Abstract
Myosin II-dependent contraction of the contractile ring drives equatorial furrowing during cytokinesis in animal cells. Nonetheless, myosin II-null cells of the cellular slime mold Dictyostelium divide efficiently when adhering to substrates by making use of polar traction forces. Here, we show that in the presence of 30 μM blebbistatin, a potent myosin II inhibitor, normal rat kidney (NRK) cells adhering to fibronectin-coated surfaces formed equatorial furrows and divided in a manner strikingly similar to myosin II-null Dictyostelium cells. Such blebbistatin-resistant cytokinesis was absent in partially detached NRK cells and was disrupted in adherent cells if the advance of their polar lamellipodia was disturbed by neighboring cells. Y-27632 (40 μM), which inhibits Rho-kinase, was similar to 30 μM blebbistatin in that it inhibited cytokinesis of partially detached NRK cells but only prolonged furrow ingression in attached cells. In the presence of 100 μM blebbistatin, most NRK cells that initiated anaphase formed tight furrows, although scission never occurred. Adherent HT1080 fibrosarcoma cells also formed equatorial furrows efficiently in the presence of 100 μM blebbistatin. These results provide direct evidence for adhesion-dependent, contractile ring-independent equatorial furrowing in mammalian cells and demonstrate the importance of substrate adhesion for cytokinesis.
INTRODUCTION
After the onset of anaphase, animal cells assemble a contractile ring comprised of actin and myosin II filaments in their equatorial region, after which active sliding between the two filament systems contributes to the constriction of a furrow that eventually yields two daughter cells. It has been thought that this “purse-string” mechanism is the conserved method by which cells ranging from yeast and slime molds to mammalian cells divide (Fishkind and Wang, 1995; Glotzer, 1997). When cultured on substrates, however, myosin II-null cells of the cellular slime mold Dictyostelium discoideum are able to divide efficiently in a manner clearly different from wild-type cells (Neujahr et al., 1997; Zang et al., 1997). Subsequent molecular genetic analyses revealed that Dictyostelium makes use of two parallel pathways leading to cell cycle-coupled division, one that involves the myosin II-dependent purse-string mechanism and another that depends on substrate adhesion and is independent of active contraction of the contractile ring (Nagasaki et al., 2002). The physical mechanism of the latter remains controversial (Gerisch and Weber, 2000; Robinson et al., 2002), but there is strong evidence to suggest that it is driven by traction forces that are generated around both poles and that move the two daughter cells away from one another (Nagasaki et al., 2002; Uyeda and Nagasaki, 2004). Consistent with this view, a recent modeling by Zhang and Robinson (2005) demonstrated that in elongated cells, such as adherent mitotic cells, a Laplace pressure resulting from material properties and the geometry of the cell generates force to help furrow ingression.
Mammalian cells also exhibit a number of behaviors that are difficult to explain on the basis of the purse-string mechanism (reviewed by Uyeda and Nagasaki, 2004). For example, when cytochalasin D, which caps the plus ends of actin filaments and inhibits their polymerization, is applied locally to the equatorial region of mitotic normal rat kidney (NRK) cells, the cleavage process is accelerated rather than inhibited, whereas local application of the drug to the polar regions inhibits the furrowing process (O'Connell et al., 2001). Those phenomena can be interpreted as suggesting that highly adherent mammalian cells are also capable of dividing in an adhesion-dependent, contractile ring-independent manner. Furthermore, it has been shown that myosin II is not essential for cytokinesis and viability in certain strains of the budding yeast Saccharomyces cerevisiae (Watts et al., 1987; Bi et al., 1998).
These and other (Young et al., 1993; O'Connell et al., 1999; Takeda et al., 2003) observations paint a complex picture in which cytokinesis in animal and fungal cells cannot be entirely explained by the simple purse-string mechanism. Recently, blebbistatin, a potent and specific inhibitor of nonmuscle myosin II ATPase activity, was identified and shown to inhibit cytokinesis in HeLa cells and cultured Xenopus tissue cells (Straight et al., 2003). This finding was interpreted as supportive of the essential role of the contractile ring in cytokinesis. But because myosin II also may be involved in postmitotic spreading (Cramer and Mitchison, 1995), spindle formation (Rosenblatt et al., 2004), and maintenance of cell polarity during interphase (Pierini et al., 2003; Straight et al., 2003), it seems premature to conclude that blebbistatin disrupts cytokinesis by inhibiting contraction of the contractile ring. Moreover, cells other than HeLa or Xenopus tissue cells might respond differently to blebbistatin treatments.
Thus, to reexamine the importance of the purse-string mechanism in cytokinesis of animal cells, we analyzed the effects of blebbistatin on cytokinesis of NRK cells, which were previously shown to exhibit behaviors that are difficult to explain in the context of the purse-string mechanism (O'Connell et al., 1999, 2001). The results of this and related experiments that we report here establish experimentally that certain mammalian cells are able to form tight equatorial furrows in a traction force-dependent and contractile ring-independent manner, under appropriate adhesion conditions.
MATERIALS AND METHODS
Culture and Observation of Cells
NRK cells (NRK-52E; Health Science Research Resources Bank, Tokyo, Japan; cell no. IF050480) were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). HT1080 cells (Rasheed et al., 1974) were cultured in RPMI 1640 medium supplemented with 10% FBS. To facilitate cell adhesion, untreated polystyrene dishes (#1000-035X; Asahi Techno Glass, Tokyo, Japan) were coated with fibronectin (Yagai Research Center, Yamagata, Japan) at 50 μg/ml or 0.01% collagen type I solution (Wako Pure Chemicals, Osaka, Japan) overnight. Trypsinized NRK cells were allowed to attach to the fibronectin matrix for 3-5 h before addition of blebbistatin or Y-27632. To assess cytokinesis in partially detached NRK cells, mitotic cells were obtained by treatment for 4 h with nocodazole (40 ng/ml), followed by washoff and transfer to Easy Grip tissue culture dishes (#353001; BD Biosciences Discovery Labware, Bedford, MA) containing medium with or without blebbistatin or Y-27632. The S-(-)-enantiomer of blebbistatin was purchased from Toronto Research Chemicals (Toronto, Ontario, Canada) and dissolved at 10 mM in dimethyl sulfoxide (DMSO). Y-27632 (Merck, Tokyo, Japan) was dissolved at 5 mM in water. Cells were maintained at constant temperature in a stage incubator (Onpu-4; Taiei Denki, Kasama, Japan) attached to an inverted microscope (IX50; Olympus, Tokyo, Japan) and observed using a 20× objective with phase-contrast optics. Images were captured with a charge-coupled device camera (C5985; Hamamatsu Photonics, Hamamatsu, Japan) and a time-lapse recording system (ARGAS-20; Hamamatsu Photonics). Using accumulation times of 1-2 s enabled us to reduce the intensity of illuminating light to minimize the phototoxic effects (Kolega, 2004).
