Abstract
The genetic loss of endothelial-derived nitric oxide synthase (eNOS) in mice impairs vascular endothelial growth factor (VEGF) and ischemia-initiated blood flow recovery resulting in critical limb ischemia. This result may occur through impaired arteriogenesis, angiogenesis, or mobilization of stem and progenitor cells. Here, we show that after ischemic challenge, eNOS knockout mice [eNOS (-/-)] have defects in arteriogenesis and functional blood flow reserve after muscle stimulation and pericyte recruitment, but no impairment in endothelial progenitor cell recruitment. More importantly, the defects in blood flow recovery, clinical manifestations of ischemia, ischemic reserve capacity, and pericyte recruitment into the growing neovasculature can be rescued by local intramuscular delivery of an adenovirus encoding a constitutively active allele of eNOS, eNOS S1179D, but not a control virus. Collectively, our data suggest that endogenous eNOS-derived NO exerts direct effects in preserving blood flow, thereby promoting arteriogenesis, angiogenesis, and mural cell recruitment to immature angiogenic sprouts.
Keywords: angiogenesis, arteriogenesis, genetics
Ischemia-induced arteriogenesis and subsequent angiogenesis are compensatory mechanisms to provide blood supply to ischemic tissues. In the coronary circulation and peripheral vasculature, ischemia-initiated opening of preexistent collaterals and arterialization of these immature vascular channels preserves blood flow and contributes to the extent of ischemic reserve capacity in the heart and leg (1). Therefore, understanding the molecular mechanisms of ischemia-initiated vascular remodeling and angiogenesis is important.
The availability of knockout mice that are developmentally “normal” permits testing of genes required for postnatal hindlimb angiogenesis and remodeling, despite their relative lack of importance during vasculogenesis. Examples of impaired ischemic limb arteriogenesis and angiogenesis can be found in mice deficient in placental-derived growth factor (2), IL-10 (3), the angiotensin II type-1 receptor (4), matrix metalloproteinase-9 (5), adiponectin (6), caveolin-1 (7), and, in some, but not all laboratories, the receptor for monocyte chemotactic peptide 1, CC-chemokine receptor 2 (8, 9). One downstream pathway shown by multiple groups to be important for ischemia-mediated arteriogenesis and blood flow stimulated angiogenesis in endothelial nitric oxide synthase. In many laboratories, endothelial-derived nitric oxide synthase (eNOS) is required for postischemic blood flow recovery, because mice deficient in eNOS show a severe form of critical limb ischemia in mouse hindlimb models (10, 11). Moreover, the ability of, statin-based drugs, angiotensin II, neuropeptide Y, and stromal cell-derived factor-1α to improve limb angiogenesis are absent in mice deficient in eNOS (12-15). The molecular mechanisms for how eNOS regulates ischemia-triggered arteriogenesis and angiogenesis is related, in part, to the inability of eNOS (-/-) mice to respond to vascular endothelial growth factor (VEGF). Indeed, VEGF-mediated permeability, angiogenesis, and endothelial cell precursor mobilization is markedly impaired in mice lacking eNOS (10, 11, 16, 17). However, given the importance of eNOS in regulating blood flow and arterial remodeling, it is possible that other mechanisms are operational.
In this study, we show that eNOS is important for contraction-evoked hyperemia in skeletal muscle, ischemia-mediated angiogenesis, and pericyte recruitment but not mobilization of endothelial precursor cells into the systemic circulation. Moreover, local adenoviral gene delivery of a constitutively active allele of eNOS (eNOS S1179D) rescues chronic limb ischemia in eNOS (-/-) mice, improves contraction induced hyperemia, lower limb angiogenesis, and pericyte recruitment, suggesting that the beneficial autocrine and paracrine actions of eNOS-derived NO sustains blood flow to severely ischemic limbs.
Methods
Mouse Hindlimb Ischemic Model and Adenovirus Gene Delivery. All animal studies were approved by the institutional animal care and use committees of Yale University. Eight- to 12-week-old male congenic (F10) eNOS (-/-) or C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) were used for all experiments. Mouse ischemic hindlimb model was performed as described in ref. 18 and detailed in Supporting Methods, which is published as supporting information on the PNAS web site.
Blood Flow Measurement and Clinical Score. Blood flow was measured by the PeriFlux system with the Laser Doppler perfusion module unit (Perimed, North Royalton, OH). A deep measurement probe was placed directly on the gastrocnemius muscle to ensure a deep muscle flow measurement. Ischemic and nonischemic limb perfusion was measured before surgery, immediately after surgery, and 3 days, 2 weeks and 4 weeks after surgery. The final blood flow values were expressed as the ratio of ischemic to nonischemic hind limb perfusion. To more precisely evaluate the mobility of mice after limb ischemia, we designed a scoring system. 0 = normal; 1 = pale foot or gait abnormalities; 2 = gangrenous tissue in less than one-half of the foot without lower limb necrosis; 3 = gangrenous tissue in less than one-half of the foot with lower limb necrosis; 4 = gangrenous tissue in greater than one-half of the foot; 5 = loss of one-half of the lower limb. Clinical outcome of all mice were observed and recorded at the same time points of blood flow measurement.
Postcontraction Hyperemia Before and After Schemia. After anesthesia, mice were placed on a heated pad. The adductor muscle group and gastrocnemius muscle were exposed by a middle-line incision of the limb. After baseline gastrocnemius blood flow was measured, adductor muscles were stimulated with two electrodes at 2 Hz and 5 mA by using the electrostimulator for 2 min. Blood flow was taken and recorded by maclab chart (ADInstruments, Grand Junction, CO) during stimulation and for 10 min after stimulation.
