export signal. Mutation of the NPF binding pocket on Sec24p led to a dramatic reduction in the capture of Sed5p into COPII vesicles, whereas packaging of other ER-Golgi SNAREs was normal. Of all the cargoes tested, only Sed5p was depleted in vesicles made with Sec24p A-site mutants. Surprisingly, vesicles generated with the mutant Sec24p were unable to fuse with the Golgi apparatus. This inability to fuse was not the result of the lack of Sed5p, because vesicles specifically depleted of Sed5p generated by antibody inhibition targeted and fused normally. We propose that the A-site of Sec24p is a multipurpose cargo-binding site that must recognize additional unidentified cargo proteins, at least one of which is essential at a late stage of vesicle fusion.
INTRODUCTION
Efficient and accurate protein secretion relies on the selective capture of properly folded, newly synthesized proteins into ER-derived vesicles that deliver these proteins to downstream compartments within the secretory pathway (Lee et al., 2004). These vesicles are generated by a set of cytoplasmic coat proteins, collectively known as the COPII coat. Vesicle biogenesis is initiated by the exchange of GDP for GTP on the small G-protein, Sar1p, resulting in the recruitment of Sar1p to the ER membrane. Activated Sar1p recruits the Sec23/24p heterodimer, which in turn binds and recruits cargo proteins into the nascent vesicle. The Sar1/Sec23/Sec24p prebudding complex then recruits Sec13/31p, a heterotetramer that is thought to cross-link prebudding complexes to generate a COPII vesicle. In addition to newly synthesized secretory proteins, the COPII coat must also recruit the machinery required for subsequent tethering and fusion of vesicles at the Golgi apparatus. This machinery includes the SNARE proteins required for the final fusion stage of ER-Golgi transport.
Genetic, biochemical, and structural analyses of cargo recruitment by the COPII coat have pinpointed the Sec24p subunit as the predominant facilitator of cargo selection (Miller et al., 2003; Mossessova et al., 2003). Three independent cargo-binding sites have been identified on Sec24p. The “A-site” interacts with a YNNSNPF motif found on the syntaxin-like SNARE, Sed5p. The “B-site” recognizes a second motif on Sed5p (LxxME) as well as a similar motif on the v-SNARE, Bet1p (LxxLE) and a “di-acidic” motif found on the Golgi protein, Sys1p (DxE; Mossessova et al., 2003). A third site, the “C-site” was identified by mutagenesis of Sec24p and appears to specifically recognize another ER-Golgi SNARE, Sec22p, through a motif that remains to be identified (Miller et al., 2003).
Mutagenesis of the Sec24p B-site confirmed that packaging of Bet1p and Sys1p is primarily driven by interaction with this site (Miller et al., 2003). Furthermore, additional proteins were impaired in their uptake into B-site mutant COPII vesicles to varying degrees: the p24 proteins were severely affected, whereas both Sed5p and Emp47p were only moderately affected. The moderate defects in Sed5p packaging are consistent with the presence of two independent coat-binding sites on Sed5p. The p24 proteins employ a hydrophobic sorting signal that would appear to be inconsistent with binding to the B-site pocket, which mediates cargo binding largely through electrostatic interactions. However, mutagenesis of the B-site on the Sec24p homolog, Lst1p/Sfb2p, which also packages p24 proteins confirmed a role for this site in uptake of this family of proteins. Surprisingly, B-site mutants displayed no defect in packaging Gap1p, another “di-acidic” signal-containing membrane protein, suggesting that two superficially similar sorting signals (DxE of Sys1p and DID of Gap1p) are sorted independently by Sec24p.
Similar mutagenesis of the A-site of Sec24p is critical in understanding both how this site interacts with Sed5p and in determining whether additional cargo proteins also employ this site. Unlike the B-site, interaction between the YNNSNPF of Sed5p and the A-site pocket on Sec24p may be driven by shape complementarity (Mossessova et al., 2003). The NPF sequence forms a β-turn and makes extensive hydrophobic contact with Sec24p. We report here that several residues in the A-site of Sec24p are remarkably impervious to alterations, both with respect to in vivo function and in vitro packaging of Sed5p. However, mutation of one critical residue, W897, which makes important hydrophobic contacts with Sed5p, dramatically impairs Sed5p uptake into COPII vesicles. Furthermore, we show that the NPF motif is the dominant ER export signal on Sed5p and is critical for Sed5p function in vivo.
MATERIALS AND METHODS
Yeast Strains and Media
Strains used in this study are listed in Table 1. Cultures were grown at 30°C in either rich medium (YPD: 1% yeast extract, 2% peptone, 2% glucose) or synthetic complete medium (SC: 0.67% yeast nitrogen base, 2% glucose, supplemented with amino acids as required). Complementation analysis was performed by streaking transformants onto SC-his supplemented with 5-fluoroorotic acid (5-FOA: 0.1% final concentration).
Table 1.