Immunofluorescence
One hour after addition of blebbistatin (100 μM), cells on fibronectin-coated glass-bottom dishes (MatTek, Ashland, MA) were fixed for 15 min in 80 mM PIPES, pH 7.0, 1 mM MgCl2, 10 mM EGTA, 0.2% Triton X-100, and 4% formaldehyde and then blocked in phosphate-buffered saline containing 0.1% Triton X-100 and 2% bovine serum albumin. Cells were stained with anti-α-tubulin (clone DM 1A; Sigma, St. Louis, MO; 1:1000 dilution) or anti-myosin IIA (M8064; Sigma; 1:1000 dilution) antibody, followed by a mixture of Alexa 488-conjugated anti-mouse-IgG or anti-rabbit-IgG antibody (Invitrogen, Carlsbad, CA), 3.3 nM rhodamine-phalloidin (Sigma), and 1 μg/ml Hoechst 33258 (Wako Pure Chemicals). Immunostained cells were observed using an inverted microscope (IX-70; Olympus) equipped with a confocal laser scanning unit (CSU 10; Yokogawa, Tokyo, Japan).
RESULTS
Effects of Blebbistatin on Cytokinesis in NRK Cells Adhering to Fibronectin-coated Surfaces
To evaluate the consequences of inhibiting myosin II-dependent contractile ring activity in NRK cells, we first examined the effects of 30 μM blebbistatin, which is sufficient to reduce myosin II ATPase activity by >90% in vitro (Straight et al., 2003; Kovacs et al., 2004). Within 5 min after addition of the drug, interphase cells lost their single leading lamellipodia and distinct polarity and instead exhibited repeated uncoordinated expansion and contraction of the cell periphery (our unpublished data).
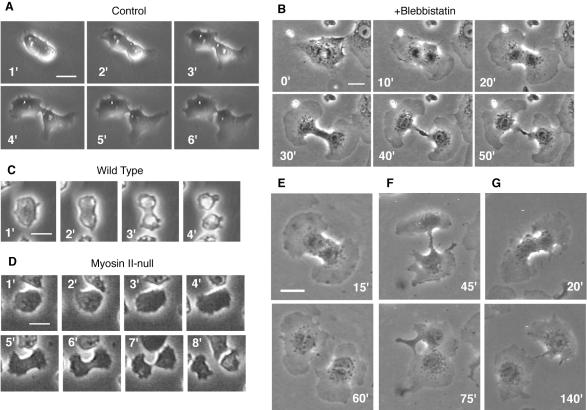
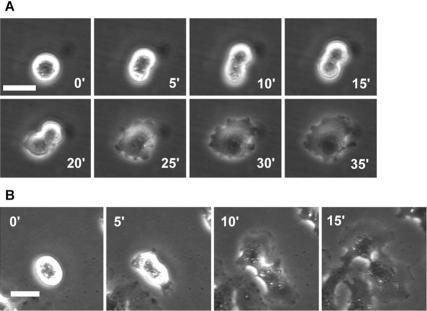
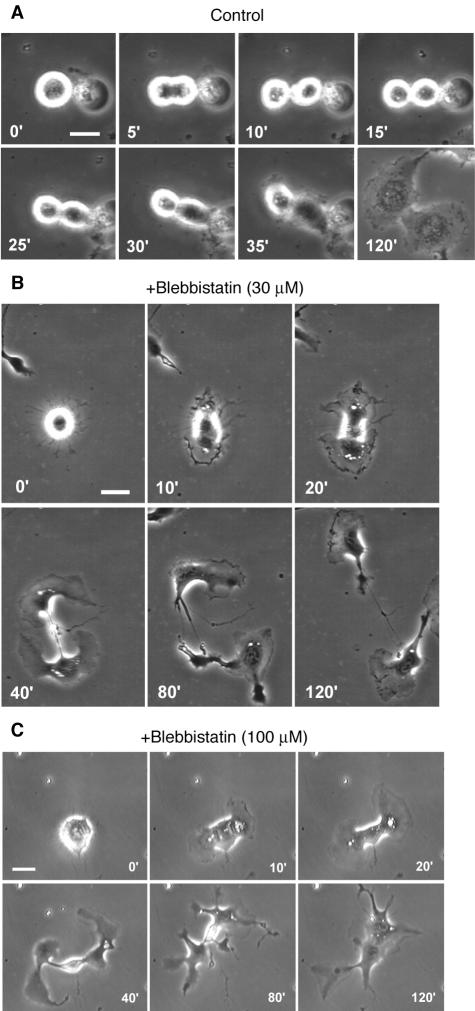
On entry into mitosis, control NRK cells in sparse cultures treated with 0.3% DMSO alone withdrew their lamellipodia and became thicker and less adherent to the substrate, as suggested by these cells being easily dislodged by shaking the culture dishes. These cells then formed cleavage furrows in their equatorial regions, respread, and divided into two daughter cells via a process that was typically completed within 10 min after the onset of anaphase (Figure 1A, Table 1, and Supplemental Movie 1). In contrast, in the presence of 30 μM blebbistatin plus 0.3% DMSO, mitotic NRK cells on fibronectin-coated substrates remained adherent, but they initiated chromosome segregation. Subsequently, the cells respread and formed fan-shaped lamellipodia around both poles. Accompanying the radial advance of the lamellipodia was a furrowing of the equatorial region that progressed over a period of ∼60 min (Figure 1B, Table 1, and Supplemental Movie 2). This sequence of morphological changes is very different from that seen in the absence of blebbistatin but is strikingly similar to the division of myosin II-null Dictyostelium cells on substrates (Figure 1D) (Neujahr et al., 1997; Zang et al., 1997; Nagasaki et al., 2002).