Histology and Immunohistochemistry. Mice were killed at 4 weeks after surgery, and muscles of the lower limbs were harvested, methanol fixed, and paraffin embedded. Tissue sections (5-μm thick) were stained by using anti-PECAM-1 antibody (Pharmingen) and anti-smooth muscle α actin (SMA) antibody (DAKO). Bound primary antibodies were detected by using avidin-biotin-peroxidase (NovaRed peroxidase substrate kit, Vector Laboratories) as described in Supporting Methods.
Gene Expression in Ischemic Muscle. Total RNA of lower limb muscles was isolated by using phenol/chloroform and isolated by using RNeasy kit with DNase I digestion (Qiagen, Valencia, CA). Reverse transcription was done by the standard procedure (Super Script First-Strand Synthesis System, Qiagen) by using 1 μg of total RNA. Quantitative real-time PCR was performed as described in Supporting Methods.
Arteriogenesis Analysis. Two and 4 weeks after surgery, mice were anesthetized and heparinzed. Mice were perfused with PBS containing the vasodilators (papaverine, 4 mg/liter; adenosine, 1 g/liter) for 3 min at physiological pressure through descending aorta, and blood was drained from the inferior vena cava. The vasculature was fixed with 2% paraformaldehyde (in PBS) for 5 min, flushed with PBS for 2 min, and infused with contrast agent (bismuth oxychloride in saline and 10% gelatin in PBS, 1:1). Mice were then immersed in ice to solidify the contrast agent. Microangiography was taken with Faxitron x-ray machine (Hewlett-Packard) at 25 kV and 3.25 mA for 3 min. Upper limb vascular density (pixel density), vessel length (average length of vessels with diameter >1 pixel), and fractal dimension were analyzed by modified imagej and matlab software (Research Services Branch, National Institute of Mental Health, Bethesda, MD).
Endothelium Progenitor Cell (EPC) Mobilization Assay. After anesthesia and heparinization, blood was drawn by cardiac puncture. Mononuclear cells were isolated by a density gradient method by using Histopaque-1077 (Sigma). Five hundred microliters of blood was mixed with 2 ml of PBS, gently added to 2 ml of Histopaque-1077, and centrifuged at 400× g for 30 min. The mononuclear fraction was collected, washed in PBS, and following red cell lysis with ammonium chloride solution (StemCell Technologies), 1 × 106 cells per cm2 were seeded on fibronectin-coated slides (Clontech). Cells were allowed to differentiate in EGM-2 SingleQuots medium (Cambrex, East Rutherford, NJ) containing VEGF-A, FGF, IGF-1, hydrocortisone, ascorbic acid, GA 1000, heparin, 1% gentamicin/streptomycin (GIBCO, Carlsbad, CA), and 5% FBS. Medium was changed every other day. After 5 days of in vitro culture, cells were incubated in 10 μg/ml Ac-Dil-LDL (Biomedical Technologies) for 4 h and Ac-Dil-LDL-positive cells were photographed and counted in 10 low power (10×) fields for each animal.
Statistical Analysis. All data are expressed as means ± SEM. Statistical differences were measured by either Student's t test or one or two-way analysis of variance followed by Bonferroni post hoc test. A value of P < 0.05 was considered as statistically significant.
Results
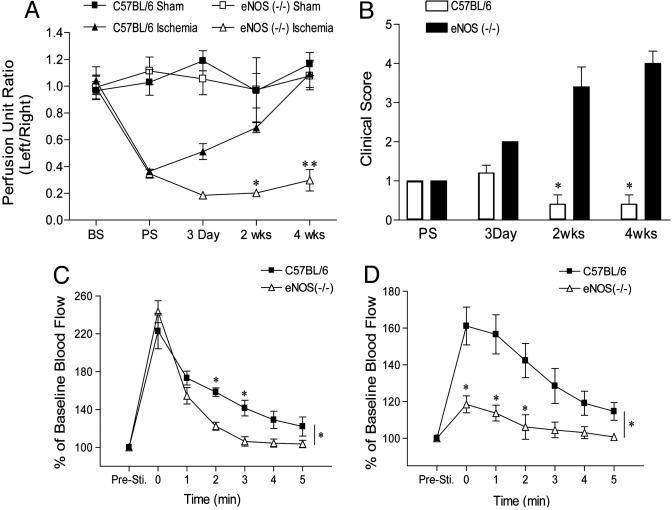
eNOS Deficiency Results in Impaired Blood Flow Recovery, Severe Limb Ischemia, and Impaired Ischemic Reserve Capacity. Surgical arteriectomy of the left femoral artery in congenic eNOS (-/-) mice results in a severe form of limb ischemia. As seen in Fig. 1A, before surgery (BS), the ratio of left leg to right leg gastrochemius blood flow by using a deep penetrating laser Doppler applied directly to the muscle is 1. Postsurgery (PS), flow drops by 80% in both C57BL/6 and eNOS (-/-) mice and, in C57BL/6 mice, returns to a ratio of 1 over the ensuing 4 weeks. In contrast, there is a marked impairment in gastrocnemius blood flow in eNOS (-/-), consistent with previous studies measuring superficial blood flow through laser Doppler perfusion imaging (10, 11). The impairment in blood flow in eNOS (-/-) is associated with a marked increase in clinical severity (Fig. 1B).
