Abstract
The effect of heat shock on centrosomes has been mainly studied in interphase cells. Centrosomes play a key role in proper segregation of DNA during mitosis. However, the direct effect and consequences of heat shock on mitotic cells and a possible cellular defense system against proteotoxic stress during mitosis have not been described in detail. Here, we show that mild heat shock, applied during mitosis, causes loss of dynamitin/p50 antibody staining from centrosomes and kinetochores. In addition, it induces division errors in most cells and in the remaining cells progression through mitosis is delayed. Expression of heat shock protein (Hsp)70 protects against most heat-induced division abnormalities. On heat shock, Hsp70 is rapidly recruited to mitotic centrosomes and normal progression through mitosis is observed immediately after release of Hsp70 from centrosomes. In addition, Hsp70 expression coincides with restoration of dynamitin/p50 antibody staining at centrosomes but not at kinetochores. Our data show that during mitosis, centrosomes are particularly affected resulting in abnormal mitosis. Hsp70 is sufficient to protect against most division abnormalities, demonstrating the involvement of Hsp70 in a repair mechanism of heat-damaged mitotic centrosomes.
INTRODUCTION
Centrosomes, the microtubule-organizing centers of the cell, are involved in proper segregation of duplicated DNA, play a role in completion of cytokinesis (Piel et al., 2001; Gromley et al., 2003), and are required for progression through G1 phase (Hinchcliffe et al., 2001; Khodjakov and Rieder, 2001; Gromley et al., 2003). It has been demonstrated that centrosomes are target organelles affected by different kinds of cellular stress responses, especially during mitosis. In Drosophila embryos, centrosome inactivation occurs in the presence of genotoxic lesions at the onset of mitosis (Sibon et al., 2000; Takada et al., 2003; Sibon and Theurkauf, 2004), a process that requires Chk2 kinase (Takada et al., 2003). In this process, multiple components of the γ-tubulin ring complex disappear from mitotic centrosomes, anastral spindles are formed, and segregation of duplicated DNA is prevented. Mammalian cells also show mitosis-specific centrosome responses to genotoxic stress. When cells enter mitosis with damaged DNA, centrosomes split into monocentriolar parts that form multiple spindle poles, resulting in mitotic catastrophe (Hut et al., 2003).
Centrosomes are not only affected after genotoxic stress but also after a proteotoxic stress such as heat shock. In Drosophila embryos, heat shock leads to severe defects in centriole organization specifically when cells are in mitosis (Debec and Marcaillou, 1997). Cellular consequences of these heat shock-affected mitotic centrosomes have not been studied in this system. In mammalian cells, it has been described that during interphase, heat shock causes alterations of centrosomes, such as increases in the electrondense material comprising the pericentriolar mass, disappearance of centrosomal proteins, and disruption of centriolar walls (Barrau et al., 1978; Malawista et al., 1983; Vidair et al., 1995, 1996; Brown et al., 1996b). In addition, the appearance of multipolar mitotic spindles in the first mitoses postheating were reported (Vidair et al., 1993; Nakahata et al., 2002).
It is known that preconditioning with a mild heat shock can induce a state of heat resistance, i.e., thermotolerance, that also protects against centrosome alterations after a second heat treatment (Vidair et al., 1995; Brown et al., 1996b). This has been demonstrated for centrosomes in interphase because thermotolerant cells display a more rapid recovery from heat-induced damage to centrosome structure (assayed by antibody staining) and centrosome function (assayed by regrowth of microtubules) (Vidair et al., 1995; Brown et al., 1996b). However, centrosome alterations in heat-treated mammalian mitotic cells have not been investigated, and the fate of cells with heat-affected centrosomes remains unknown.
Thermotolerance is tightly correlated with the elevated expression of heat shock proteins (Hsps) both in interphase (Li and Werb, 1982) and in mitosis (Borrelli et al., 1996). Associations of heat shock proteins with mitotic centrosomes have been observed previously (Rattner, 1991; Perret et al., 1995; Brown et al., 1996a; Lange et al., 2000; Agueli et al., 2001), suggesting a role for heat shock proteins in assisting repair of centrosomes after heat shock. Microinjection of Hsp73 antibodies retarded recovery of interphase centrosome structure and microtubule regrowth abilities after heat shock, whereas injection of purified Hsp73 before heat shock enhanced these processes (Brown et al., 1996b). Because heat shock proteins function as molecular chaperones to repair protein damage (Lindquist, 1986; Kampinga, 1993), it can be suggested that protein aggregation may underlie the centrosome abnormalities after heat shock. Indeed, electron microscopical studies suggested the presence of protein aggregates at the pericentriolar mass after heat shock (Vidair et al., 1996).
Most of the above-mentioned studies have concentrated on effects of severe heat treatments on interphase centrosome structure and function in fixed cells. Here, by performing time-lapse studies, we aimed to address what the effects are of mild and severe heat shock on mitotic centrosomes, what the fate is of mitotic cells with heat-affected centrosomes, and whether heat shock proteins can protect heat shock affected centrosomes in mitotic cells.
We show that severe heat treatment resulted in the loss of γ-tubulin and pericentrin antibody labeling at centrosomes and loss of dynamitin/p50 (a 50-kDa subunit of dynactin) antibody labeling at centrosomes and at kinetochores. Under these conditions, CREST antiserum did not show an altered localization pattern. However, the organization of mitotic spindles was altered, mitotic progression was strongly delayed, and division errors occurred. After mild heat shock, only dynamitin/p50 antibody labeling disappeared, and none of the other protein alterations found after severe heat shock were detected. Nonetheless, under these mild heat shock conditions, delays in mitotic progression and mitotic failures were observed. In thermotolerant cells, the length of heat shock-induced mitotic delays and the frequency of heat shock-induced division errors were reduced. The reduction in abnormalities correlated with the association of Hsp70, but not Hsp27 or Hsp40, with centrosomes. Overexpression of Hsp70 restored localization of dynamitin/p50 antibody labeling at centrosomes and protected cells against most heat-induced mitotic abnormalities. Our data suggest a link between protein damage at centrosomes and centrosome function.