Figure 1.
Cytokinesis in NRK and Dictyostelium cells and the effects of blebbistatin. (A and B) Cytokinesis in sparsely cultured NRK cells on fibronectin-coated substrates in the presence of 0.3% DMSO (A) or 30 μM blebbistatin and 0.3% DMSO (B). (C and D) Cytokinesis in wild-type (C) and myosin II-null (D) Dictyostelium cells (data modified from Nagasaki et al., 2002). (E-G) Three representative patterns of cytokinesis in NRK cells cultured as in B. See text for details. Numbers indicate time in minutes after onset of anaphase; bars, 20 μm (A, B, E-G) or 10 μm (C and D).
Table 1.
Effects of blebbistatin on three steps of cytokinesis of adherent NRK and HT1080 cells
| Cell | Cell density | No. of cells examined | Blebbistatin (μM) | Karyokinesis (%) | Furrow formationa (%) | Scissionb (%) | Regressionb (%) | Bridgedb (%) |
|---|---|---|---|---|---|---|---|---|
| NRK | Sparse | 9 | 0 | ND | 100 | 100 | 0 | 0 |
| NRK | Sparse | 16 | 30 | 100 | 100 | 44 | 19 | 38 |
| NRK | Sparse | 15 | 100 | 59c | 80 | 0 | 7 | 73 |
| HT1080 | Sparse | 7 | 0 | ND | 100 | 100 | 0 | 0 |
| HT1080 | Sparse | 11 | 30 | 100 | 91 | 9 | 18 | 64 |
| HT1080 | Sparse | 11 | 100 | ND | 64 | 0 | 45 | 18 |
| NRK | Subconfluent | 15 | 0 | ND | 100 | 100 | 0 | 0 |
| NRK | Subconfluent | 10 | 30 | ND | 0 |
ND, not determined.
Frequencies of furrow formation among cells that completed normal karyokinesis
Fractions of cells among those that formed tight furrows that completed successful scission, regressed to form binucleate cells, or remained connected by cytoplasmic bridges
This datum was obtained in an experiment involving 22 cells. The other data in this line are from a separate experiment involving 15 cells
Interestingly, only seven of the 16 cells that formed tight furrows in the presence of 30 μM blebbistatin completed the division to yield separate daughter cells (Figure 1E); three started to form furrows, but one of the polar lamellipodia stopped migrating, after which the furrow slowly regressed, resulting in binucleate cells (Figure 1F). The oppositely oriented locomotion of daughter cells away from one another usually stopped within 2 h, and in the remaining six cells, thin cytoplasmic bridges connecting the daughter cells persisted during the 2-h observation period, even though cleavage seemed almost complete (Figure 1G). Two of those pairs subsequently fused back to form binucleate cells; the fate of the remaining four pairs was not determined.
Cytokinesis in Subconfluent NRK Cultures in the Presence of Blebbistatin
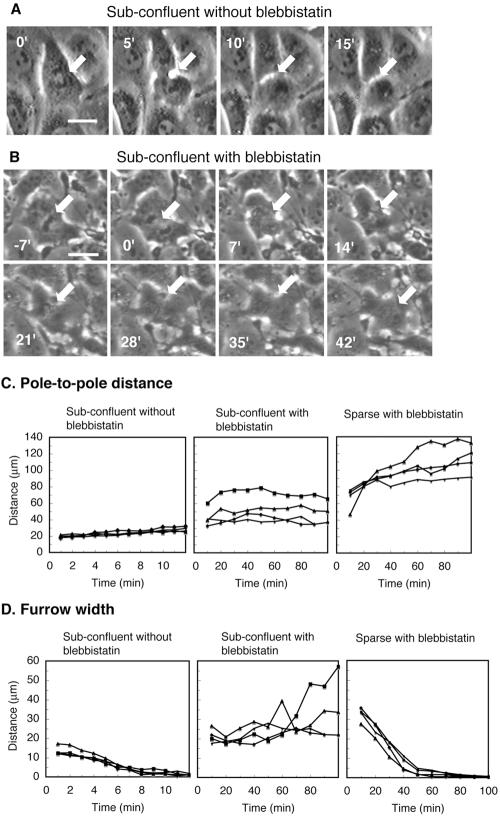
In subconfluent cultures treated with 30 μM blebbistatin, the polar lamellipodium of a mitotic cell typically encountered a neighboring cell before a tight equatorial furrow was formed, which stopped the advancement, and the cell failed to form a furrow or divide (Figure 2, B-D, Table 1, and Supplemental Movie 3). In contrast, all control cells divided successfully in subconfluent cultures, despite the lack of polar advancement (Figure 2, A, C, and D, and Table 1). This suggests that for cytokinesis to be carried out in the presence of blebbistatin, it is necessary that lamellipodia are formed around both poles and that they migrate away from one another while front-to-rear polarity is continuously maintained in both daughter cells. It further suggests that this mode of cytokinesis is dependent on polar advancement and expansion of the mitotic cell, rather than on active contraction of the contractile ring.
Figure 2.
Failed cytokinesis in subconfluent cultures of NRK cells on fibronectin-coated substrates in the presence of 30 μM blebbistatin. (A and B) Mitotic cells in the presence of 0.3% DMSO (A) or 30 μM blebbistatin and 0.3% DMSO (B). Arrows, the original position of the cell's equator; numbers, time in minutes after onset of anaphase; bars, 20 μm. (C and D) Changes in the distances between the poles of the daughter cells (C) and the widths of the furrows (D) in four individual mitotic cells in each of three groups: DMSO-treated cells in subconfluent cultures (left), blebbistatin-treated cells in subconfluent cultures (middle), and blebbistatin-treated cells in sparse cultures (right).