Fig. 1.
eNOS (-/-) mice are a model of critical limb ischemia with impaired hyperemia. (A and B) C57BL/6 or congenic eNOS (-/-) mice were exposed to sham surgery (squares) or surgical arteriectomy (triangles) and gastrocnemius blood flow (A) and clinical score (B) assessed over 4 weeks. (C) The adductor muscle groups of mice were electro-stimulated, and the increase in gastronemius blood flow was recorded. (D) The identical experiment was performed after 2 weeks of ischemia in C57BL/6 and eNOS (-/-) mice. Data represent mean ± SEM; n = 4 mice per group for sham surgery and n = 10 mice per strain for arteriectomy in A and B; n = 5 mice per strain in C and D; *, P < 0.05.
The above data suggests that eNOS (-/-) may lack the ability to use preexisting collaterals to supply blood flow to the lower leg secondary to ischemia. To assess the capacity to recruit blood flow through preexisting collaterals to ischemic tissue, we examined skeletal muscle contraction-stimulated hyperemia in the gastrocnemius muscle in C57BL/6 and eNOS (-/-) mice at baseline and after ischemia. Electrical stimulation of the adductor muscle groups in the upper legs of C57BL/6 (squares) and eNOS (-/-; triangles) mice results in a marked increase in peak blood flow measured in the gastrocnemius muscle group of both strains [compare before stimulation (PreSti) to time 0, Fig. 1C]. However, the return to baseline flow is markedly accelerated in eNOS (-/-) mice compared with C57BL/6 mice, suggesting that eNOS-derived NO is critical for maintaining vasodilation necessary for the gradual return of flow back to normal. Next, we measured the same physiological response after limb ischemia in C57BL/6 and eNOS (-/-) mice at 1 and 2 weeks after ischemia. At 1 week after ischemia, despite a return of resting blood flow in the gastrocnemius in C57BL/6 mice (see Fig. 1A), we were unable to measure a hyperemic response in both strains (data not shown). However, after 2 weeks of ischemia, electrical stimulation of the adductor muscle groups in the upper leg resulted in a 60% increase in peak blood flow measured in the gastrocnemius muscle group of C57BL/6 mice and a markedly diminished peak response in eNOS (-/-) mice (≈20%, Fig. 1D). The kinetics of blood flow recovery after hyperemia were similar in ischemic C57BL/6 mice compared with their nonischemic counterparts, an effect dramatically attenuated in eNOS (-/-) mice. These data show that nonischemic eNOS (-/-) mice have a normal peak hyperemic response but reduced blood flow after stimulation and ischemic eNOS (-/-) mice have markedly reduced flow both during and after stimulation.
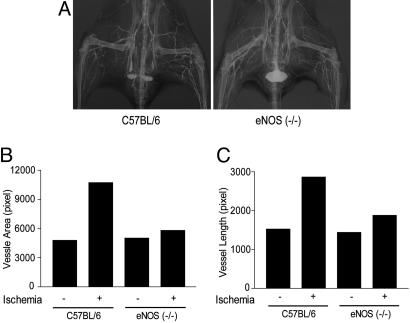
Postischemic Arteriogenesis Is Impaired in eNOS (-/-) Mice. Next, we examined ischemia-initiated arteriogenesis in C57BL/6 and eNOS (-/-) mice by quantitative angiography. As seen in Fig. 2A, after 4 weeks of ischemia in C57BL/6 mice (left panel), there was an increase in tracer perfusion on the ischemic side (Left) compared with the contralateral leg, reflecting an increase in the density and length of vessels. This result was quantified as an increased vessel area and length in the adductor regions of interest (Fig. 2 B and C). The increase in total arterial dimensions was not due to changes in branching patterns because the fractal dimensions were similar on the right and left legs (fractal dimension for C57BL/6 mice were 1.52 on the ischemic leg and 1.46 in the contralateral leg). In contrast, ischemia did not increase vascular density or length to the same extent in eNOS (-/-), documenting for the first time an impairment in arteriogenesis in these mice (2A Right as quantified in B and C). Again, the changes in arterial dimensions were not due to changes in branching patterns as the fractal dimensions were similar on the right and left legs [fractal dimension for eNOS (-/-) were 1.40 in the ischemic leg and 1.52 in the contralateral leg]. These data document the impaired arteriogenesis in eNOS (-/-) mice but no gross changes in vascular patterning.
Fig. 2.
Angiographic evidence for impaired arteriogenesis in eNOS (-/-) mice. (A) Arterial phase angiograms from C57BL/6 (Left) and eNOS (-/-) (Right) mice after 4 weeks of left limb ischemia. (B and C) eNOS (-/-) mice show reduced quantification of vessel area (B) and total length (C) compared with C57BL/6 mice. Data are mean values from two angiograms.