MATERIALS AND METHODS
Cell Lines and Culturing
OT70 hamster lung fibroblasts were cultured as described previously (Nollen et al., 1999) and maintained at 5% CO2 in a humidified 37°C incubator. Transient transfections were performed by using Lipofectamine according to the procedure of the manufacturer (Invitrogen, Paisley, United Kingdom). Per transfection experiment, the total amount of plasmid DNA was kept constant at 1 μg of pDNA per coverslip. The pBOS-H2BGFP construct was purchased from BD Biosciences PharMingen (San Diego, CA). The pHsp70-enhanced yellow fluorescent protein (EYFP) was constructed, and the fusion protein Hsp70-EYFP has been shown to have similar chaperone activity as the untagged Hsp70 (Rujano, Salomons, and Kampinga, unpublished data).
Heat Shock Treatment
To induce heat shock, Petri dishes containing coverslips were preincubated in a water bath at 37°C and transferred to a second water bath at the indicated temperatures ± 0.1°C for the indicated times. Thermotolerance was induced by exposing the cells to a priming heat shock of 43°C for 15 min followed by 16- to 20-h recovery at 37°C.
Western Blot Analysis
Proteins were separated in SDS/10% polyacrylamide gels and transferred to nitrocellulose membranes. Heat-inducible proteins Hsp70, Hsp40, and Hsp27 were detected on the same membrane with anti-Hsp70 mouse (SPA810; Stressgen Biotechnologies, Victoria, British Columbia, Canada), anti-Hsp40 rabbit (SPA400; Stressgen), and anti-Hsp27 rabbit (SPA801; Stressgen, Biotechnologies) primary antibodies. As secondary antibodies, horseradish peroxidase-conjugated goat anti-mouse and anti-rabbit were used (Amersham Biosciences, Piscataway, NJ). Signals were detected by enhanced chemiluminescence (Amersham Biosciences).
Immunofluorescence Analysis
Cells were fixed using methanol/acetone as described previously (Hut et al., 2003). To visualize the centrosomes, polyclonal anti-γ-tubulin (T3559; Sigma, St. Louis MO) was used in combination with swine anti-rabbit fluorescein isothiocyanate (FITC) (DakoCytomation Denmark, Glostrup, Denmark), monoclonal anti-γ-tubulin (T6557; Sigma) in combination with goat antimouse CY3 (Amersham Biosciences), or polyclonal anti-pericentrin (BAbCO, Richmond, CA) in combination with goat anti-rabbit CY3 (Amersham Biosciences). To visualize the mitotic spindles, monoclonal anti-α-tubulin (T5168; Sigma) was used in combination with goat anti-mouse CY3 (Amersham Biosciences). To visualize kinetochore components, cells were formaldehyde fixed and labeled with CREST (kindly provided by B. R. Brinkley; Valdivia and Brinkley, 1985) in combination with secondary antibody rabbit antihuman (A0190; DakoCytomation Denmark) and tertiary antibody swine anti-rabbit FITC (DakoCytomation Denmark), or cells were labeled with monoclonal anti-dynamitin/p50 (611002; BD Biosciences PharMingen) in combination with secondary antibody goat anti-mouse CY3 (Amersham Biosciences) or goat anti-mouse FITC (The Jackson Laboratory, Bar Harbor, ME). For visualizing heat shock proteins, anti-Hsp70 mouse (SPA810; Stressgen Biotechnologies) was used in combination with anti-mouse CY3 (Amersham Biosciences), anti-Hsc70 rat (SPA815; Stressgen Biotechnologies) in combination with rabbit anti-rat FITC (DakoCytomation Denmark), anti-Hsp40 rabbit (SPA400; Stressgen Biotechnologies) in combination with swine anti-rabbit FITC (DakoCytomation Denmark), or anti-Hsp27 rabbit (SPA801; Stressgen Biotechnologies) in combination with swine anti-rabbit FITC (DakoCytomation Denmark). To visualize the DNA, cells were stained for 10 min with 0.2 μg/ml 4,6-diamidino-2-phenylindole (DAPI). Images were obtained with a confocal laser scanning microscope (Leica TCS SP2; Leica Microsystems, Heidelberg, Germany) with 351/364-, 488-, and 543-nm lasers.
Time-Lapse Imaging
Images were made with a confocal laser scanning microscope (Leica TCS SP2). To keep cells alive during the experiment, cells were cultured on round coverslips in a special chamber with 2 ml of medium. The chamber was put in a 37°C-heated microscope stage. A mixture of air with 5% CO2 was blown into the heated stage. The 63× oil immersion lens also were heated to 37°C. Cells were imaged in 8–10 optical planes (thickness 1–2 μm). In heat shock experiments, time-lapse recordings were started within 10 min after heat shock. The cells were imaged every 2 min, for up to 3 h. Cells expressing green fluorescent protein (GFP)-fusion proteins were excited with a 488 laser, and emission was measured with a BP 500–550 filter. Cells expressing yellow fluorescent protein (YFP)-fusion proteins were excited with a 514 laser, and emission was measured with a BP 500–550 filter. Data were analyzed using the Leica simulator. Maximum projections were made, and progression through mitosis was scored as normal when DNA aligned in the metaphase plate and two identical daughter cells were formed. Progression through mitosis was scored as abnormal when two daughter cells were formed containing micronuclei, when more than two daughter cells were formed, when anaphase onset did not occur within 2 h after heat shock, or when chromatin prematurely decondensed without preceding division of chromosomes.