Strains and plasmids
| Strain | Genotype | Source |
|---|---|---|
| RSY620 | MATα leu2-3112 ura3-52 ade2-1 trp1-1 his3-11,15 pep4::TRP1 | Schekman lab strain |
| YTB1 | MATα can1-100 leu2-2112 ura3-1 ade2 trp1-1 his3-11,15 sec24::LEU2 carrying pLM22 (CEN SEC24-URA3) | Miller et al. (2003) |
| YTB20 | MATα can1-100 leu2-2112 ura3-1 ade2 trp1-1 his3-11,15 sec24::LEU2 iss1::KanR carrying pLM22 (CEN SEC24-URA3) | Miller et al. (2003) |
| RSY1289 | MATα leu2,3-112 trp1-Δ901 lys2-801 suc2-Δ9 ura-52 his3-Δ200 sed5-1 | Pelham lab |
| Plasmid | Description | Source |
|---|---|---|
| pLM22 | 4.2-kb XhoI-SpeI fragment containing 6× His-tagged SEC24 in pRS316 | Miller et al. (2003) |
| pLM23 | 4.2-kb XhoI-SpeI fragment containing 6× His-tagged SEC24 in pRS313 | Miller et al. (2003) |
| pLM129 | 2.8-kb XbaI-HindIII fragment containing 6× His-tagged SEC24 in p425GAL1 | Miller et al. (2003) |
| pLM212 | SEC24-A1 (M721A,R724A) mutation in pLM23 | This study |
| pLM213 | SEC24-A2 (H732R) mutation in pLM23 | This study |
| pLM214 | SEC24-A3 (T893K) mutation in pLM23 | This study |
| pLM251 | SEC24-A4 (W897A) mutation in pLM23 | This study |
| pLM298 | SEC24-A5 (W897F) mutation in pLM23 | This study |
| pLM299 | SEC24-A6 (W897M) mutation in pLM23 | This study |
| pLM215 | SEC24-A1 (M721A,R724A) mutation in pLM129 | This study |
| pLM216 | SEC24-A2 (H732R) mutation in pLM129 | This study |
| pLM217 | SEC24-A3 (T893K) mutation in pLM129 | This study |
| PLM253 | SEC24-A4 (W897A) mutation in pLM129 | This study |
| pLM291 | SEC24-A5 (W897F) mutation in pLM129 | This study |
| pLM292 | SEC24-A6 (W897M) mutation in pLM129 | This study |
| pLM222 | 3× HA-tagged SED5 inserted into pMET25-HA | This study |
| pLM225 | SED5A (N206A,P207A,F208A) mutation in pLM222 | This study |
| pLM226 | SED5B (M241A,E242A) mutation in pLM222 | This study |
| pLM237 | SED5AB (N206A,P207A,F208A,M241A,E242A) mutation in pLM222 | This study |
Plasmid Construction
Plasmids used in this study are listed in Table 1. Point mutations in SEC24 were introduced by QuikChange mutagenesis (Stratagene, La Jolla, CA) using primers containing the desired changes and plasmids pLM23 or pLM129 as template (Miller et al., 2003). The Sed5p open reading frame was amplified by PCR and subcloned into the pMET25-HA vector using BglII/HindIII restriction sites. This created an in-frame fusion of a triple-HA tag to the N-terminus of Sed5p, generating plasmid pLM222. Mutations in the Sec24p-binding motifs of Sed5p were introduced into pLM222 by QuikChange mutagenesis (Stratagene).
Protein Purification
Sar1p, Sec13/31p, and wild-type Sec23/24p were prepared as described (Barlowe et al., 1994). Mutant versions of Sec24p were coexpressed with Sec23p under the control of the GAL1 promoter in RSY620 and were purified as described (Miller et al., 2003). Uso1p was purified from yeast cytosol as described (Barlowe, 1997).
In Vitro Vesicle Budding, Tethering, and Fusion
Microsomal membranes were prepared from WT cells (RSY620) and used in vesicle budding and fusion assays as described (Barlowe et al., 1994). Vesicle tethering was assessed using semi-intact cells supplemented with COPII proteins and Uso1p as described (Cao et al., 1998). Antibody inhibition of Sly1p/Sed5p packaging was performed by preincubating membranes with anti-Sly1p antibodies before addition of COPII proteins. In some experiments, COPII vesicles were purified after budding by flotation through a Nycodenz gradient (Barlowe et al., 1994). Nycodenz-purified vesicles were then added to a fusion reaction containing WT acceptor membranes, cytosol, and energy. Subsequent fusion of COPII vesicles with Golgi membranes was assessed by measuring the α-1,6-mannose modification of α-factor by immunoprecipitation with a specific antiserum (Barlowe et al., 1994).
RESULTS
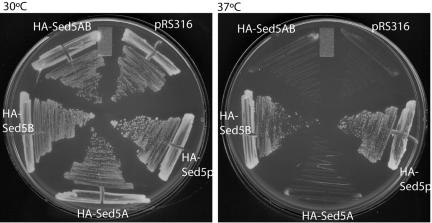
Biochemical and structural analysis of the Sed5p-Sec24p interactions revealed two independent binding sites(Mossessova et al., 2003). To investigate the role of each of these two sites on ER-Golgi traffic of Sed5p, we created an epitope-tagged version of Sed5p (Table 1) and introduced mutations that alter either the NPF motif, which interacts with the Sec24p A-site (HA-Sed5A); the LxxME motif, which interacts with the Sec24p B-site (HA-Sed5B); or both sites (HA-Sed5AB). We tested the ability of each of these proteins to complement a sed5-1 temperature-sensitive allele. Both HA-Sed5p and HA-Sed5B were able to confer viability at the restrictive temperature, whereas HA-Sed5A and HA-Sed5AB mutants were inviable (Figure 1).