Cytokinesis in Partially Detached NRK Cells in the Presence of Blebbistatin
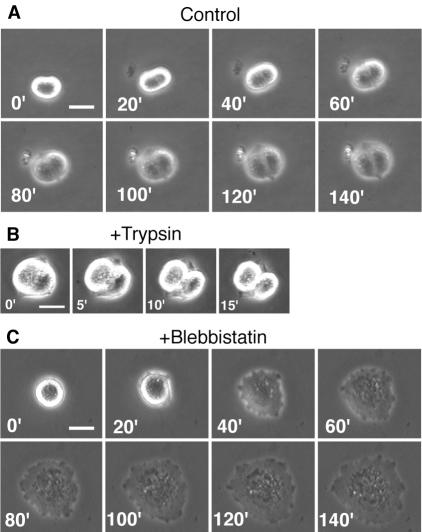
To rule out the possibility that cytokinesis in the presence of 30 μM blebbistatin was driven by residual myosin II activity, we sought conditions under which cytokinesis in NRK cells was clearly dependent on the activity of the contractile ring. For example, in Dictyostelium, cytokinesis in cells in suspension requires active contraction of the contractile ring (De Lozanne and Spudich, 1987; Knecht and Loomis, 1987; Manstein et al., 1989; Zang et al., 1997). However, consistent with previous reports on mammalian cells (Ben-Ze'ev and Raz, 1981; Winklbauer, 1986), we found that when mitotic NRK cells were transferred to agarose plates, to which they could not adhere, cytokinesis was completely blocked, even in the absence of blebbistatin (our unpublished data). We then transferred mitotic NRK cells to fresh Easy Grip tissue culture dishes containing medium and asked whether these cells could divide while they were still in the process of secreting extracellular matrix protein to establish adhesion. Time-lapse observation of individual cells demonstrated that successful cytokinesis under these conditions yields pairs of semicircular cells (7/14 cells; Figure 3A and Supplemental Movie 4), whereas failure yields single, round, binucleate cells (7/14 cells). Within 10 min of replacement of the culture medium with a trypsin-EDTA solution, all of the semicircular cells detached from the substrate to become two separated spherical cells (Figure 3B), demonstrating that these pairs of semicircular cells have indeed completed division. We thus used these morphological features to distinguish between successful and unsuccessful cytokinesis in 1000 cells (200 cells from five independent plates) surveyed 5-10 h after transfer to fresh dishes in the presence or absence of 30 μM blebbistatin. In the absence of blebbistatin, 60 ± 14% (average ± SD) of mitotic NRK cells divided successfully, but in the presence of blebbistatin, 96 ± 2% of cells failed to divide when in this partially detached condition. Time-lapse observation of 18 individual cells showed that no noticeable equatorial furrows or polar lamellipodia were formed in the presence of blebbistatin; those cells eventually respread as binucleate cells (Figure 3C and Supplemental Movie 5).
Figure 3.
Effects of blebbistatin on cytokinesis in partially detached NRK cells. Mitotic cells were harvested and replated on fresh Easy Grip tissue culture dishes. (A) Successful cytokinesis of a control cell. (B) Successful cytokinesis in A was confirmed by the formation of two spherical cells after trypsinization. (C) Failed cytokinesis in the presence of 30 μM blebbistatin. Numbers, time in minutes after onset of anaphase (A and C) or after addition of trypsin (B); bars, 20 μm.
Thus, 30 μM blebbistatin was clearly sufficient to inhibit contractile ring-dependent cytokinesis, which confirms that the successful cytokinesis on fibronectin-coated surfaces in the presence of 30 μM blebbistatin was mostly independent of contractile ring activity.
Effects of a High Concentration of Blebbistatin on Cytokinesis in NRK Cells
We also examined cytokinesis of NRK cells in the presence of 100 μM blebbistatin, which reduces myosin II ATPase by >95% in vitro (Straight et al., 2003; Kovacs et al., 2004). Rosenblatt et al. (2004) recently reported that myosin II is required for efficient karyokinesis in cells in which the nuclear envelope breaks down. In that regard, we also found in a preliminary experiment that the tighter inhibition of myosin II activity achieved with the higher dose of blebbistatin resulted in frequent (41%) failure of karyokinesis, halting the process before anaphase (Table 1). Therefore, to focus on the effects of 100 μM blebbistatin on the furrowing process, we analyzed in a separate experiment only those cells that initiated anaphase normally. Of 15 such cells, 12 formed lamellipodia around both poles and formed tight furrows in their equatorial regions (Table 1). In the remaining three cells, a lamellipodium was formed all along the cell after karyokinesis, but no noticeable furrows were formed. In five of the 12 cells that formed furrows, lamellipodia were not formed simultaneously around each pole. This caused the furrows to be misplaced, forming between a binucleate fragment and an anucleate fragment.
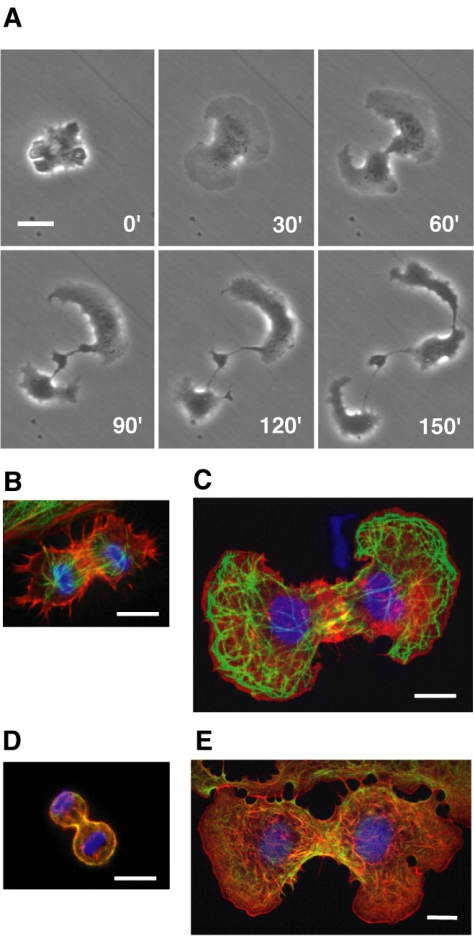
None of the cells that formed furrows in the presence of 100 μM blebbistatin ultimately divided to two separate cells within the 2-h observation period. The majority (11/12) retained the furrow (Table 1), but the daughter cells gradually lost their front-to-rear polarity, and their lamellipodial movements became fragmented and uncoordinated. This led to fragmentation of the daughter cells into several pieces connected by thin cytoplasmic strands (Figure 4A and Supplemental Movie 6).