RESULTS
Heat Shock Affects Mitosis in a Dose-dependent Manner
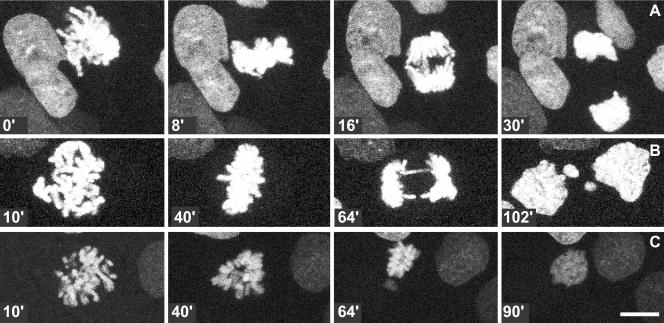
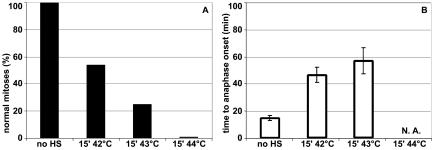
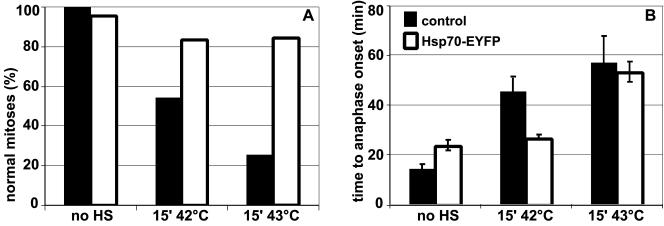
To investigate whether and how heat shock affects mitotic cells, time-lapse confocal microscopy was performed. Cells were transiently transfected with the Histon2B-GFP construct to visualize the organization and dynamics of chromosomes. Under control conditions, H2B-GFP–expressing cells progress normally through mitosis (Figure 1A; http://coo.med.rug.nl/sscb/video.htm, Figure 1), and on average, these cells proceed from early metaphase to anaphase within 15 min (Table 1). This indicates that the intensity of the laser beam used did not cause cell cycle arrest or any abnormalities in cell cycle progression. To analyze the effects of heat shock, cells were heated and immediately thereafter, mitotic cells were selected and progression through mitosis was followed using time-lapse analysis. A variety of abnormalities was seen, including division of cells in two daughter cells containing micronuclei (Figure 1B; http://coo.med.rug.nl/sscb/video.htm, Figure 2), formation of multipolar spindles (our unpublished data), lack of progression into anaphase within 2 h after heat shock (our unpublished data), or decondensation of chromatin without a preceding division of chromosomes (Figure 1C; http://coo.med.rug.nl/sscb/video.htm, Figure 3). Quantitative analysis showed a heat-dose dependent decline in normal mitoses (Figure 2A and Table 1). For those cells that did complete cytokinesis normally, i.e., after relatively mild heat treatments such as 15′ 42°C or 15′ 43°C, the time from early metaphase to anaphase was significantly increased (Figure 2B and Table 1), indicating that even after mild heat shock treatments every mitotic cell was affected leading to a delay in progression through mitosis.
Figure 1.
Heat shock during mitosis causes division abnormalities. Cells expressing H2B-GFP under control conditions (A) or after heat shock for 15′ at 43°C (B and C). Time is given in minutes after heat shock. Under control conditions mitotic progression occurred normally (A). After heat shock, mitotic abnormalities were observed such as the formation of two daughter cells with micronuclei (B) or premature decondensation of chromatin (C). Bar, 10 μm. For movies, see http://coo.med.rug.nl/sscb/video.htm, Figures 1, 2, 3.
Table 1.
Division errors observed after heat shock
| Cells | Treatment | Normal mitoses (%) | Avg time to anaphase onset (min) | n | Formation of micronuclei (%) | Multipolar (%) | No anaphase onset (%) | Prematurely decondensed DNA (%) |
|---|---|---|---|---|---|---|---|---|
| — | No | 100 | 15 | 20 | 0 | 0 | 0 | 0 |
| Hsp70-EYFP | No | 95 | 24 | 22 | 5 | 0 | 0 | 0 |
| — | 15′ 42°C | 54 | 46 | 35 | 6 | 17 | 6 | 17 |
| Hsp70-EYFP | 15′ 42°C | 83 | 27 | 36 | 17 | 0 | 0 | 0 |
| TT | 15′ 42°C | 73 | 38 | 37 | 8 | 13 | 3 | 3 |
| — | 15′ 43°C | 25 | 58 | 20 | 30 | 10 | 0 | 35 |
| Hsp70-EYFP | 15′ 43°C | 85 | 54 | 20 | 10 | 5 | 0 | 0 |
| — | 15′ 44°C | 0 | ND | 24 | 0 | 0 | 8 | 92 |
| — | 30′ 44°C | 0 | ND | 20 | 0 | 0 | 0 | 100 |
Overview of division errors observed in control cells (—), in thermotolerant cells (TT), and in cells expressing Hsp70-EYFP (Hsp70-EYFP) under control conditions and after heat treatments. ND, not determined.
Figure 2.
Heat shock during mitosis leads to a heat-dose–dependent decrease in normal mitoses and to an increase in mitotic delay. Mitotic progression of cells expressing H2B-GFP was recorded by time-lapse confocal microscopy. The percentage of normal mitoses (A) and the time in minutes from early metaphase to anaphase onset (B) is given in control cells (no HS), after 15′ heat shock at 42°C (15′ 42°C), after 15′ heat shock at 43°C (15′ 43°C) and after 15′ heat shock at 44°C (15′ 44°C). For each condition, >20 mitoses were recorded. Data represent mean ± SD.
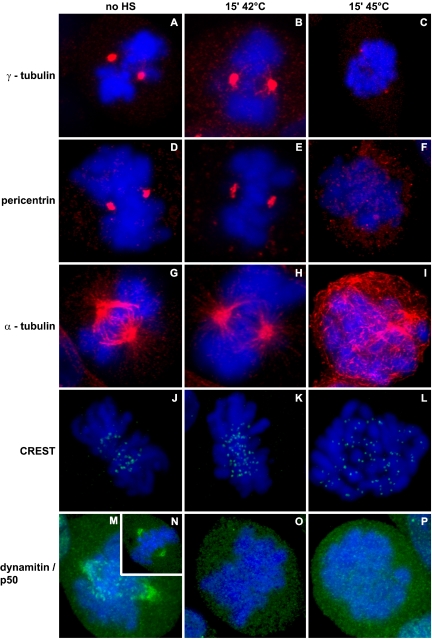
Figure 3.
Severe, but not mild, heat shock affects antibody staining of centrosome proteins and organization of microtubules, whereas both severe and mild heat shock affects antibody staining of dynamitin/p50. Cells were untreated (no HS), mildly heat shocked (15′ 42°C), or severely heat shocked (15′ 45°C). Cells were fixed and labeled with antibodies to γ-tubulin (A–C, red), to pericentrin (D–F, red), or to α-tubulin (G–I, red), with CREST antiserum (J–L, green), or with antibodies to dynamitin/p50 (M–P, green). DNA was stained by DAPI (A–P, blue).