Figure 1.
In vivo function of ER export motifs on Sed5p. A temperature-sensitive sed5-1 strain was transformed with either empty plasmid (pRS316), wild-type epitope-tagged Sed5p (HA-Sed5), or mutant versions of sed5 that contained alterations in either the NPF motif that interacts with the Sec24p A-site (HA-Sed5A), in the LxxME motif that interacts with the Sec24p B-site (HA-Sed5B) or in both motifs (HA-Sed5AB). Growth was tested at both permissive (30°C; left panel) and restrictive (37°C; right panel) temperatures.
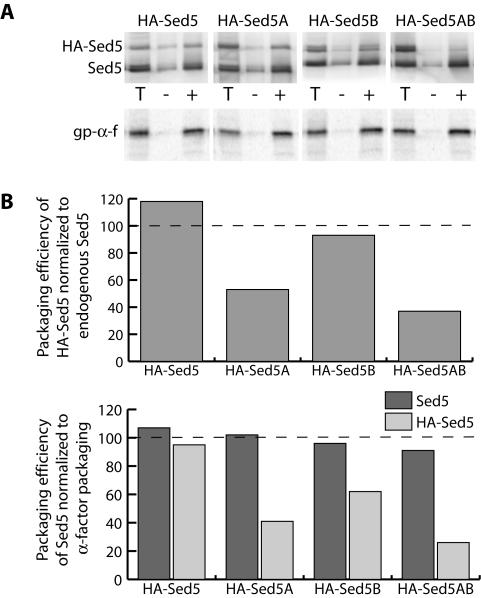
We next assessed the packaging efficiency of each of the Sed5p proteins using in vitro budding assays from microsomes expressing both endogenous WT Sed5p and the HA-tagged copy of Sed5p (Figure 2). The presence of the HA tag conferred a slower gel mobility on the plasmid-borne copies of Sed5p, allowing us to monitor packaging of both endogenous Sed5p and the mutant forms. For comparison, we also monitored packaging of the model cargo protein, gp-α-factor (Figure 2A). The different microsomal membrane preparations yielded slightly different vesicle budding efficiencies, as monitored both by endogenous Sed5p and gp-α-factor packaging. Therefore, we normalized the packaging of each of the tagged forms of Sed5p to the endogenous copy of Sed5p in order to compensate for these variations. Both wild-type HA-Sed5 and HA-Sed5B were packaged to a similar extent as endogenous Sed5p, whereas both HA-Sed5A and HA-Sed5AB were impaired in their uptake into COPII vesicles (Figure 2B). Similar results were obtained when we normalized HA-Sed5p packaging to gp-α-factor. Packaging of endogenous Sed5p, normalized to gp-α-factor packaging, remained unchanged irrespective of the mutation introduced to the HA-tagged copy of Sed5p. Together, these data support the observation that the A-site on Sec24p has a higher affinity for Sed5p than the B-site (Mossessova et al., 2003) and suggest that Sed5p primarily uses the A-site for uptake into COPII vesicles.
Figure 2.
In vitro budding defects are associated with Sed5p mutants. (A) Microsomal membranes were prepared from strains bearing an HA-tagged copy of wild-type or mutant Sed5p as indicated. Total membranes (T) and vesicles were collected from reactions that were generated in the absence (-) or presence (+) of wild-type Sec23/24p. Packaging of both endogenous Sed5p and HA-Sed5p was detected by anti-Sed5p immunoblots. Release of glycosylated pro-α-factor (gp-α-f) was detected by autoradiography. (B) Packaging of the HA-tagged copy of Sed5p was quantified by chemiluminescence and normalized to the amount of endogenous Sed5p released in the same budding reaction (top panel). HA-Sed5A and HA-Sed5AB were dramatically reduced in their packaging efficiency relative to endogenous Sed5p. Similar results were seen when Sed5p packaging was normalized to gp-α-factor release (bottom panel), whereas normalized endogenous Sed5p packaging was unaffected by mutation of the HA-tagged version.
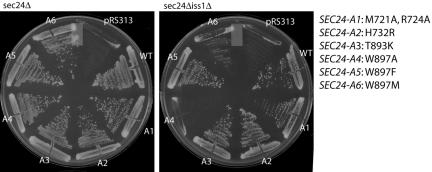
B-site mutants of Sec24p are only partially defective in Sed5p packaging (Miller et al., 2003). We sought to characterize the role of the Sec24p A-site in ER export by making complementary mutations in the NPF-binding site on Sec24p. The Sed5p-binding pocket of Sec24p (the “A-site”) is lined with a number of amino acid residues that we targeted for site-directed mutagenesis. Plasmids bearing the resulting SEC24-A mutants were introduced into strains that had chromosomal deletions in either SEC24 alone, or in both SEC24 and its closely related homolog, ISS1. Because SEC24 is an essential gene, viability in these strains is maintained by the presence of a wild-type copy of SEC24 borne on a plasmid marked with the URA3 gene, which allows for counterselection on the drug 5-FOA. Cells grow in the presence of 5-FOA only if a mutated version of SEC24 is able to function in place of the wild-type plasmid-borne gene. Conversely, a mutant that cannot provide essential functions will not sustain growth on 5-FOA. All of the A-site mutants of SEC24 were able to function in place of SEC24, but showed various phenotypes in a sec24Δiss1Δ background (Figure 3). The majority of the mutations we tested were fully functional in this double mutant background, but changes in W897 were less well tolerated. Mutation of W897 to either alanine or methionine (SEC24-A4 and -A6, respectively) yielded proteins that were unable to support viability. However, the more conservative change of W897 to phenylalanine (SEC24-A5) conferred complementation of the loss of SEC24. These phenotypes are reminiscent of those observed with Sec24p B-site mutants (Miller et al., 2003) and implicate the Sec24p A-site as an important cargo-binding site in vivo.