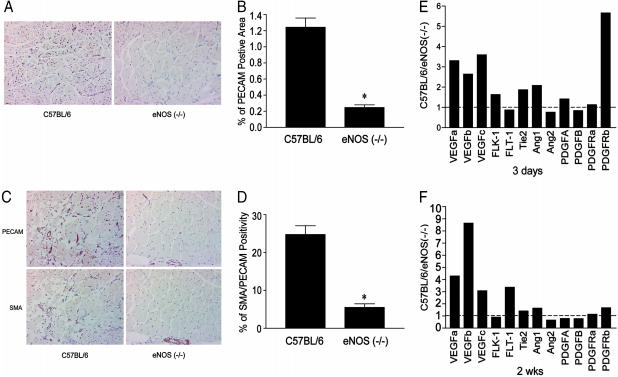
Postischemic Angiogenesis, Recruitment of Pericytes, and Gene Expression Is Impaired in eNOS (-/-) Mice. Secondary to upper limb ischemia, shear stress-dependent changes in blood flow promotes capillary angiogenesis in the lower leg. As seen in Fig. 3A, after 4 weeks of ischemia, there was an increase in PECAM-1-positive endothelial cells surrounding the skeletal muscle myocytes in C57BL/6, an effect markedly diminished in eNOS (-/-) mice (as quantified in Fig. 3B). Stable angiogenesis is believed to occur contemporaneously with pericyte recruitment mediated by platelet-derived growth factor (PDGF) and/or angiopoietin-1. To examine the recruitment of pericytes to angiogenic capillary sprouts after ischemia, thin, serial sections were immunostained with PECAM-1 or SMA antibodies. As seen in Fig. 3 C and D, ≈25% of PECAM-1-positive skeletal muscle capillaries and vessels were colabeled with SMA in cross sections of ischemic gastrocnemius muscles from C57BL/6 mice. In contrast, 5% of PECAM-1-positive structures colabeled with SMA in gastrocnemius muscles from eNOS (-/-) mice. Next, we examined the difference in gene expression of several growth factors and their cognate receptors implicated in angiogenesis and pericyte recruitment by using quantitative PCR. The levels of gene expression were compared in ischemic and contralateral, nonischemic gastrocnemius muscle groups 3 days and 2 weeks after ischemia in both strains of mice. As seen in Fig. 3E, a value of 1 reflects the normalized level of gene expression in nonsurgically manipulated muscles (comparing left with right leg). After 3 days of ischemia, the levels of VEGF isoforms (VEGF-A, -B and -C) were 2- to 3-fold higher in C57BL/6 mice; however, the levels of the genes for VEGF receptors (Flk-1, Flt-1) were not different. Angiopoeitin-1 (Ang-1) and its receptor Tie-2 were slightly increased, whereas angiopoeitin-2 (Ang-2) levels were similar. The genes for PDGF-A and -B chains and the PDGF-α receptor (PDGFR-α) did not change. However, there was a marked increase in PDGF-β receptor (PDGFR-β) gene expression. After 2 weeks of ischemia (Fig. 3F) in C57BL/6 mice, VEGF isoform levels remained elevated and, in particular, VEGF-B was markedly increased in C57BL/6 mice compared with eNOS (-/-) mice. The levels of the VEGF receptor Flt-1 also were higher. All other genes remained remarkably consistent. Collectively, these data suggest both the impaired ischemia driven angiogenesis and immature, SMA deficient vessels in eNOS (-/-) mice correlates with reductions in the mRNAs for both VEGF and PDGF signaling pathways.
Fig. 3.
Impaired ischemia-induced angiogenesis, pericyte recruitment, and gene expression in eNOS (-/-) mice. Gastrocnemius muscles of ischemic mice were immunostained for PECAM-1 (an endothelial cell marker) only (A with quantification in B) or PECAM-1 and SMA (a smooth muscle/pericyte marker in C with quantification in D). Data represent mean ± SEM; n = 8 mice per strain in B and D; *, P < 0.05. The expression of angiogenic and remodeling genes were assessed by qPCR in the gastronemius after 3 days (E) and two weeks (F) after ischemia. Data represent the differences in gene expression in C57BL/6 compared with eNOS (-/-) mice. Data are average determinations of relative gene expression in ischemic/nonischemic limbs from RNA pooled from three mice per strain per time point.
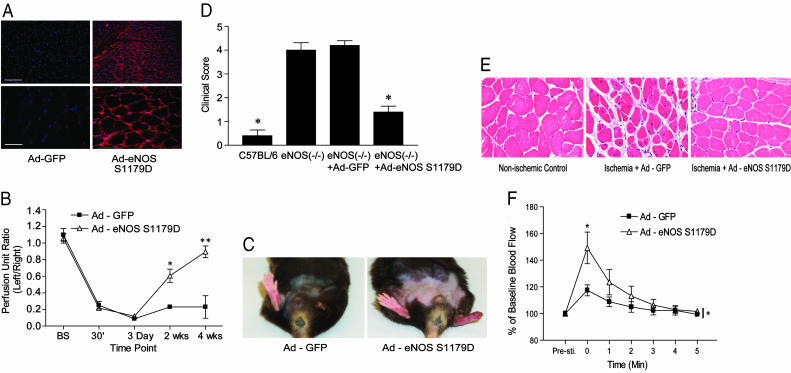
Adenoviral Delivery of Constitutively Active eNOS Rescues Limb Ischemia in eNOS (-/-) Mice. To examine whether local gene delivery of eNOS could rescue the critical limb ischemia in eNOS (-/-) mice, we used a constitutively active form of eNOS bearing a mutation of a key phosphorylation site, Serine 1179 to an aspartate residue (Ad-eNOS S1179D). Serine 1179 is phosphorylated by several kinases including AMPK, Akt, PKG, and PKA, and phosphorylation of this residue increases the basal synthesis of NO (19, 20). Moreover, substitution of aspartate for serine mimics the negative charge imparted by the phosphate and renders eNOS constitutively active by increasing the rate of electron flux through the protein and increases basal NO production severalfold (21). The delivery method and activity of this construct has been well characterized (22-24). As seen in Fig. 4A, intramuscular injection Ad-eNOS S1179D into the adductor muscle group of ischemic eNOS (-/-) resulted in expression of eNOS (detected at 4 days after infection by immunofluoresence microscopy) in the sarcolemma of myocytes and in adjacent vasculature (Right), whereas injection of a control virus expressing Ad-GFP did not result in eNOS labeling (Left). These data verify adenoviral delivery of eNOS into cells in the adductor muscle group. Administration of Ad-eNOS S1179D, but not Ad-GFP, into the adductor muscle group of eNOS (-/-) mice at the time of surgery markedly improved blood flow recovery at 2 and 4 weeks after ischemia (Fig. 4B). Remarkably, the improvement of blood flow partially rescued the severely ischemic limb (Fig. 4C), clinical scores (Fig. 4D), and skeletal myocyte damage (Fig. 4E). More significantly, Ad-eNOS S1179D partially rescued skeletal muscle contraction-stimulated hyperemia in the gastrocnemius muscle (Fig. 4F), consistent with eNOS gene transfer improving resting blood flow and clinical outcome.