It has already been demonstrated that heat shock induces alterations of centrosome structure (Barrau et al., 1978; Malawista et al., 1983; Vidair et al., 1995, 1996; Brown et al., 1996b; Debec and Marcaillou, 1997). Also, heat has profound effects on cytoskeleton elements (Malawista et al., 1983; Knox et al., 1991; Vidair et al., 1993), including effects on mitotic spindles (Coss et al., 1982; Debec and Marcaillou, 1997). To test whether heat-induced alterations of centrosomes are the cause of abnormal mitoses, cells were fixed within 10 min after heat shock and immunolabeling was performed using anti-γ-tubulin antibodies and anti-pericentrin antibodies. Like interphase cells, γ-tubulin and pericentrin staining decreased in mitotic cells after severe heat shock (Figure 3, C and F). But, no alterations in γ-tubulin and pericentrin staining were observed after a mild heat shock (compare Figures 3, B and E, to 3, A and D).
Comparable with the dose-dependent, heat-induced modifications of centrosomal proteins, the organization of microtubules also was found to be affected after a severe heat shock (Figure 3I), whereas no structural changes of microtubules were observed after a mild heat shock (Figure 3H). These results demonstrate that division errors after severe heat shock can be explained by the observed changes in centrosomal content and spindle organization; however, to explain the division errors after mild heat shock, further investigation is required.
Kinetochores are functionally important for proper mitosis because they are involved in attachment of chromosomes to spindle microtubule plus ends, contribute to force generation for chromosome movement, and play a major role in the mitotic spindle checkpoint (DeLuca and Salmon, 2004; Hauf and Watanabe, 2004; Shah et al., 2004). To investigate whether mitotic division errors as observed after heat shock are associated with alterations in localization of kinetochore components, immunolabeling was performed using antibodies to detect dynamitin/p50 and by using CREST antiserum. Dynamitin/p50 is a 50-kDa subunit of dynactin, a component of the kinetochore outer core that contains binding sites for the plus ends of kinetochore microtubules (Rieder and Salmon, 1998). CREST antiserum recognizes CENP-A, -B, and -C, components of the kinetochore inner core (Valdivia and Brinkley, 1985; Maney et al., 2000). CREST labeling was unaffected after both mild and severe heat shock compared with control conditions (Figure 3, J–L). Staining of unheated mitotic cells with the anti-dynamitin/p50 antibody revealed staining at centrosomes and kinetochores in prometaphase cells (Figure 3M). No staining was found when chromosomes were aligned at the metaphase plate (Figure 3N), consistent with previously published results (Echeverri et al., 1996). After both mild and severe heat shock, dynamitin/p50 localization was absent from centrosomes and kinetochores of nonaligned chromosomes during prometaphase (Figure 3, O and P), in contrast to CREST antiserum labeling. So, loss of dynamitin/p50 from kinetochores and centrosomes is associated with the mitotic delays and division errors induced by both severe and mild heat shock.
Thermotolerance Protects Cells against Heat-induced Mitotic Abnormalities
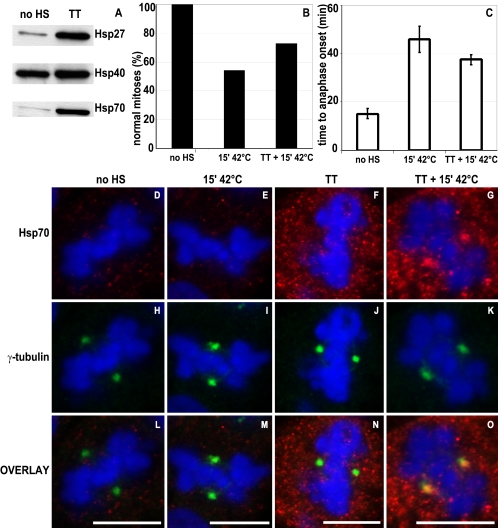
One clear response to protein damage is the induction of heat shock proteins (Morimoto, 1991) leading to a transient state of thermotolerance during which cellular structures are protected against subsequent heat challenges. Recruitment of (induced) heat shock proteins to a specific site can indicate the presence of damaged proteins at that specific site and a need for protection against subsequent heat challenges (Kampinga, 1993). To test whether induced expression of heat shock proteins can protect mitotic cells against division errors as observed after heat shock treatments, we generated thermotolerant cells by pretreating cells at 43°C for 15 min followed by incubation at 37°C overnight. Typically, Hsp27, Hsp40, and Hsp70 expression is increased in thermotolerant cells (Figure 4A). Thermotolerant cells were treated with a second, mild heat shock after which mitotic cells were selected and followed by time-lapse analysis. The percentage of normal mitoses in thermotolerant cells was higher compared with nonthermotolerant cells after the same heat shock (Figure 4B and Table 1). In addition, thermotolerant cells in which cytokinesis occurred normally showed less delay in progression through mitosis (Figure 4C and Table 1). These data show the presence of a mechanism in thermotolerant cells that protects against most heat shock-induced mitotic abnormalities. This protective mechanism may involve expression of heat shock proteins and/or recruitment of these heat shock proteins to mitotic structures suffering from heat shock-induced protein damage.
Figure 4.
Thermotolerance protects against heat-induced mitotic abnormalities. Cells were left untreated (no HS) or preconditioned to induce thermotolerance (TT). Expression of Hsp27, Hsp40, and Hsp70 was analyzed by Western blotting (A), demonstrating that in TT cells Hsp27, Hsp40, and Hsp70 are induced. Heat shock was given to H2B-GFP–expressing cells (15′ 42°C) or H2B-GFP–expressing TT cells (TT + 15′ 42°C), and mitotic progression was recorded by time-lapse confocal microscopy. Per condition, >20 cells were recorded. The percentage of normal mitoses (B) and mean time ± SD in minutes from early metaphase to anaphase onset (C) are given. After the indicated treatments, cells were methanol/acetone fixed and labeled with monoclonal antibody to Hsp70 (D–G, red) and polyclonal antibody to γ-tubulin (H–K, green). DNA was stained by DAPI (D–O, blue). Overlays of the images are shown in L–O. Bar, 10 μm.
To test this idea, recruitment of individual heat shock proteins to mitotic structures was examined in thermotolerant cells. As shown in Figure 4, Hsp70 colocalized with γ-tubulin in heat-shocked, thermotolerant cells (Figure 4, G, K, and O) but not in unheated thermotolerant cells (Figure 4, F, J, and N), indicating that Hsp70 is dynamically recruited to mitotic centrosomes after heat shock. Hsp70 localization at centrosomes was not observed in unheated or heated nonthermotolerant cells (Figure 4, D, E, H, I, L, and M) in which Hsp70 is not expressed, indicating that the labeling is specific. None of the other heat shock proteins tested (Hsp27, Hsp40, and Hsc70) showed this localization pattern to centrosomes after heat shock in thermotolerant cells (our unpublished data), indicating that reallocation of Hsp70 to centrosomes upon heat shock could be sufficient to protect against heat-induced mitotic division errors.