Figure 3.
In vivo phenotypes of Sec24p A-site mutants. Strains containing chromosomal deletions in either SEC24 alone (left panel), or SEC24 and it homolog ISS1 (right panel) were transformed with either empty vector, wild-type SEC24, or SEC24 mutants as indicated. All mutants were able to complement the sec24Δ strain whereas the SEC24-A4 and -A6 mutants were unable to confer viability in a sec24Δiss1Δ background.
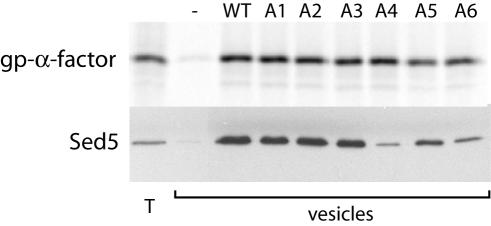
To further characterize the effects of these mutations in the Sec24p A-site on COPII vesicle formation, we overexpressed hexahistidine-tagged versions of the mutant proteins and purified Sec23/24p complexes. Wild-type and mutant Sec23/24p complexes were used in in vitro budding reactions that generate COPII vesicles from urea-washed microsomal membranes. Each of the mutant proteins was able to generate COPII vesicles, as determined by the specific release of glycosylated pro-α-factor into the vesicle fraction (Figure 4). The ability of the mutant proteins to package Sed5p corresponded closely with the in vivo phenotypes: Sec24-A1, -A2, -A3, and -A5 packaged Sed5p to the same extent as wild-type Sec24p, whereas the Sec24-A4 and -A6 mutant proteins were severely impaired in their ability to package Sed5p (Figure 4). The severity of packaging defects associated with the A4 and A6 mutations were equivalent, and we used these two mutant proteins interchangeably in further characterization of the Sec24p A-site.
Figure 4.
Sed5p packaging defects are associated with A-site mutations in Sec24p. Total membranes (T) and budded vesicles were collected from in vitro budding reactions containing Sar1p (10 μg/ml), Sec13/31p (20 μg/ml), and GMP-PNP (0.1 mM) supplemented with wild-type or mutant Sec23/24p (10 μg/ml) as indicated. Radiolabeled glycosylated pro-α-factor (gp-α-factor) was detected by autoradiography and serves as an indicator of general budding efficiency. Sed5p, detected by immunoblotting, was dramatically reduced in vesicles generated with either Sec24-A4 or -A6.
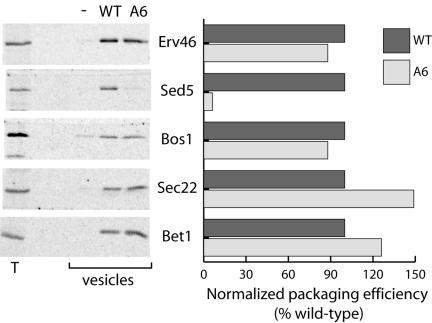
Of particular interest were the effects of A-site mutations on packaging of other cargo proteins, and specifically on the uptake of the two SNAREs, Sec22p and Bos1p. Biochemical characterization of the affinity of Sec24p for the two binding sites found on Sed5p indicated that Sec24p binding was dependent on the conformation of Sed5p (Mossessova et al., 2003). Like all syntaxin-type SNAREs, Sed5p has a large N-terminal regulatory domain (NRD) that is thought to fold back over the SNARE domain to regulate SNARE complex formation. When the NRD is engaged with Sed5p (i.e., in the “closed” conformation), the NPF motif is not accessible to Sec24p. However, when Sed5p is in the “open” conformation, the NRD no longer occludes the SNARE domain, allowing SNARE complex formation and rendering the NPF motif accessible. This suggests that Sed5p in complex with Sec22p and Bos1p may be preferentially packaged into CO-PII vesicles (Mossessova et al., 2003). Quantitative immunoblots of COPII vesicles generated with either WT or Sec24-A6 revealed that of all the cargo proteins analyzed, only Sed5p was disrupted in its uptake into the vesicle fraction (Figure 5). The observation that Sec22p and Bos1p were not affected by mutation of the A-site suggests that these two proteins do not rely on interaction with Sed5p for their uptake into a COPII vesicle. Sec24-A4 and -A6 were indistinguishable in this respect (Figure 5 and unpublished data).
Figure 5.