Fig. 4.
Intramuscular injection of Ad-eNOS S1179D, rescues ischemic defect in flow, clinical outcome, and hyperemia. (A) eNOS (-/-) mice were injected with Ad-GFP or Ad-eNOS S1179D and eNOS levels examined in the adductor muscle group by fluorescence microscopy after 4 days of infection. (Scale bars: Upper Left, 200 μm; Lower Left, 50 μm.) Ad-eNOS S1179D improved the time course of blood flow recovery (B) and clinical outcome (D). Images in C and E reflect improvement of limb appearance and muscle histology after Ad-eNOS S1179D infection. (F) Ad-eNOS S1179D gene transfer into eNOS (-/-) mice also improves postcontraction hyperemia. Data are mean ± SEM; n = 5 mice per group in B, D, and F; *, P < 0.05.
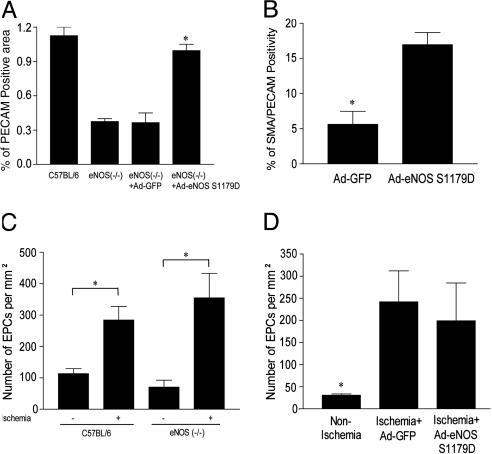
Ad-eNOS S1179D Gene Transfer Promotes Postischemic Angiogenesis and Recruitment of Pericytes in eNOS (-/-) but Does Not Effect EPC Mobilization. Next, we examined capillary angiogenesis and pericyte recruitment in eNOS (-/-) transduced with Ad-eNOS S1179D or Ad-GFP. Administration of Ad-GFP did not influence the number of PECAM-1-positive vascular structures (Fig. 5A, third column) or SMA positive, PECAM-1 positive, mature vessels in the gastrocnemius muscle (Fig. 5B). However, Ad-eNOS S1179D gene transfer increased the number of PECAM-1-positive structures as well SMA/PECAM-1-positive structures. These data demonstrate that local delivery of eNOS is sufficient to rescue both the anatomical and functional deficiencies in eNOS (-/-) mice.
Fig. 5.
Ad-eNOS S1179D improves angiogenesis but does not influence EPC mobilization. Gastrocnemius muscles of ischemic C57BL/6, eNOS (-/-), and eNOS (-/-) mice injected with Ad-GFP or Ad-eNOS S1179D were immunostained for PECAM-1 (an endothelial cell marker) (A) or serial sections colabeled with PECAM-1 and SMA antibodies (a smooth muscle/pericyte marker) (B). Ischemia mediated increases in EPC mobilization into peripheral blood in C57BL/6 and eNOS (-/-) mice (C), and the beneficial effects of Ad-eNOS S1179D (D) are not different. Data are mean ± SEM; n = 5 mice per treatment in A, B, and D and 6 mice per group in C; *, P < 0.05.
Previous work has demonstrated that basal numbers of circulating EPCs do not differ between eNOS (-/-) and control mice. However, after i.v. injection of VEGF, the mobilization of EPCs, as isolated from the spleen, is markedly impaired in eNOS (-/-) mice, suggesting that eNOS influences the recruitment of EPCs. In addition, the defect in hindlimb flow recovery in ischemic eNOS (-/-) mice could be rescued by i.v. infusion of in vitro expanded EPCs (11) but not wild-type bone marrow, suggesting that progenitor cells mobilization from bone marrow is impaired in eNOS (-/-) mice. However, there are no data examining ischemia-mediated mobilization of EPCs into the blood in eNOS (-/-) mice. Therefore, we assessed whether endogenous eNOS and/or local delivery of Ad-eNOS S1179D influences ischemia-mediated peripheral EPC mobilization into peripheral blood. In contrast to defects in VEGF-stimulated mobilization of splenic EPCs seen in eNOS (-/-) mice, limb ischemia triggered similar levels of EPC mobilization in C57BL/6 and eNOS (-/-) mice (Fig. 5C). Moreover, intramuscular delivery of Ad-GFP or Ad-eNOS S1179D did not modify ischemia-induced mobilization of peripheral EPCs in eNOS (-/-) mice (Fig. 5D). These data indicate that the improved structural and functional recovery in eNOS (-/-) mice administered with Ad-eNOS S1179D is unlikely related to an effect on EPC mobilization but to the local actions of eNOS-derived NO on blood flow, angiogenesis, and vessel maturation.