Figure 4.
Failed cytokinesis in NRK cells on fibronectin-coated substrates in the presence of 100 μM blebbistatin. (A) Time-lapse microscopy of an abnormal division. Numbers indicate times in minutes after onset of anaphase. (B-E) Cytoskeletal organization of cells treated with 1% DMSO as a control (B and D) or 100 μM blebbistatin plus 1% DMSO (C and E) during cytokinesis. Cells were immunostained with anti-tubulin antibody (B and C) or anti-myosin-IIA antibody (D and E), followed by a mixture of Alexa 488-conjugated secondary antibodies (green), Hoechst 33258 for DNA (blue), and rhodamine-phalloidin for actin filaments (red). Bars, 20 μm (A) or 10 (B-E) μm.
Immunofluorescence microscopy revealed that high concentrations of actin and myosin II filaments were present in the equatorial regions of cells treated with 100 μM blebbistatin (Figure 4, C and E), which was similar to control cells treated with DMSO alone (Figure 4, B and D). These cells also showed extensive arrays of astral microtubules behind the lamellipodia, and in the later phases of the separation of the daughter cells, the nuclear envelopes reformed and the microtubule cytoskeletons became more like those of interphase cells (Figure 4C).
Effects of Y-27632 on Cytokinesis in NRK Cells
Y-27632 inhibits Rho kinase (ROCK) and thus disturbs actomyosin ring contraction by inhibiting the phosphorylation of the light chain of myosin II (Kosako et al., 2000; Glotzer, 2001; Yoshizaki et al., 2004). We therefore examined its effects on cytokinesis in NRK cells. Under the conditions used to produce partial detachment (see above), 17/17 mitotic cells failed to divide and subsequently respread as binucleate cells in the presence of 40 μM Y-27632. Four of these cells showed a slight equatorial furrowing (Figure 5A), which eventually regressed, but the remaining 13 did not form detectable furrows. We next evaluated the effects of the inhibitor on cytokinesis in cells adhering to fibronectin-coated substrates. In this case, 9/9 mitotic cells divided successfully in the presence of 40 μM Y-27632, albeit somewhat more slowly than in its absence (Figure 5B). These effects of Y-27632 are similar to, although weaker than, those of blebbistatin, which suggests that the drug effects are not artifacts arising from some side effects of blebbistatin or Y-27632.
Figure 5.
Effects of 40 μM Y-27632 on cytokinesis in NRK cells. (A) Failed cytokinesis in a partially detached cell. (B) Successful cytokinesis in a cell on a fibronectin-coated substrate. Numbers, time in minutes after onset of anaphase; bars, 20 μm.
Adhesion-dependent and Contractile Ring-independent Equatorial Furrowing in HT1080 Cells
It is important to know whether other mammalian cells are also capable of carrying out adhesion-dependent and contractile ring-independent cytokinesis. In that regard, we tested the effects of blebbistatin on cytokinesis in highly adherent human HT1080 fibrosarcoma cells (Rasheed et al., 1974) on substrates coated with collagen type I. On entry into mitosis, 7/7 control cells treated with 1% DMSO rounded up, formed cleavage furrows in their equatorial regions, respread, and successfully divided into two daughter cells (Figure 6A and Table 1). In contrast, in the presence of 30 μM blebbistatin, HT1080 cells rounded up slightly and then respread without forming equatorial furrows. The majority (10/11) subsequently formed lamellipodia around both poles and tight furrows in their equatorial regions (Figure 6B and Table 1), whereas one failed to form lamellipodia around both poles and a tight equatorial furrow. The cells that formed tight furrows lost their oppositely oriented polarity of the presumptive daughter cells immediately after the formation of cytoplasmic bridges connecting the daughter cells. Only one cell completed the division, and two cells fused back to form binucleate cells, within the 2-h observation period. In the remaining seven cells, the cytoplasmic bridges persisted for at least 2 h (Figure 6B and Table 1). Two of those pairs subsequently fused back to form binucleate cells, and one pair eventually completed the division; the fate of the remaining pairs was not determined.
Figure 6.
Cytokinesis in HT1080 cells on substrates coated with type I collagen in the presence of 1% DMSO (A), 30 μM blebbistatin plus 0.3% DMSO (B), or 100 μM blebbistatin plus 1% DMSO (C). Numbers, time in minutes after onset of anaphase; bars, 20 μm.
We also examined cytokinesis of HT1080 cells that initiated anaphase in the presence of 100 μM blebbistatin. Like the cells treated with 30 μM blebbistatin, these cells first rounded up and then respread. Of 11 cells, seven subsequently formed lamellipodia around both poles and tight furrows in their equatorial regions (Figure 6C and Table 1), whereas four failed to form lamellipodia around both poles and a tight equatorial furrow. Daughter cells of two of the seven cells that formed tight furrows eventually lost distinct polarity and became fragmented, as was the case in NRK cells in 100 μM blebbistatin. In the remaining five cells, the daughter cells fused back to form binucleate cells within the 2-h observation period (Figure 6C and Table 1).