Hsp70 Is Sufficient to Protect against Heat-induced Division Errors
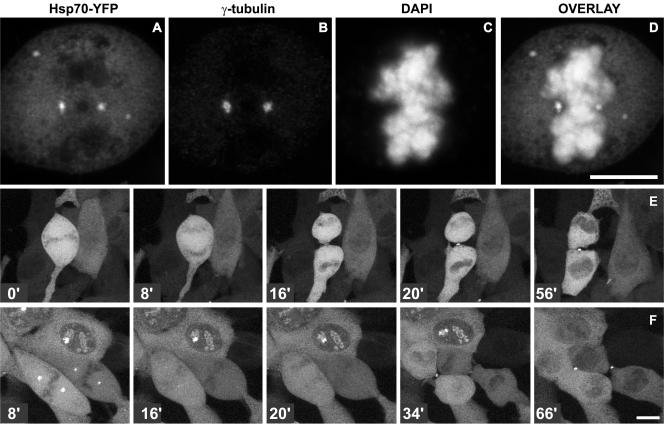
To gain insight in the putative protective role of Hsp70 against mitotic errors after heat shock, cells were transiently transfected with Hsp70-EYFP. First, we confirmed that in these cells, Hsp70-EYFP colocalized to centrosomes after heat shock by staining centrosomes with γ-tubulin antibodies (Figure 5, A–D). Next, we studied the dynamics of Hsp70 using time-lapse analysis (Figure 5, E and F; http://coo.med.rug.nl/sscb/video.htm, Figures 4 and 5). In unheated Hsp70-EYFP–expressing cells, Hsp70-EYFP was diffusely distributed in mitotic cells, and Hsp70-EYFP–expressing cells progressed through mitosis normally (Figure 5E and Table 1; http://coo.med.rug.nl/sscb/video.htm, Figure 4). In heated Hsp70-EYFP–expressing cells, Hsp70-EYFP was present at mitotic centrosomes immediately after heating and disappeared just before the onset of anaphase (Figure 5F, 16 min) after which the cells went through mitosis normally (Figure 5F, 20–66 min). Note that Hsp70-EYFP also decorated centrosomes in interphase cells, and this localization was more prolonged than Hsp70-EYFP localization to mitotic centrosomes (Figure 5F, 8–34 min).
Figure 5.
Hsp70 localizes transiently to centrosomes of heat shocked mitotic cells. Cells expressing Hsp70-EYFP were left untreated (E) or treated with heat shock (15′ 42°C; A–D, F). Immediately after heat shock, cells were fixed with methanol/acetone (A–D) and labeled with monoclonal anti-γ-tubulin (B). DNA was stained with DAPI (C). Hsp70-EYFP (A) colocalizes with γ-tubulin in heat-shocked mitotic cells (D). Mitotic cells expressing Hsp70-EYFP were selected for time-lapse analysis under control conditions (E) or after heat shock (F). Time is given in minutes after heat shock. Note that Hsp70-EYFP only localizes to centrosomes of heat-shocked cells (F, 8′) and disappears from centrosomes just before anaphase onset in mitotic cells (F, 16′) and later in interphase cells (F, 66′). Bar, 10 μm. For movies of the time-lapse data in E and F, see http://coo.med.rug.nl/sscb/video.htm, Figures 4 and 5.
These time-lapse data suggested that, like in thermotolerant cells, expression of Hsp70 might protect against heat-induced mitotic abnormalities. Quantitative analysis indeed confirmed that expression of Hsp70-EYFP resulted in a decrease of mitotic abnormalities (Figure 6A and Table 1) and that, at least after mild heating at 42°C, no mitotic delay was observed (Figure 6B and Table 1). Interestingly, irrespective of the heat treatment used, in >75% of the observed normal mitoses after heat shock, loss of Hsp70-EYFP localization at centrosomes preceded or coincided with the onset of anaphase (Figure 5F).
Figure 6.
Hsp70 protects against heat-induced mitotic abnormalities and reduces heat-induced delay in mitotic progression. Mitotic progression of cells expressing H2B-GFP (control) or Hsp70-EYFP (Hsp70-EYFP) was recorded by time-lapse confocal microscopy. The percentage of normal mitoses (A) and the time in minutes from early metaphase to anaphase onset (B) are given in control cells (no HS), after 15′ heat shock at 42°C (15′ 42°C), and after 15′ heat shock at 43°C (15′ 43°C). For each condition, >20 mitoses were recorded. Data represent mean ± SD.
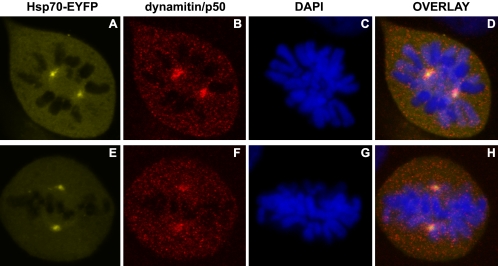
Because dynamitin/p50 localization at kinetochores and at centrosomes was not detectable in mitotic cells as a result of mild heat shock (Figure 3, O and P), we investigated whether Hsp70-EYFP expression would restore the localization pattern of dynamitin/p50 after heat shock. In Hsp70-EYFP–expressing cells, centrosomal localization of dynamitin/p50 was indeed restored (compare Figures 7, B and F, and 3O), but a pronounced kinetochore labeling as observed under control conditions (Figure 3M) was not observed in mitotic cells regardless of whether the DNA was congressed (Figure 7, E–H) or not congressed (Figure 7, A–D) at the metaphase plate. Our data strongly suggest that Hsp70 plays a role in the repair of centrosome damage induced by heat shock and thereby facilitates normal progression through mitosis.
Figure 7.
Hsp70-expressing cells show dynamitin/p50 antibody labeling at centrosomes but not at kinetochores after mild heat shock. Cells expressing Hsp70-EYFP (A and E) were mildly heat shocked (15′ 42°C). Cells were fixed and labeled with antibodies to dynamitin/p50 (B and F), and DNA was stained with DAPI (C and G). Overlays of the images are shown in D and H.