Discussion
The most salient findings of this study are that eNOS (-/-) have a defective postcontraction hyperemic response at rest and exhibit a severe form of limb ischemia due to impaired ischemic flow reserve, arteriogenesis, angiogenesis, and pericyte stabilization of angiogenic vasculature; defects consistent with impairments of gene expression for components of VEGF and PDGF pathways. Remarkably, most of these functional and structural defects can be rescued by local delivery of an adenovirus expressing a constitutively active form of eNOS. A priori, correction of the severe limb ischemia exhibited in eNOS (-/-) with Ad-eNOS S1179D (this study) but not VEGF [protein or adenovirus (10), statins (13), or SDF-1α (15)] argues in favor of eNOS-derived NO as a critical second messenger and paracrine mediator of adaptive arteriogenesis and angiogenesis during limb ischemia in mice.
Ischemia-triggered arteriogenesis is believed to mediate a series of factors including monocyte/macrophage derived cytokines, such as monocyte chemotactic peptide 1 or VEGF, followed by changes in shear stress in preexisting collaterals that would activate eNOS acutely and induce its gene expression after chronic changes in blood flow as seen during exercise training (1, 25). eNOS-derived NO can serve in its well known capacity as a vasodilator to reduce vascular resistance, improve blood flow, and maintain proportional remodeling of blood vessels during changes in blood flow (26). In addition, eNOS exerts a second messenger role in VEGF signaling and is necessary for many of the actions of VEGF in cultured endothelial cells or in postnatal mice (27-29). Most directly, work from several laboratories has shown that VEGF-induced angiogenesis, vascular leakage, and EPC isolation from spleen are dramatically reduced in eNOS (-/-) mice or by NOS inhibitors in normal mice (10, 11, 16, 17). The relevance of the VEGF/NO link has been manifested clinically because a primary side effect in cancer patients treated with anti-VEGF therapy is hypertension, likely due to a deficit in VEGF coupling to eNOS activation and NO release (30). Once formed, NO can positively and negatively regulate the synthesis of VEGF depending on the levels of NO produced and oxygen tensions (31-34). Our data support the idea that eNOS-derived NO promotes VEGF production because VEGF isoform mRNA levels were reduced in ischemic limbs from eNOS (-/-) mice compared with wild-type mice. Whether this occurs as a direct or indirect consequence of NO in vivo is not known. Collectively, these data support multiple links between eNOS and VEGF signaling pathways but are in stark contrast to the lack of importance of eNOS during vascular development compared with the essential role for VEGF and its receptors. eNOS (-/-) mice have smaller litter sizes but do not exhibit gross defects in vasculogenesis (35, 36), whereas genetic loss of VEGF164 or its receptors in mice triggers defects in embryonic vasculogenesis and angiogenesis. This paradox provides an important example demonstrating that genes required for embryonic vascular patterning or angiogenesis may overlap but also differ from those used in postnatal paradigms of angiogenesis. Our data showing the essential role of eNOS for various aspects of ischemic arteriogenesis/angiogenesis and the rescue of ischemic defects by reintroduction of eNOS provides a solid, genetic argument supporting the critical role of eNOS.
Although cytokines are implicated as initiators of ischemic arteriogenesis and angiogenesis, the role of mechanical forces and shear stress in shaping and/or remodeling growing collaterals or capillaries should not be underestimated. Clearly, in adult mice, changes in blood flow, in the absence of overt changes in inflammatory cytokines, are sufficient to remodel large conduit and resistance blood vessels and promote capillary angiogenesis (37, 38). Similar evidence for flow-mediated remodeling and arterialization is beginning to be embraced as a determinant of vascular patterning during vasculogenesis (39, 40). The role of eNOS in flow-regulated, noninflammatory angiogenesis has been shown because the genetic loss of eNOS attenuated flow mediated increases in capillary to fiber ratio in certain muscle groups (41). Our data supports the importance of flow as an important regulator of ischemic arteriogenesis and angiogenesis, because we detected a subtle defect in blood flow after stimulation in nonischemic eNOS (-/-) mice, akin to endothelial dysfunction, and a grossly impaired ischemia initiated blood flow reserve capacity in ischemic eNOS (-/-) mice. We interpret these data as follows: after ischemic challenge, eNOS (-/-) mice lack the ability to redirect blood flow through preexisting collaterals (through impaired downstream vasodilation), thereby reducing shear dependent remodeling of collaterals and the attendant lower limb angiogenesis. The reduction in flow reduces shear stress-dependent eNOS activation and subsequent NO release, thus retarding further growth of the collaterals and flow-dependent angiogenesis and remodeling of angiogenic sprouts in the lower limb. At the same time, it is clear the eNOS (-/-) mice are defective in their responses to exogenous VEGF and SDF-1 (10, 11, 15), two cytokines implicated in ischemic remodeling, angiogenesis, and mobilization of bone marrow endothelial progenitor cells; however, in our hands, ischemia triggered mobilization of EPCs into peripheral blood were not different between wild-type and eNOS (-/-) mice. Moreover, we can partially correct defects in arteriogenesis, angiogenesis, and limb ischemia by adenoviral eNOS S1179D into eNOS (-/-) mice without affecting ischemia-triggered EPC mobilization. Considering that a majority of the adenovirally transduced cells are myocytes, we interpret the functional recovery after gene transfer to a bystander effect, i.e., NO diffusion from the site of synthesis to adjacent vasculature to improve flow reserve and shear-dependent remodeling and angiogenesis.