DISCUSSION
We have shown here that NRK and HT1080 cells are able to form tight furrows in an adhesion-dependent manner, even when myosin II activity is greatly suppressed by blebbistatin. Furthermore, in the presence of 30 μM blebbistatin, the majority of the daughter NRK cells maintained front-to-rear polarity after the furrow was formed, and the resultant migration away from one another played an important role in completing the separation of the two cells. These results are inconsistent with the view that active contraction of the contractile rings is essential for equatorial furrowing and cytokinesis of mammalian cells, and they suggest the critical importance of substrate adhesion in cytokinesis of adherent animal cells.
Straight et al. (2003) observed that blebbistatin completely inhibits contraction of the contractile rings and cytokinesis in HeLa cells and Xenopus tissue culture cells. We were able to confirm a strong inhibitory effect of blebbistatin on cytokinesis of HeLa cells, which round up and mostly detach from substrates during cytokinesis (our unpublished data). Therefore, our data support the view of Straight et al. (2003) that active contraction of contractile rings is inhibited by blebbistatin and hence requires the activity of myosin II. They are also consistent with the data of Rosenblatt et al. (2004) and Komatsu et al. (2000), who observed that efficient karyokinesis in mammalian cells requires myosin II. On the other hand, our findings differ from those of Cramer and Mitchison (1995), who observed that the respreading of PtK 2 cells after karyokinesis was inhibited by butanedione monoxime and suggested that this process is myosin II dependent. We found that even in the presence of 100 μM blebbistatin, the majority of the NRK and HT1080 cells that successfully initiated anaphase respread and formed lamellipodia, which indicates that this process is not critically dependent on myosin II in these cells. A similar result also was obtained by Pelham and Wang (1999)) using 3T3 cells.
In addition, we found that NRK and HT1080 daughter cells were unable to maintain large, fan-shaped lamellipodia in the presence of 100 μM blebbistatin; instead, the uncoordinated formation of leading edges resulted in fragmentation of the cells into smaller pieces connected by cytoplasmic strands, whereas the original furrow was maintained. We therefore suggest that either a trace amount of myosin II activity and/or some other factor that is inhibited by a high concentration of blebbistatin is required for maintenance of the appropriate polarity of the daughter cells. Consistent with the former possibility, it has been demonstrated that filaments of myosin II accumulate at the bases of lamellipodia in PtK 1 and REF 52 cells (Ponti et al., 2004; Verkhovsky et al., 1995), and inhibition of myosin light chain kinase by ML-7 resulted in the loss of polarity in interphase neutrophils (Pierini et al., 2003). On the other hand, Shu et al. (2005) recently reported that 100 μM blebbistatin inhibits motility of mutant Dictyostleium cells lacking myosin II, supporting the possibility that 100 μM blebbistatin interfered with myosin II-independent processes in NRK and HT1080 cells.
In summary, our results suggest that three aspects of cytokinesis in NRK cells require myosin II: karyokinesis, active contraction of the contractile rings, and maintenance of cell polarity after furrow formation. Moreover, comparison of the differential sensitivities to 30 and 100 μM blebbistatin suggests that a higher level of myosin II activity is required to maintain contraction of the contractile rings than is necessary for karyokinesis or appropriate cell polarization. Most importantly, however, equatorial furrow formation in appropriately adherent cells does not require myosin II in either NRK cells or HT1080 fibrosarcoma cells.
This adhesion-dependent, contractile ring-independent mode of cytokinesis was first characterized in the cellular slime mold D. discoideum (Neujahr et al., 1997; Zang et al., 1997; Nagasaki et al., 2002). It was named “attachment-assisted mitotic cleavage” (Neujahr et al., 1997) or “cytokinesis B” to distinguish it from “cytokinesis A,” which refers to the classic, adhesion-independent, contractile ring-dependent cytokinesis (Zang et al., 1997; Nagasaki et al., 2002). It has been suggested that cytokinesis B is a more primitive process than cytokinesis A (Gerisch and Weber, 2000; Nagasaki et al., 2002). Although there are a number of observations in the literature that can be interpreted as suggesting that mammalian cells also have the capacity for cytokinesis B (reviewed by Uyeda et al., 2004), the present study provides the first direct demonstration that particular mammalian cell types are able to carry it out.
The phenotypes of certain mutant animals also support the possibility that cytokinesis B plays important roles in animal cells. For example, knocking out the single nonmuscle myosin II heavy chain gene in Drosophila is lethal, but developing larvae seem to die because of failure in morphogenetic cell sheet movements, whereas cytokinesis proceeds normally (Young et al., 1993). It is possible that maternal material present in larvae provided sufficient amounts of myosin II to drive cytokinesis, but then cytokinesis must require less myosin II activity than cell sheet movements. Furthermore, cardiomyocytes of mice lacking the gene for nonmuscle myosin IIB heavy chain show only moderate degrees of cytokinetic failure (Takeda et al., 2003). These cells do not express nonmuscle myosin IIA, and therefore cytokinesis of these mutant cardiomyocytes should be due either to myosin IIC expressed at low levels or to the cytokinesis B mechanism.
It has been suggested that active Rho-dependent phosphorylation of myosin II by ROCK has a facilitating, but not an essential, role in cytokinesis, so that inhibition of ROCK by Y-27632 results in a delay but not blockage of cytokinesis (Kosako et al., 2000; Piekny et al., 2000; Yoshizaki et al., 2004; reviewed by Glotzer, 2001). We were able to confirm that cytokinesis is slowed by Y-27632 in adherent NRK cells. However, in partially detached NRK cells, cytokinesis was completely inhibited by 40 μM Y-27632. It may be that NRK cells are somehow more sensitive to Y-27632 than other types of cells. Alternatively, cytokinesis B may contribute to the Y-27632-resistant, slower cytokinesis reported previously.