Evaluation of the extent of cargo-packaging defects associated with Sec24-A6. Vesicles were generated from microsomal membranes using either wild-type Sec24p or Sec24-A6, as described in Figure 2. The presence of a range of cargo proteins was detected by immunoblotting (left panel) and quantified using a radiolabeled secondary antibody (right panel). Only Sed5p was impaired in its uptake into vesicles generated with Sec24-A6.
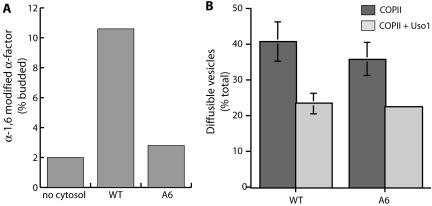
To further explore the role of the Sec24p A-site in ER-Golgi transport, we next asked whether vesicles generated with Sec24-A6 were capable of fusing with the Golgi apparatus in vitro. COPII vesicles carrying radiolabeled gp-α-factor were generated with either WT Sec24p or Sec24-A6 and incubated with acceptor membranes, energy, and cytosol. Fusion with the Golgi apparatus was monitored by immunoprecipitation with antibodies directed against an α-1,6-mannose epitope, which is added to glycoproteins in the yeast Golgi complex. Vesicles generated with WT Sec24p were capable of fusion in a cytosol-dependent manner, whereas vesicles generated with either Sec24-A4 or -A6 were defective for fusion (Figure 6A, and unpublished data). To address whether this Golgi delivery defect reflected a deficiency early in the reaction, during the tethering stage, or later during membrane fusion, we assessed the tethering efficiency of vesicles generated with either WT or mutant Sec24p using a two-stage reaction. Vesicles generated by incubating semi-intact cells with purified COPII proteins are readily separated from Golgi membranes by differential centrifugation. Addition of the tethering factor, Uso1p, during a second-stage incubation, before centrifugation, results in the association of a significant population of these vesicles with the heavier Golgi membranes causing an apparent reduction in the diffusible vesicle population (Cao et al., 1998). COPII budding reactions performed with either WT or Sec24-A6 generated a significant pool of diffusible vesicles that tethered to heavier membranes in a Uso1p-dependent manner with equivalent efficiency (Figure 6B). These data suggest that vesicles generated with Sec24p A-site mutants are defective in fusion with the Golgi apparatus at a relatively late stage during the reaction, after the vesicle tethering event.
Figure 6.
Vesicles generated with Sec24-A6 fail to fuse with the Golgi but are capable of tethering to acceptor membranes. (A) Vesicles containing 35S-labeled gp-α-factor were generated with either wild-type Sec24p or Sec24-A6. Vesicles were incubated with acceptor membranes and ATP and a regenerating system in the presence or absence of cytosol. Fusion of vesicles with Golgi membranes was determined by precipitation with antibodies against an α-1,6-mannose moiety and scintillation counting. (B) Semi-intact cells containing 35S-labeled gp-α-factor were incubated with COPII or CO-PII plus Uso1p to monitor vesicle budding and tethering. The amount of radiolabeled gp-α-factor present in freely diffusible vesicles was quantified by ConA precipitation. The Uso1p-dependent reduction of gp-α-factor in the vesicle fraction corresponds to vesicle tethering to larger Golgi membranes.
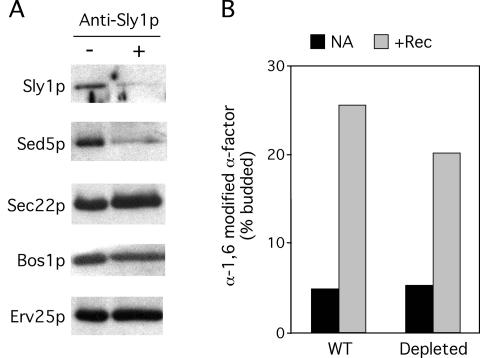
Because Sed5p was specifically depleted from vesicles generated with Sec24p A-site mutants, which were subsequently unable to fuse, we sought to determine whether the fusion defect was simply the result of the absence of Sed5p. As an independent test for the requirement of Sed5p on the vesicle population, we used antibodies to inhibit uptake of Sed5p during the vesicle-budding step. Antibodies against Sed5p did not dramatically inhibit Sed5p packaging (unpublished data), but preincubation of membranes with antibodies against the Sed5p effector protein, Sly1p, quantitatively depleted both Sed5p and Sly1p from the vesicle population (Figure 7A). Although the depletion of Sed5p with anti-Sly1p antibodies was not 100% efficient, the amount of remaining Sed5p in anti-Sly1p-depleted vesicles was comparable to that seen with the Sec24-A6 mutant protein (compare Figures 4 and 7A). Vesicles generated in the presence of anti-Sly1p antibodies were purified by flotation through a Nycodenz gradient to remove excess antibodies that might interfere with downstream fusion events. Nycodenz-purified vesicles were incubated with acceptor membranes, cytosol, and ATP, and delivery of gp-α-factor to the Golgi was monitored by α-1,6-mannose immunoprecipitation. Vesicles depleted of Sed5p by anti-Sly1 antibodies were capable of undergoing fusion with the Golgi apparatus, showing only a modest reduction in fusion efficiency compared with untreated vesicles (Figure 7B). This contrasts with vesicles generated with Sec24-A6, which were depleted of Sed5p to a similar extent, yet were incapable of fusion. These data support previous experiments that used temperature-sensitive SNARE alleles to examine the requirements for each of the ER-Golgi SNAREs in mediating fusion of COPII vesicles with the Golgi apparatus, which indicated that Sed5p was required only on the Golgi acceptor membranes (Cao and Barlowe, 2000). Thus the absence of Sed5p on ER-derived vesicles is not the primary cause of the fusion deficiency of vesicles generated with Sec24p A-site mutants.