Collectively, our data strongly support the role of eNOS-derived NO as a critical gene in regulating many aspects of blood flow control and a second messenger for pathways leading arteriogenesis and stable angiogenesis. Considering the prevalence of endothelial dysfunction (manifested by impaired eNOS/NO bioavailability) associated with aging, cardiovascular diseases, and peripheral vascular insufficiency, our results imply that therapeutic approaches aimed at improving arteriogenesis in patients with peripheral vascular disease by using a single cytokine will have limited utility unless impairments in eNOS function are taken into account and corrected.
Supplementary Material
Acknowledgments
We thank Drs. Carsten Schorr and Kenneth Walsh (Boston University) for help in establishing peripheral EPC isolation and Dr. Al Sinusas (Yale University) for help with angiography. This work was funded by National Institutes of Health Grants R01 HL64793, R01 HL 61371, R01 HL 57665, and P01 HL 70295, and National Heart, Lung, and Blood Institute-Yale Proteomics Contract N01-HV-28186 (to W.C.S.).
Author contributions: K.K., G.M.R., H.S.Q., and W.C.S. designed research; J.Y., E.D.d., Z.Z., H.S.Q., T.M., and B.E. performed research; E.D.d., Z.Z., M.D., T.M., and B.E. contributed new reagents/analytic tools; J.Y., M.D., and W.C.S. analyzed data; and K.K., G.M.R., and W.C.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: eNOS, endothelial NOS; EPC, endothelium progenitor cell; PDGF, platelet-derived growth factor; SMA, anti-smooth muscle α actin; VEGF, vascular endothelial growth factor.
References
- 1.Heil, M. & Schaper, W. (2004) Circ. Res. 95, 449-458. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet, P., Moons, L., Luttun, A., Vincenti, V., Compernolle, V., De Mol, M., Wu, Y., Bono, F., Devy, L., Beck, H., et al. (2001) Nat. Med. 7, 575-583. [DOI] [PubMed] [Google Scholar]
- 3.Silvestre, J. S., Mallat, Z., Duriez, M., Tamarat, R., Bureau, M. F., Scherman, D., Duverger, N., Branellec, D., Tedgui, A. & Levy, B. I. (2000) Circ. Res. 87, 448-452. [DOI] [PubMed] [Google Scholar]
- 4.Sasaki, K., Murohara, T., Ikeda, H., Sugaya, T., Shimada, T., Shintani, S. & Imaizumi, T. (2002) J. Clin. Invest. 109, 603-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson, C., Sung, H. J., Lessner, S. M., Fini, M. E. & Galis, Z. S. (2004) Circ. Res. 94, 262-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata, R., Ouchi, N., Kihara, S., Sato, K., Funahashi, T. & Walsh, K. (2004) J. Biol. Chem. 279, 28670-28674. [DOI] [PubMed] [Google Scholar]
- 7.Sonveaux, P., Martinive, P., DeWever, J., Batova, Z., Daneau, G., Pelat, M., Ghisdal, P., Gregoire, V., Dessy, C., Balligand, J. L. & Feron, O. (2004) Circ. Res. 95, 154-161. [DOI] [PubMed] [Google Scholar]
- 8.Heil, M., Ziegelhoeffer, T., Wagner, S., Fernandez, B., Helisch, A., Martin, S., Tribulova, S., Kuziel, W. A., Bachmann, G. & Schaper, W. (2004) Circ. Res. 94, 671-677. [DOI] [PubMed] [Google Scholar]
- 9.Tang, G., Charo, D. N., Wang, R., Charo, I. F. & Messina, L. (2004) J. Vasc. Surg. 40, 786-795. [DOI] [PubMed] [Google Scholar]
- 10.Murohara, T., Asahara, T., Silver, M., Bauters, C., Masuda, H., Kalka, C., Kearney, M., Chen, D., Symes, J. F., Fishman, M. C., Huang, P. L. & Isner, J. M. (1998) J. Clin. Invest. 101, 2567-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aicher, A., Heeschen, C., Mildner-Rihm, C., Urbich, C., Ihling, C., Technau-Ihling, K., Zeiher, A. M. & Dimmeler, S. (2003) Nat. Med. 9, 1370-1376. [DOI] [PubMed] [Google Scholar]
- 12.Tamarat, R., Silvestre, J. S., Kubis, N., Benessiano, J., Duriez, M., deGasparo, M., Henrion, D. & Levy, B. I. (2002) Hypertension 39, 830-835. [DOI] [PubMed] [Google Scholar]
- 13.Sata, M., Nishimatsu, H., Suzuki, E., Sugiura, S., Yoshizumi, M., Ouchi, Y., Hirata, Y. & Nagai, R. (2001) FASEB J. 15, 2530-2532. [DOI] [PubMed] [Google Scholar]
- 14.Lee, E. W., Michalkiewicz, M., Kitlinska, J., Kalezic, I., Switalska, H., Yoo, P., Sangkharat, A., Ji, H., Li, L., Michalkiewicz, T., et al. (2003) J. Clin. Invest. 111, 1853-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiasa, K., Ishibashi, M., Ohtani, K., Inoue, S., Zhao, Q., Kitamoto, S., Sata, M., Ichiki, T., Takeshita, A. & Egashira, K. (2004) Circulation 109, 2454-2461. [DOI] [PubMed] [Google Scholar]