Nonetheless, the physiological significance of cytokinesis B in mammalian cells is not clear at this point. We speculate that the conservation of the cytokinesis B mechanism in both cellular slime molds and mammalian cells suggests that cytokinesis B has some important role. Burton and Taylor (1997)) reported division of a fibroblast that was driven by oppositely oriented traction forces after regression of the initial equatorial furrow. We also observed that mitotic NRK cells that failed to constrict a furrow in the absence of blebbistatin or Y-27632 respread and formed prominent lamellipodia that moved apart in opposite directions (Kanada and Uyeda, unpublished observations). Therefore, there seems to be a correlation between the failure of cytokinesis A and the subsequent activation of the cytokinesis B mechanism, which suggests the possibility that cytokinesis B in mammalian cells may be a backup mechanism when cells fail to undergo cytokinesis A. Piel et al. (2001) reported that postanaphase repositioning of the centriole to the midbody triggers the completion of cell division and facilitates severing of the midbody. Furthermore, in adherent and flattened L929 and 3T3 cells on fibronectin-coated substrates, postanaphase centrioles did not reposition to the midbody and rather migrated toward the lamellipodia, and the cytoplasmic bridge was severed only after migration of the daughter cells away from one another. This behavior of L929 and 3T3 cells on fibronectin-coated substrates is reminiscent of NRK cells undergoing cytokinesis B, and it is interesting to speculate that the cytokinesis B mechanism is activated by failure of the postanaphase centriole to reposition to the midbody.
Finally, because astral microtubules are dynamic (Canman et al., 2003) and the distal ends of dynamic microtubules in interphase cells are known to activate Rac1 (Waterman-Storer et al., 1999), we speculate that cytokinesis B is dependent on polar Rac activation by astral microtubules. Furthermore, in certain types of cells, antagonistic relationships between the activities of Rac and Rho-type GTPase proteins have been demonstrated, suggesting that equatorial activation of Rho-type GTPase proteins might elicit polar Rac activation, or vice versa (reviewed by Uyeda et al., 2004). In addition, activation of integrin by extracellular matrix also leads to activation of Rac1 (del Pozo et al., 2004), and this may be how adhesion to a fibronectin-coated substrate contributes to efficient cytokinesis B in NRK cells. It thus seems that to fully understand the interdependent network of regulatory pathways leading to successful cytokinesis in animal cells and, more broadly, the origin and principles of cytokinesis, it will be necessary to understand the mechanism of cytokinesis B.
Supplementary Material
Acknowledgments
We thank Dr. Y. Nishimura for useful suggestions on NRK cells, Dr. E. D. Korn for constructive comments on the manuscript, and Dr. T. Mizuno for providing reagents.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0233) on June 8, 2005.
Abbreviations used: NRK, normal rat kidney; ROCK, Rho-kinase).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Ben-Ze'ev, A., and Raz, A. (1981). Multinucleation and inhibition of cytokinesis in suspended cells: reversal upon reattachment to a substrate. Cell 26, 107-115. [DOI] [PubMed] [Google Scholar]
- Bi, E., Maddox, P., Lew, D. J., Salmon, E. D., McMillan, J. N., Yeh, E., and Pringle, J. R. (1998). Involvement of an actomyosin contractile ring in Saccharomyces cerevisiae cytokinesis. J. Cell Biol. 142, 1301-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, K., and Taylor, D. L. (1997). Traction forces of cytokinesis measured with optically modified elastic substrata. Nature 385, 450-454. [DOI] [PubMed] [Google Scholar]
- Canman, J. C., Cameron, L. A., Maddox, P. S., Straight, A., Tirnauer, J. S., Mitchison, T. J., Fang, G., Kapoor, T. M., and Salmon, E. D. (2003). Determining the position of the cell division plane. Nature 424, 1074-1078. [DOI] [PubMed] [Google Scholar]
- Cramer, L. P., and Mitchison, T. J. (1995). Myosin is involved in postmitotic cell spreading. J. Cell Biol. 131, 179-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lozanne, A., and Spudich, J. A. (1987). Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 236, 1086-1091. [DOI] [PubMed] [Google Scholar]
- del Pozo, M. A., Alderson, N. B., Kiosses, W. B., Chiang, H. H., Anderson, R. G., and Schwartz, M. A. (2004). Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839-842. [DOI] [PubMed] [Google Scholar]
- Fishkind, D. J., and Wang, Y. L. (1995). New horizons for cytokinesis. Curr. Opin. Cell Biol. 7, 23-31. [DOI] [PubMed] [Google Scholar]
- Gerisch, G., and Weber, I. (2000). Cytokinesis without myosin II. Curr. Opin. Cell Biol. 12, 126-132. [DOI] [PubMed] [Google Scholar]
- Glotzer, M. (1997). The mechanism and control of cytokinesis. Curr. Opin. Cell Biol. 9, 815-823. [DOI] [PubMed] [Google Scholar]
- Glotzer, M. (2001). Animal cell cytokinesis. Annu. Rev. Cell Dev. Biol. 17, 351-386. [DOI] [PubMed] [Google Scholar]
- Knecht, D. A., and Loomis, W. F. (1987). Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science 236, 1081-1086. [DOI] [PubMed] [Google Scholar]
- Kolega, J. (2004). Phototoxicity and photoinactivation of blebbistatin in UV and visible light. Biochem. Biophys. Res. Commun. 320, 1020-1025. [DOI] [PubMed] [Google Scholar]
- Komatsu, S., Yano, T., Shibata, M., Tuft, R. A., and Ikebe, M. (2000). Effects of the regulatory light chain phosphorylation of myosin II on mitosis and cytokinesis of mammalian cells. J. Biol. Chem. 275, 34512-34520. [DOI] [PubMed] [Google Scholar]
- Kosako, H., Yoshida, T., Matsumura, F., Ishizaki, T., Narumiya, S., and Inagaki, M. (2000). Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 19, 6059-6064. [DOI] [PubMed] [Google Scholar]