Figure 7.
Specific depletion of Sed5p from COPII vesicles does not block fusion with Golgi membranes. Vesicles containing 35S-gp-α-factor were generated from microsomes using wild-type COPII proteins in the absence or presence of anti-Sly1p antibodies and purified by flotation through a Nycodenz gradient. (A) Specific proteins released into purified vesicles were monitored by immunoblotting. Note that Sly1p and Sed5p were specifically depleted from vesicles generated in the presence (+) of anti-Sly1p antibodies. (B) Purified vesicles were incubated with acceptor membranes, energy and no addition (NA) or fusion reconstitution proteins (Rec) at 25°C for 70 min. Fusion of vesicles with the Golgi membranes was monitored by α-1,6-mannose immunoprecipitation and scintillation counting.
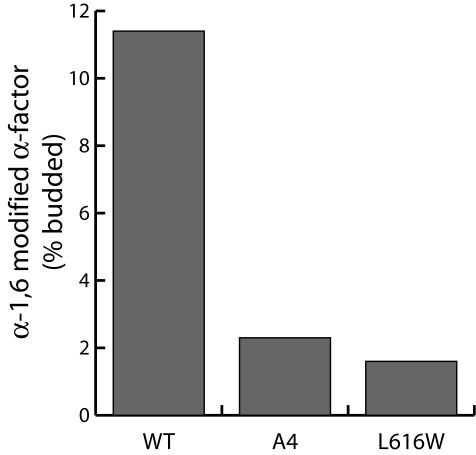
Having ruled out that the fusion defects associated with the Sec24p A-site mutants were caused by the absence of Sed5p, we considered the possibility that an interaction between Golgi-localized Sed5p and vesicle-borne Sec24p might function in regulating membrane fusion by triggering coat dissociation. The mechanism by which the COPII coat is released from vesicles after fission is not well understood, but likely depends on GTP hydrolysis by Sar1p. However, even after Sar1p is released from COPII vesicles, significant amounts of Sec23/24p and Sec13/31p copurify with the vesicle fraction, suggesting that a physical barrier may persist that would retard membrane fusion (Barlowe et al., 1994). If Golgi-localized Sed5p specifically interacted with vesicle-borne Sec24p, vesicle uncoating would be coupled to membrane fusion, promoting the specific fusion of ER-derived vesicles with the appropriate Golgi compartment. Thus, Sec24p A-site mutants, unable to bind to Golgi-localized Sed5p, might retain the COPII coat, thereby preventing vesicle fusion. We sought to test this model by physically stripping the COPII coat from vesicles generated with either WT or Sec24-A4. Nycodenz flotation of COPII vesicles generated in the presence of GTP efficiently removes the coat proteins (Barlowe et al., 1994). Nycodenz-purified vesicles were incubated with acceptor membranes, cytosol, and energy and the fusion of vesicles monitored by α-1,6-mannose immunoprecipitation. Removal of the coat from vesicles generated with Sec24-A4 failed to reverse the fusion defect, suggesting that the coat was not acting to sterically inhibit fusion (Figure 8). Similar results were obtained with vesicles generated with a different Sec24p mutant, disrupted in the B-site, which lack the SNARE, Bet1p, known to be required on the vesicle fraction for efficient fusion.
Figure 8.
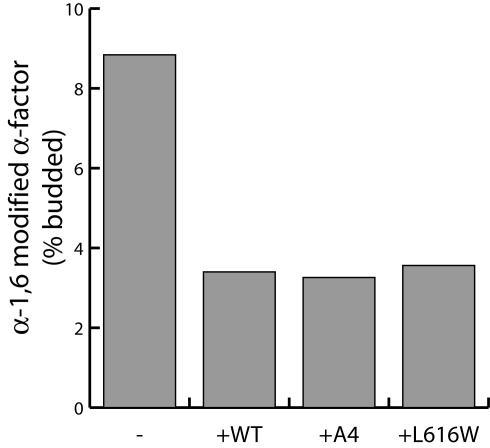
Fusion defects associated with Sec24p A-site mutants are not due to a regulatory role for Sed5p-Sec24p interaction. Vesicles generated with either wild-type Sec24p, Sec24-A4, or Sec24L616W were purified by flotation through Nycodenz before addition to a vesicle fusion reaction as described in Figure 6A. Removal of the COPII coat before fusion did not bypass the fusion defect associated with either Sec24p mutant.