- 16.Fukumura, D., Gohongi, T., Kadambi, A., Izumi, Y., Ang, J., Yun, C. O., Buerk, D. G., Huang, P. L. & Jain, R. K. (2001) Proc. Natl. Acad. Sci. USA 98, 2604-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratton, J. P., Lin, M. I., Yu, J., Weiss, E. D., Jiang, Z. L., Fairchild, T. A., Iwakiri, Y., Groszmann, R., Claffey, K. P., Cheng, Y. C. & Sessa, W. C. (2003) Cancer Cell 4, 31-39. [DOI] [PubMed] [Google Scholar]
- 18.Couffinhal, T., Silver, M., Zheng, L. P., Kearney, M., Witzenbichler, B. & Isner, J. M. (1998) Am. J. Pathol. 152, 1667-1679. [PMC free article] [PubMed] [Google Scholar]
- 19.Dimmeler, S., Fleming, I., Fisslthaler, B., Hermann, C., Busse, R. & Zeiher, A. M. (1999) Nature 399, 601-605. [DOI] [PubMed] [Google Scholar]
- 20.Fulton, D., Gratton, J. P., McCabe, T. J., Fontana, J., Fujio, Y., Walsh, K., Franke, T. F., Papapetropoulos, A. & Sessa, W. C. (1999) Nature 399, 597-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCabe, T. J., Fulton, D., Roman, L. J. & Sessa, W. C. (2000) J. Biol. Chem. 275, 6123-6128. [DOI] [PubMed] [Google Scholar]
- 22.Gratton, J. P., Yu, J., Griffith, J. W., Babbitt, R. W., Scotland, R. S., Hickey, R., Giordano, F. J. & Sessa, W. C. (2003) Nat. Med. 9, 357-363. [DOI] [PubMed] [Google Scholar]
- 23.Scotland, R. S., Morales-Ruiz, M., Chen, Y., Yu, J., Rudic, R. D., Fulton, D., Gratton, J. P. & Sessa, W. C. (2002) Circ. Res. 90, 904-910. [DOI] [PubMed] [Google Scholar]
- 24.Akiyama, M., Eguchi, D., Weiler, D., O'Brien, T., Kovesdi, I., Scotland, R. S., Sessa, W. C. & Katusic, Z. S. (2002) Stroke 33, 1071-1076. [DOI] [PubMed] [Google Scholar]
- 25.Sessa, W. C., Pritchard, K., Seyedi, N., Wang, J. & Hintze, T. H. (1994) Circ. Res. 74, 349-353. [DOI] [PubMed] [Google Scholar]
- 26.Rudic, R. D. & Sessa, W. C. (1999) Am. J. Hum. Genet. 64, 673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziche, M., Morbidelli, L., Masini, E., Amerini, S., Granger, H. J., Maggi, C. A., Geppetti, P. & Ledda, F. (1994) J. Clin. Invest. 94, 2036-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziche, M., Morbidelli, L., Choudhuri, R., Zhang, H. T., Donnini, S., Granger, H. J. & Bicknell, R. (1997) J. Clin. Invest. 99, 2625-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papapetropoulos, A., Garcia-Cardena, G., Madri, J. A. & Sessa, W. C. (1997) J. Clin. Invest. 100, 3131-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurwitz, H., Fehrenbacher, L., Novotny, W., Cartwright, T., Hainsworth, J., Heim, W., Berlin, J., Baron, A., Griffing, S., Holmgren, E., et al. (2004) N. Engl. J. Med. 350, 2335-2342. [DOI] [PubMed] [Google Scholar]
- 31.Tsurumi, Y., Murohara, T., Krasinski, K., Chen, D., Witzenbichler, B., Kearney, M., Couffinhal, T. & Isner, J. M. (1997) Nat. Med. 3, 879-886. [DOI] [PubMed] [Google Scholar]
- 32.Kimura, H., Weisz, A., Ogura, T., Hitomi, Y., Kurashima, Y., Hashimoto, K., D'Acquisto, F., Makuuchi, M. & Esumi, H. (2001) J. Biol. Chem. 276, 2292-2298. [DOI] [PubMed] [Google Scholar]
- 33.Kimura, H., Weisz, A., Kurashima, Y., Hashimoto, K., Ogura, T., D'Acquisto, F., Addeo, R., Makuuchi, M. & Esumi, H. (2000) Blood 95, 189-197. [PubMed] [Google Scholar]
- 34.Mateo, J., Garcia-Lecea, M., Cadenas, S., Hernandez, C. & Moncada, S. (2003) Biochem. J. 376, 537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang, P. L., Huang, Z., Mashimo, H., Bloch, K. D., Moskowitz, M. A., Bevan, J. A. & Fishman, M. C. (1995) Nature 377, 239-242. [DOI] [PubMed] [Google Scholar]
- 36.Shesely, E. G., Maeda, N., Kim, H. S., Desai, K. M., Krege, J. H., Laubach, V. E., Sherman, P. A., Sessa, W. C. & Smithies, O. (1996) Proc. Natl. Acad. Sci. USA 93, 13176-13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudlicka, O. (1991) J. Physiol. 444, 1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudlicka, O. & Brown, M. D. (1996) J. Vasc. Res. 33, 266-287. [DOI] [PubMed] [Google Scholar]
- 39.le Noble, F., Moyon, D., Pardanaud, L., Yuan, L., Djonov, V., Matthijsen, R., Breant, C., Fleury, V. & Eichmann, A. (2004) Development (Cambridge, U.K.) 131, 361-375. [DOI] [PubMed] [Google Scholar]
- 40.le Noble, F., Fleury, V., Pries, A., Corvol, P., Eichmann, A. & Reneman, R. S. (2005) Cardiovasc. Res. 65, 619-628. [DOI] [PubMed] [Google Scholar]
- 41.Baum, O., Da Silva-Azevedo, L., Willerding, G., Wockel, A., Planitzer, G., Gossrau, R., Pries, A. R. & Zakrzewicz, A. (2004) Am. J. Physiol. 287, H2300-H2308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.