- Kovacs, M., Toth, J., Hetenyi, C., Malnasi-Csizmadia, A., and Sellers, J. R. (2004). Mechanism of blebbistatin inhibition of myosin II. J. Biol. Chem. 279, 35557-35563. [DOI] [PubMed] [Google Scholar]
- Manstein, D. J., Titus, M. A., De Lozanne, A., and Spudich, J. A. (1989). Gene replacement in Dictyostelium: generation of myosin null mutants. EMBO J. 8, 923-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki, A., de Hostos, E., and Uyeda, T.Q.P. (2002). Genetic and morphological evidence for two parallel pathways of cell-cycle-coupled cytokinesis in Dictyostelium. J. Cell Sci. 115, 2241-2251. [DOI] [PubMed] [Google Scholar]
- Neujahr, R., Heizer, C., and Gerisch, G. (1997). Myosin II-independent processes in mitotic cells of Dictyostelium discoideum: redistribution of the nuclei, re-arrangement of the actin system, and formation of the cleavage furrow. J. Cell Sci. 110, 123-137. [DOI] [PubMed] [Google Scholar]
- O'Connell, C. B., Warner, A. K., and Wang, Y. (2001). Distinct roles of the equatorial and polar cortices in the cleavage of adherent cells. Curr. Biol. 11, 702-707. [DOI] [PubMed] [Google Scholar]
- O'Connell, C. B., Wheatley, S. P., Ahmed, S., and Wang, Y. L. (1999). The small GTP-binding protein Rho regulates cortical activities in cultured cells during division. J. Cell Biol. 144, 305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, R.J.J., and Wang, Y. (1999). High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol. Biol. Cell 10, 935-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekny, A. J., Wissmann, A., and Mains, P. E. (2000). Embryonic morphogenesis in Caenorhabditis elegans integrates the activity of LET-502 Rho-binding kinase, MEL-11 myosin phosphatase, DAF-2 insulin receptor and FEM-2 PP2c phosphatase. Genetics 156, 1671-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel, M., Nordberg, J., Euteneuer, U., and Bornens, M. (2001). Centrosome-dependent exit of cytokinesis in animal cells. Science 291, 1550-1553. [DOI] [PubMed] [Google Scholar]
- Pierini, L. M., Eddy, R. J., Fuortes, M., Seveau, S., Casulo, C., and Maxfield, F. R. (2003). Membrane lipid organization is critical for human neutrophil polarization. J. Biol. Chem. 278, 10831-10841. [DOI] [PubMed] [Google Scholar]
- Ponti, A., Machacek, M., Gupton, S. L., Waterman-Storer, C. M., and Danuser, G. (2004). Two distinct actin networks drive the protrusion of migrating cells. Science 305, 1782-1786. [DOI] [PubMed] [Google Scholar]
- Rasheed, S., Nelson-Rees, W. A., Toth, E. M., Arnstein, P., and Gardner, M. B. (1974). Characterization of a newly derived human sarcoma cell line (HT-1080). Cancer 33, 1027-1033. [DOI] [PubMed] [Google Scholar]
- Robinson, D. N., Girard, K. D., Octtaviani, E., and Reichl, E. M. (2002). Dictyostelium cytokinesis: from molecules to mechanics. J. Muscle Res. Cell Motil. 23, 719-727. [DOI] [PubMed] [Google Scholar]
- Rosenblatt, J., Cramer, L. P., Baum, B., and McGee, K. M. (2004). Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell 117, 361-372. [DOI] [PubMed] [Google Scholar]
- Straight, A. F., Cheung, A., Limouze, J., Chen, I., Westwood, N. J., Sellers, J. R., and Mitchison, T. J. (2003). Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science 299, 1743-1747. [DOI] [PubMed] [Google Scholar]
- Shu, S., Liu, X., and Korn, E. D. (2005). Blebbistatin and blebbistatin-inactivated myosin II inhibit myosin II-independent processes in Dictyostelium. Proc. Natl. Acad. Sci. USA 102, 1472-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda, K., Kishi, H., Ma, X., Yu, Z. X., and Adelstein, R. S. (2003). Ablation and mutation of nonmuscle myosin heavy chain II-B results in a defect in cardiac myocyte cytokinesis. Circ. Res. 93, 330-337. [DOI] [PubMed] [Google Scholar]
- Uyeda, T.Q.P., Nagasaki, A., and Yumura, S. (2004). Multiple parallelisms in animal cytokinesis. Int. Rev. Cytol. 240, 377-432. [DOI] [PubMed] [Google Scholar]
- Uyeda, T.Q.P., and Nagasaki, A. (2004). Variations on a theme: the many modes of cytokinesis. Curr. Opin. Cell Biol. 16, 55-60. [DOI] [PubMed] [Google Scholar]
- Verkhovsky, A. B., Svitkina, T. M., and Borisy, G. G. (1995). Myosin II filament assemblies in the active lamella of fibroblasts: their morphogenesis and role in the formation of actin filament bundles. J. Cell Biol. 131, 989-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer, C. M., Worthylake, R. A., Liu, B. P., Burridge, K., and Salmon, T. D. (1999). Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1, 45-50. [DOI] [PubMed] [Google Scholar]
- Watts, F. Z., Shiels, G., and Orr, E. (1987). The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 6, 3499-3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winklbauer, R. (1986). Cell proliferation in the ectoderm of the Xenopus embryo: development of substratum requirements for cytokinesis. Dev. Biol. 118, 70-81. [DOI] [PubMed] [Google Scholar]
- Yoshizaki, H., Ohba, Y., Parrini, M. C., Dulyaninova, N. G., Bresnick, A. R., Mochizuki, N., and Matsuda, M. (2004). Cell type-specific regulation of RhoA activity during cytokinesis. J. Biol. Chem. 279, 44756-44762. [DOI] [PubMed] [Google Scholar]
- Young, P., Richman, A., Ketchum, A., and Kiehart, D. (1993). Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 7, 29-41. [DOI] [PubMed] [Google Scholar]
- Zang, J. H., Cavet, G., Sabry, J. H., Wagner, P., Moores, S. L., and Spudich, J. A. (1997). On the role of myosin-II in cytokinesis: division of Dictyostelium cells under adhesive and nonadhesive conditions. Mol. Biol. Cell 8, 2617-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W., and Robinson, D. N. (2005). Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. Proc. Natl. Acad. Sci. USA 102, 7186-7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.