DISCUSSION
In this study, we show that severe heat treatment during mitosis induces loss of γ-tubulin and pericentrin from centrosomes and loss of dynamitin/p50 antibody labeling from centrosomes and kinetochores. After mild heat shock, only dynamitin/p50 antibody labeling disappeared, and none of the other protein alterations found after severe heat shock were detected. Severe and mild heat shock caused delays in normal mitotic progression and resulted in a variety of mitotic abnormalities. Hsp70 expression is sufficient to protect against most heat-induced division errors because in Hsp70-expressing cells, the percentage of division errors is decreased compared with non-Hsp70–expressing cells after heat shock. After heat shock, Hsp70 is transiently recruited to centrosomes, and in most cells, normal mitotic progression occurs immediately after exit of Hsp70 from the centrosomes. Expression of Hsp70 and its protection against division errors coincide with restored staining of the dynamitin/p50 antibody at centrosomes but not at kinetochores, suggesting that dynamitin/p50 localization at centrosomes improves the rate of normal mitoses and dynamitin/p50 localization at kinetochores is not essential for mitotic progression. Our findings imply that centrosomes are affected by heat shock and that the observed division errors are consequences of heat-damaged mitotic centrosomes.
What Are the Cellular Responses to Heat Shock?
The main molecular event after heat shock is denaturation and subsequent aggregation of proteins. Expression of heat shock proteins can protect the cells from these effects (Fischer et al., 2002). The recruitment of Hsp70 after heat shock to centrosomes, but not to microtubules or kinetochores, suggests that such heat-induced protein damage mainly affects centrosomes and no other mitotic structures. Indeed, ultrastructural analysis of interphase centrosomes after severe heat shock revealed the presence of high electron-dense material indicative of protein aggregates (Barrau et al., 1978; Vidair et al., 1996). Also, in the studies by the group of Watanabe (Nakahata et al., 2002), mitotic abnormalities were linked to abnormalities in centrosome structure. Our findings that overexpression of Hsp70 restores dynamitin/p50 antibody labeling at centrosomes and protects against most heat-induced division errors support the view that Hsp70 repairs protein damage at the centrosomes, most likely via its well-known chaperone activity (Lindquist, 1986; Kampinga, 1993).
Why Are Centrosomes Sensitive to Heat Shock?
Because our results indicate that damage to centrosomes is crucial for the heat-induced mitotic defects, the question arises why centrosomes are so extremely sensitive to heat shock. First, it is possible that centrosomal proteins are labile and readily unfold during heat shock and can rapidly form aggregates with other proteins, soluble or insoluble. The decreased staining of several centrosome-specific antibodies after heat shock (Figure 3, C, F, O, and P) (Malawista et al., 1983; Vidair et al., 1995, 1996; Brown et al., 1996b) may be explained by the presence of these aggregates at centrosomes. A second explanation could be that centrosomes are specific sites for storage of heat-induced misfolded proteins (Johnston et al., 1998). Indeed, misfolded proteins were shown to accumulate at the centrosomes in interphase cells (Vidair et al., 1996). These so-called aggresomes (Johnston et al., 1998) may enable cells to dispose damaged proteins at specific cellular sites, thereby preventing random protein aggregation and cellular dysfunction.
What Is Causing the Mitotic Delay in Heat-shocked Cells?
Our findings suggest that cells contain a mechanism that senses heat damage at centrosomes and induces mitotic delay. We have shown that Hsp70 is recruited to centrosomes in response to heat shock and that progression through mitosis hardly ever occurs as long as Hsp70 is present at the centrosomes. Mitotic progression occurs in most cases immediately after the disappearance of Hsp70 from centrosomes. Severe heat shock induces a longer mitotic delay compared with mild heat shock, implying that the more heat-induced damage to centrosomes the longer the mitotic delay. Directly after heat shock, Hsp70 moves to the centrosomes most likely to repair protein damage and to restore centrosome function. Restoration of centrosome function or disappearance of Hsp70 from centrosomes may trigger the progression through anaphase. However the mitotic delay also is induced in the absence of Hsp70; this indicates that Hsp70 is not required for the delay and additional factors may regulate heat-induced mitotic delay.
Previously, it has been reported that hypothermia induces mitotic delay, and this delay is Mad2 dependent (Shannon et al., 2002). It remains to be investigated whether the observed heat shock-induced mitotic delay is also Mad2 dependent.
What Explains the Formation of Multipolar Spindles and Division Errors in Heat-shocked Mitotic Cells?
We show that heat shock during mitosis not only causes mitotic delays but also can result in the formation of multipolar spindles. There is an extensive list of publications in which depletion or mutations in proteins from the centrosome, kinetochore, or microtubule-associated proteins induce the formation of multipolar spindles (Garrett et al., 2002; Gruber et al., 2002; Bharadwaj et al., 2004; Cassimeris and Morabito, 2004; Holmfeldt et al., 2005). Therefore, it can be speculated that multipolar spindles arise during heat shock due to heat inactivation of one of these proteins. Hsp70 expression restored localization of dynamitin/p50 at centrosomes but not at kinetochores, and this restored localization at centrosomes coincides with protection against most heat-induced mitotic division errors. These data imply that centrosomal dynamitin/p50, but not kinetochore dynamitin/p50, is involved in improving the fidelity of mitoses. After mild heat shock, 17% of the mitoses in Hsp70-expressing cells results in the formation of micronuclei. It may be possible that this missegregation of DNA is due to incorrect localization of dynamitin/p50 (and perhaps other proteins) at kinetochores, because previously it has been demonstrated that other kinetochore proteins (such as CENP-E, ZW10, and Rod) are required for accurate chromosome segregation (Starr et al., 1998; Scaerou et al., 1999; Tanudji et al., 2004).
Another abnormality observed in mitotic heat-shocked cells is decondensation of chromatin without a preceding division of chromosomes. This premature mitotic exit also was observed in Xenopus tissue culture cells injected with function-blocking antibodies against two proteins (xNdc80 and xNuf2) that localize to kinetochores from prometaphase through anaphase (McCleland et al., 2003). It may be possible that the heat shock conditions used in our experiments inactivate Ndc80, Nuf2, or proteins with a related function, thereby causing premature mitotic exit.