As a second test for a role for a Sed5p-Sec24p interaction in regulating vesicle fusion, we examined the effect of excess Sec24p on the fusion reaction. Excess Sec23/24p inhibits a late stage of the vesicle fusion reaction, presumably by binding to either the vesicle or acceptor membranes and occluding the fusion machinery (Barlowe, 1997). Wild-type Sec24p and the A- and B-site mutant proteins were equally effective at inhibiting the fusion reaction (Figure 9). Together, our data indicate that it is unlikely that the fusion defects associated with Sec24p A-site mutants are caused either by the absence of Sed5p in vesicles, or by the failure to undergo vesicle uncoating before fusion. Alternatively, it seems likely that additional unidentified cargo proteins are missing in vesicles generated with Sec24p A-site mutants that are required for late stages in the vesicle fusion reaction.
Figure 9.
Mutant Sec23/24p inhibits fusion to the same extent as wild-type Sec23/24p. COPII vesicles were generated with wild-type coat proteins and then incubated with acceptor membranes, ATP, and cytosol in the absence or presence of excess wild-type or mutant Sec23/24p as indicated. Fusion was detected by α-1,6-mannose immunoprecipitation.
DISCUSSION
The combination of genetic, biochemical and structural characterization of cargo-coat interactions has been particularly fruitful in elucidating the molecular mechanisms that drive selective capture of proteins into nascent vesicles. We describe here a mutational analysis of one of three independent cargo-binding sites on the COPII coat subunit, Sec24p, which drives cargo capture into ER-derived vesicles. The Sec24p “A-site” mediates interaction with a motif found on the SNARE, Sed5p, largely through hydrophobic interactions (Mossessova et al., 2003). Alterations in residues that line the A-site pocket were introduced into yeast and the ability of the mutant protein to complement the loss of wild-type Sec24p was assessed. Mutations in hydrophilic residues that line this pocket showed no visible phenotype, either in vivo or using in vitro vesicle-budding assays. Conversely, mutation of Trp897 to either alanine or methionine caused a dramatic decline in Sed5p packaging in vitro and resulted in a lethal phenotype in a sec24Δiss1Δ genetic background. The interplay between a NPF motif and a binding pocket that contains a critical Trp residue is reminiscent of the interaction between EH domains and NPF ligands (de Beer et al., 2000). Such interactions are important in a variety of cellular functions, including endocytosis, growth factor signaling, and neuronal development.
Analysis of cargo packaging defects associated with the Sec24W897 mutants indicated that of the cargoes tested, only Sed5p was depleted in COPII vesicles made with these mutants. Interestingly, the severity of the reduction in Sed5p packaging with the Sec24p A-site mutants was greater than that observed with B-site mutants, which showed only a 50% reduction in Sed5p packaging (Miller et al., 2003). That Sed5p interacts with Sec24p predominantly through the A-site is consistent with a higher affinity for this site (∼19-fold higher than for the B-site; Mossessova et al., 2003). Furthermore, our analysis of the in vivo and in vitro function of Sed5p mutants altered in each of the two Sec24p-interacting motifs confirmed a dominant role for the NPF-A-site interaction.
Of particular interest was the observation that COPII vesicles generated with the Sec24p A-site mutants contained wild-type levels of two other ER-Golgi SNAREs, Sec22p and Bos1p, in the absence of Sed5p packaging. Previous biochemical characterization of SNARE-Sec24p interactions indicated that the Sed5p NPF motif would only be available to interact with Sec24p when Sed5p was assembled in SNARE bundle. Furthermore, the absence of a detectable interaction between Sec24p and Bos1p led to the suggestion that Bos1p would be largely captured into COPII vesicles by virtue of its interaction with Sed5p rather than through direct interaction with Sec24p (Mossessova et al., 2003). Our data suggest that it is unlikely that Bos1p and Sec22p uptake into COPII vesicles is dependent on Sed5p. Similarly, mutations in the SNARE domain of Sec22p that disrupt SNARE complex formation but do not affect uptake into COPII vesicles further support a Sed5p-independent mechanism for packaging of these nonsyntaxin SNAREs (Liu et al., 2004).
Although our experiments demonstrate that Bos1p and Sec22p can be packaged independently of Sed5p, we have not addressed whether the predominant form of Sed5p that enters vesicles is associated with a SNARE complex. Because we have defined the NPF motif as the dominant ER export signal on Sed5p, it seems likely that the majority of ER-localized Sed5p is not in the “closed” conformation, which occludes this motif (Mossessova et al., 2003). Introducing mutations in Sed5p that disrupt SNARE complex formation would be useful in further elucidating the role of assembly in the capture of SNARE proteins into COPII vesicles. However, because Sed5p is a syntaxin-like SNARE and also interacts with additional effector proteins, such mutations may have pleiotropic defects. Indeed, interactions with Sly1p seem likely to directly influence packaging into COPII vesicles. Our antibody inhibition experiments made use of anti-Sly1p antibodies to dramatically deplete COPII vesicles of Sed5p, suggesting that the majority of ER-localized Sed5p is in complex with Sly1p. Thus one function of Sly1p may be to maintain this ER pool of Sed5p in an “open” conformation such that the dominant NPF motif is accessible to Sec24p even in the absence of SNARE complex formation.