The formation of multipolar spindles and micronuclei indicate that although heat shock itself does not cause DNA damage and thereby is not considered to be a genotioxic stress, genome instability may arise as a secondary consequence of heat-induced centrosomal protein damage (Nakahata et al., 2002; this study). It is likely that most daughter cells that arise after these heat-induced division errors will not be viable and will be eliminated. However, it may be possible that aneuploid cells sporadically are able to divide, survive, and finally give rise to cells with a transformed phenotype. Previously, an increased tumor incidence was observed after irradiation in combination with heat treatment compared with irradiation alone (Sminia et al., 1996). The effect of Hsp70 on viability of heat shock-induced aneuploid cells remains to be investigated.
In summary, our data show that heat shock affects mitotic cells predominantly at centrosomes and that this heat damage results in division errors. An Hsp70-dependent cellular defense system protects heat-damaged mitotic cells by facilitating restoration of centrosomes, and, after a mitotic delay, allowing normal progression through mitosis.
Acknowledgments
We thank Willy Lemstra and Rita Setroikromo for excellent technical assistance. This work was supported by the Netherlands Organization for Scientific Research Grant NWO (901-01-221).
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-01-0038) on June 1, 2005.
References
- Agueli, C., Geraci, F., Giudice, G., Chimenti, L., Cascino, D., and Sconzo, G. (2001). A constitutive 70 kDa heat-shock protein is localized on the fibres of spindles and asters at metaphase in an ATP-dependent manner: a new chaperone role is proposed. Biochem. J. 360, 413-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrau, M. D., Blackburn, G. R., and Dewey, W. C. (1978). Effects of heat on the centrosomes of Chinese hamster ovary (CHO) cells. Cancer Res. 38, 2290-2294. [PubMed] [Google Scholar]
- Bharadwaj, R., Qi, W., and Yu, H. (2004). Identification of two novel components of the human NDC80 kinetochore complex. J. Biol. Chem. 279, 13076-13085. [DOI] [PubMed] [Google Scholar]
- Borrelli, M. J., Stafford, D. M., Karczewski, L. A., Rausch, C. M., Lee, Y. J., and Corry, P. M. (1996). Thermotolerance expression in mitotic CHO cells without increased translation of heat shock proteins. J. Cell Physiol. 169, 420-428. [DOI] [PubMed] [Google Scholar]
- Brown, C. R., Doxsey, S. J., Hong-Brown, L. Q., Martin, R. L., and Welch, W. J. (1996a). Molecular chaperones and the centrosome. A role for TCP-1 in microtubule nucleation. J. Biol. Chem. 271, 824-832. [DOI] [PubMed] [Google Scholar]
- Brown, C. R., Hong-Brown, L. Q., Doxsey, S. J., and Welch, W. J. (1996b). Molecular chaperones and the centrosome. A role for HSP 73 in centrosomal repair following heat shock treatment. J. Biol. Chem. 271, 833-840. [DOI] [PubMed] [Google Scholar]
- Cassimeris, L., and Morabito, J. (2004). TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly. Mol. Biol. Cell 15, 1580-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss, R. A., Dewey, W. C., and Bamburg, J. R. (1982). Effects of hyperthermia on dividing CHO cells and on microtubules in vitro. Cancer Res. 42, 1059-1071. [PubMed] [Google Scholar]
- Debec, A., and Marcaillou, C. (1997). Structural alterations of the mitotic apparatus induced by the heat shock response in Drosophila cells. Biol. Cell 89, 67-78. [DOI] [PubMed] [Google Scholar]
- DeLuca, J. G., and Salmon, E. D. (2004). Kinetochores: if you build it, they will come. Curr. Biol. 14, R921-R923. [DOI] [PubMed] [Google Scholar]
- Echeverri, C. J., Paschal, B. M., Vaughan, K. T., and Vallee, R. B. (1996). Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132, 617-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, D., Matten, J., Reimann, J., Bonnemann, C., and Schroder, R. (2002). Expression, localization and functional divergence of alphaB-crystallin and heat shock protein 27 in core myopathies and neurogenic atrophy. Acta Neuropathol. 104, 297-304. [DOI] [PubMed] [Google Scholar]
- Garrett, S., Auer, K., Compton, D. A., and Kapoor, T. M. (2002). hTPX2 is required for normal spindle morphology and centrosome integrity during vertebrate cell division. Curr. Biol. 12, 2055-2059. [DOI] [PubMed] [Google Scholar]
- Gromley, A., Jurczyk, A., Sillibourne, J., Halilovic, E., Mogensen, M., Groisman, I., Blomberg, M., and Doxsey, S. (2003). A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J. Cell Biol. 161, 535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber, J., Harborth, J., Schnabel, J., Weber, K., and Hatzfeld, M. (2002). The mitotic-spindle-associated protein astrin is essential for progression through mitosis. J. Cell Sci. 115, 4053-4059. [DOI] [PubMed] [Google Scholar]
- Hauf, S., and Watanabe, Y. (2004). Kinetochore orientation in mitosis and meiosis. Cell 119, 317-327. [DOI] [PubMed] [Google Scholar]
- Hinchcliffe, E. H., Miller, F. J., Cham, M., Khodjakov, A., and Sluder, G. (2001). requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 291, 1547-1550. [DOI] [PubMed] [Google Scholar]
- Holmfeldt, P., Zhang, X., Stenmark, S., Walczak, C. E., and Gullberg, M. (2005). CaMKIIgamma-mediated inactivation of the Kin I kinesin MCAK is essential for bipolar spindle formation. EMBO J. 24, 1256-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut, H.M.J., Lemstra, W., Blaauw, E. H., Van Cappellen, G. W., Kampinga, H. H., and Sibon, O.C.M. (2003). Centrosomes split in the presence of impaired DNA integrity during mitosis. Mol. Biol. Cell 14, 1993-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, J. A., Ward, C. L., and Kopito, R. R. (1998). Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 143, 1883-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga, H. H. (1993). Thermotolerance in mammalian cells. Protein denaturation and aggregation, and stress proteins. J. Cell Sci. 104, 11-17. [DOI] [PubMed] [Google Scholar]
- Khodjakov, A., and Rieder, C. L. (2001). Centrosomes enhance the fidelity of cytokinesis in vertebrates and are required for cell cycle progression. J. Cell Biol. 153, 237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox, J. D., Mitchel, R. E., and Brown, D. L. (1991). Effects of hyperthermia on microtubule organization and cytolytic activity of murine cytotoxic T lymphocytes. Exp. Cell Res. 194, 275-283. [DOI] [PubMed] [Google Scholar]
- Lange, B. M., Bachi, A., Wilm, M., and Gonzalez, C. (2000). Hsp90 is a core centrosomal component and is required at different stages of the centrosome cycle in Drosophila and vertebrates. EMBO J. 19, 1252-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. C., and Werb, Z. (1982). Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc. Natl. Acad. Sci. USA 79, 3218-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist, S. (1986). The heat-shock response. Annu. Rev. Biochem. 55, 1151-1191. [DOI] [PubMed] [Google Scholar]
- Malawista, S. E., Boisfleury-Chevance, A., Maunoury, R., and Bessis, M. (1983). Heat as a probe of centrosomal function: a phase-contrast and immunofluorescent study of human blood monocytes. Blood Cells 9, 443-453. [PubMed] [Google Scholar]
- Maney, T., Ginkel, L. M., Hunter, A. W., and Wordeman, L. (2000). The kinetochore of higher eucaryotes: a molecular view. Int. Rev. Cytol. 194, 67-131. [DOI] [PubMed] [Google Scholar]
- McCleland, M. L., Gardner, R. D., Kallio, M. J., Daum, J. R., Gorbsky, G. J., Burke, D. J., Stukenberg, P. T. (2003). The highly conserved Ndc80 complex is required for kinetochore assembly, chromosome congression, and spindle checkpoint activity. Genes Dev. 17, 101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto, R. I. (1991). Heat shock: the role of transient inducible responses in cell damage, transformation, and differentiation. Cancer Cells 3, 295-301. [PubMed] [Google Scholar]
- Nakahata, K., Miyakoda, M., Suzuki, K., Kodama, S., and Watanabe, M. (2002). Heat shock induces centrosomal dysfunction, and causes non-apoptotic mitotic catastrophe in human tumour cells. Int. J. Hyperthermia 18, 332-343. [DOI] [PubMed] [Google Scholar]
- Nollen, E. A., Brunsting, J. F., Roelofsen, H., Weber, L. A., and Kampinga, H. H. (1999). In vivo chaperone activity of heat shock protein 70 and thermotolerance. Mol. Cell Biol. 19, 2069-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret, E., Moudjou, M., Geraud, M. L., Derancourt, J., Soyer-Gobillard, M. O., and Bornens, M. (1995). Identification of an HSP70-related protein associated with the centrosome from dinoflagellates to human cells. J. Cell Sci. 108, 711-725. [DOI] [PubMed] [Google Scholar]
- Piel, M., Nordberg, J., Eutaneuer, U., and Bornens, M. (2001). centrosome-dependent exit of cytokinesis in animal cells. Science 291, 1550-1553. [DOI] [PubMed] [Google Scholar]
- Rattner, J. B. (1991). hsp70 is localized to the centrosome of dividing HeLa cells. Exp. Cell Res. 195, 110-113. [DOI] [PubMed] [Google Scholar]
- Rieder, C. L., and Salmon, E. D. (1998). The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 8, 310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaerou, F., Aguilera, I., Saunders, R., Kane, N., Blottiere, L., and Karess, R. (1999). The rough deal protein is a new kinetochore component required for accurate chromosome segregation in Drosophila. J. Cell Sci. 112, 3757-3768. [DOI] [PubMed] [Google Scholar]
- Shah, J. V., Botvinick, E., Bonday, Z., Furnari, F., Berns, M., and Cleveland, D. W. (2004). Dynamics of centromere and kinetochore proteins; implications for checkpoint signaling and silencing. Curr. Biol. 14, 942-952. [DOI] [PubMed] [Google Scholar]
- Shannon, K. B., Canman, J. C., and Salmon, E. D. (2002). Mad2 and BubR1 function in a single checkpoint pathway that responds to a loss of tension. Mol. Biol. Cell 13, 3706-3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibon, O.C.M., Kelkar, A., Lemstra, W., and Theurkauf, W. E. (2000). DNA-replication/DNA-damage-dependent centrosome inactivation in Drosophila embryos. Nat. Cell Biol. 2, 90-95. [DOI] [PubMed] [Google Scholar]
- Sibon, O.C.M., and Theurkauf, W. E. (2004). Centrosomal regulation in response to environmental and genotoxic stress. In: Centrosomes in Development and Disease, ed. E. A. Nigg, Weinheim, Austria: Wiley-VCH, 211-224.
- Sminia, P., van der Kracht, A. H., Frederiks, W. M., and Jansen, W. (1996). Hyperthermia, radiation carcinogenesis and the protective potential of vitamin A and N-acetylcysteine. J. Cancer Res. Clin. Oncol. 122, 343-350. [DOI] [PubMed] [Google Scholar]
- Starr, D. A., Williams, B. C., Hays, T. S., and Goldberg, M. L. (1998). ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 142, 763-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada, S., Kelkar, A., and Theurkauf, W. E. (2003). Drosophila checkpoint kinase 2 couples centrosome function and spindle assembly to genomic integrity. Cell 113, 87-99. [DOI] [PubMed] [Google Scholar]
- Tanudji, M., Shoemaker, J., L'Italien, L., Russell, L., Chin, G., and Schebye, X. M. (2004). Gene silencing of CENP-E by small interfering RNA in HeLa cells leads to missegregation of chromosomes after a mitotic delay. Mol. Biol. Cell 15, 3771-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia, M. M., and Brinkley, B. R. (1985). Fractionation and initial characterization of the kinetochore from mammalian metaphase chromosomes. J. Cell Biol. 101, 1124-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidair, C. A., Doxsey, S. J., and Dewey, W. C. (1993). Heat shock alters centrosome organization leading to mitotic dysfunction and cell death. J. Cell Physiol. 154, 443-455. [DOI] [PubMed] [Google Scholar]
- Vidair, C. A., Doxsey, S. J., and Dewey, W. C. (1995). Thermotolerant cells possess an enhanced capacity to repair heat-induced alterations to centrosome structure and function. J. Cell Physiol. 163, 194-203. [DOI] [PubMed] [Google Scholar]
- Vidair, C. A., Huang, R. N., and Doxsey, S. J. (1996). Heat shock causes protein aggregation and reduced protein solubility at the centrosome and other cytoplasmic locations. Int. J. Hyperthermia 12, 681-695. [DOI] [PubMed] [Google Scholar]