An additional striking feature of vesicles generated with Sec24p A-site mutants is the inability of these vesicles to undergo fusion with the Golgi apparatus. The role of each ER-Golgi SNARE in fusion of COPII vesicles with Golgi membranes has previously been characterized using membranes isolated from temperature-sensitive mutants (Cao and Barlowe, 2000). Reactions containing mutant donor vesicles or mutant acceptor Golgi membranes permitted the role of each SNARE to be defined. Under these conditions, Sed5p was not required on the vesicle population but was required on the acceptor Golgi membranes. Using antibodies to inhibit uptake of Sed5p into COPII vesicles made with wild-type Sec24p, we were able to largely deplete the vesicle population of Sed5p, similar to the degree of depletion seen with the Sec24 A-site mutants. The ability of these immunodepleted vesicles to deliver pro-α-factor to the Golgi apparatus confirms previous observations that Sed5p is not required on the donor vesicle population for Golgi fusion.
We considered the possibility that an interaction between Golgi-localized Sed5p and vesicle-borne Sec24p might play a regulatory role in promoting vesicle uncoating before fusion. COPII vesicles purified gently by gel filtration retain the Sec23/24p and Sec13/31p subunits (Barlowe et al., 1994), suggesting that these coat components may persist on vesicles after fission from the ER and release of Sar1p. We tested whether fusion of Sec24p A-site mutant vesicles was impaired as a result of a persistent COPII coat by physically stripping the coat from vesicles before fusion, anticipating that if vesicle uncoating was initiated by Sec24p-Sed5p interaction that we could bypass the fusion defects associated with the mutant Sec24p. However, vesicles generated with the mutant Sec24p were still incapable of fusion after purification, suggesting a more fundamental defect associated with these vesicles. Thus it seems most likely that an additional, unidentified protein is absent in vesicles generated with Sec24p A-site mutants that plays an important role in mediating vesicle fusion at a posttethering stage in ER-Golgi transport. Proteomic and genetic approaches should be useful in identifying the putative missing fusion factor.
We have now examined each of the three known cargo-binding sites on Sec24p by mutagenesis and have described mutations that disrupt packaging at each site. Disruption of the B-site, which recognizes cargoes largely through electrostatic interactions had the most profound effect on cargo packaging: the most severe mutant was incapable of complementing Sec24p and showed pleiotropic cargo selection defects (Miller et al., 2003). Conversely, the A- and C-sites so far seem to bind only to single cargo proteins, although these analyses have been limited to the characterization of proteins for which antibodies or epitope-tagged copies are available. More sensitive proteomic analysis of the contents of vesicles generated with the various Sec24p mutants is likely to reveal additional cargo defects. Finally, there are certainly additional cargo-binding sites that remain to be identified: packaging of a number of proteins, including the model cargo, gp-α-factor through its receptor, Erv29p, the abundant vesicle proteins Erv41/46p, and the amino acid permeases remain unaffected by any of the mutations we have generated to date. Understanding how each of these different classes of cargo interacts with the multiple binding sites on the COPII coat is a fundamental part of elucidating the molecular mechanisms that drive efficient and selective ER-Golgi transport.
Acknowledgments
We thank Crystal Chan for preparing wild-type COPII proteins and Jonathan Goldberg for suggesting several of the Sec24p A-site mutations. We are grateful to members of the Schekman lab for support, especially Alenka Copic and Silvere Pagant for comments on the manuscript. This work was supported by the Howard Hughes Medical Institute (R.S.) and by the National Institutes of Health (C.B.). During the initial stages of this work, E.A.M. was supported by a fellowship from the Jane Coffin Childs Memorial Fund for Medical Research.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0262) on June 1, 2005.
Abbreviations used: COPII, coat protein complex type II; gp-α-factor, glycosylated pro-α-factor.
References
- Barlowe, C. (1997). Coupled ER to Golgi transport reconstituted with purified cytosolic proteins. J. Cell Biol. 139, 1097-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M. F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895-907. [DOI] [PubMed] [Google Scholar]
- Cao, X., Ballew, N., and Barlowe, C. (1998). Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 17, 2156-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, X., and Barlowe, C. (2000). Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J. Cell Biol. 149, 55-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beer, T., Hoofnagle, A. N., Enmon, J. L., Bowers, R. C., Yamabhai, M., Kay, B. K., and Overduin, M. (2000). Molecular mechanism of NPF recognition by EH domains. Nat. Struct. Biol. 7, 1018-1022. [DOI] [PubMed] [Google Scholar]
- Lee, M. C., Miller, E. A., Goldberg, J., Orci, L., and Schekman, R. (2004). Bi-directional protein transport between the ER and Golgi. Annu. Rev. Cell Dev. Biol. 20, 87-123. [DOI] [PubMed] [Google Scholar]
- Liu, Y., Flanagan, J. J., and Barlowe, C. (2004). Sec22p export from the endoplasmic reticulum is independent of SNARE pairing. J. Biol. Chem. 279, 27225-27232. [DOI] [PubMed] [Google Scholar]
- Miller, E. A., Beilharz, T. H., Malkus, P. N., Lee, M. C., Hamamoto, S., Orci, L., and Schekman, R. (2003). Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114, 497-509. [DOI] [PubMed] [Google Scholar]
- Mossessova, E., Bickford, L.C., and Goldberg, J. (2003). SNARE selectivity of the COPII coat. Cell 114, 483-495. [DOI] [PubMed] [Google Scholar]