Abstract
Purpose
Patient-reported outcomes following head and neck cancer are of great importance given the functional, psychological, and social impacts of the disease and its treatment. With an increasing number of publications on HRQOL following head and neck cancer and a growing awareness of the potential role of HRQOL in practice, it was our aim to investigate head and neck functional mobility that is often not taking into account in HRQOL scores.
Methods
In this prospective study, three different groups of 32 patients each were included. Any patient who had histologically confirmed invasive OSCC in the anterior floor of the mouth was eligible. All patients were examined by a standardized test assessing function, including the distance of mouth opening, extension, flexion, and rotation of the head.
Results
A total of 96 patients were included in this study. The mean age was 62.79 ± 8.93 years. Head and neck mobility measured in patients is presented and analyzed. Compared to the baseline, a significant reduction of mouth opening and head and neck mobility was noted in all groups.
Conclusions
Although both treatment options (surgery and surgery with radiotherapy) were performed according to the tumor stage of patients, there are significant differences in the functional outcome of these patients as observed in this study. There is a lack of a measuring instrument that will be the “gold standard” in the assessment of head and neck functional mobility. This study will allow the reflection of our current practice and may stimulate further well-designed prospective studies.
Keywords: Mobility, Neck dissection, Radiation therapy, Osteoradionecrosis, Surgical resection, Oral cancer
Introduction
Radical surgical resection of oral cancer combined with or without radiation and chemotherapy is the established curative treatment for OSCC (Forastiere et al. 2003; Pignon et al. 2000; Shah and Gil 2009). Wakefield Goals of therapy are cure, organ preservation, restoration of form and function, reduction of the morbidities associated with therapy, and improvement or maintenance of quality of life. Nevertheless, the extent of surgical resection, the necessity for neck dissection, the resulting defect, the type of surgical reconstruction, and the application of radiation therapy all contribute to the patients’ postoperative functional outcome, depending on the extent of the tumor and its location in the oral cavity (Mücke et al. 2010).
Primary treatment aims to maximize tumor-free survival with the least noticeable impact of the patients’ quality of life. Although head and neck cancer is treated by a combination of ablative and reconstructive surgery, focused radiotherapy, and chemotherapy with evidence of improved survival with increasingly radical therapy, the oral, head and neck functions are impaired often exponentially. Oncologic combined with reconstructive surgery is able to reconstruct the resulting defects (Mücke et al. 2010), but the functional outcome depends on the amount of resected or adjacent remaining functional tissue, especially the mandible and tongue (Rogers et al. 2002). Radiotherapy causes xerostomia, tissue fibrosis, trismus, osteoradionecrosis, and radiation caries (Chambers et al. 2004; Mücke et al. 2011). Deterioration of swallowing is caused by a combination of all of the effective oncological therapies and has a negative effect on nutrition, speech, and therefore social rehabilitation of the patients. Clinical, physical, and sociodemographic factors also contribute to patients’ outcome after treatment and should be taken into the patients’ oncologic concept (Karvonen-Gutierrez et al. 2008). Although several questionnaires to assess the quality of life exist, an objective and reproducible assessment of the physiological function in the head and neck region is not available (Rogers et al. 2002; Tschiesner et al. 2011). For that reason, an objective functional status was developed and examination of different patients undergoing different treatments performed.
The aim of the present study was to objectively assess the postinterventional mobility in the head and neck region compared to preoperative examination results in relation to the performed oncologic treatment in patients with oral squamous cell carcinomas of the anterior floor of the mouth.
Patients and methods
A prospective study with three different groups of 32 patients each was performed. The patients were subdivided into patients 1. undergoing surgery for resection of OSCC (group 1), 2. undergoing surgery and adjuvant therapy (group 2), and 3. undergoing surgery and adjuvant therapy who developed osteoradionecrosis (group 3).
All patients were examined by a standardized test assessing function, including the distance of mouth opening, extension, flexion, and rotation of the head. Baseline assessment was performed at the time of histological confirmation of OSCC. In patients receiving surgery followed by postoperative radiation therapy, the functional assessment was performed 2 weeks after surgery and 24 months after completion of radiation therapy. In addition, all patients were asked whether they would be willing to undergo the procedure again if they had to. The patients were also asked which treatment modality, either operation or radiation therapy, was severely associated with increased impairment of subjectively assessed function in the head and neck region. The examination of patients and the questionnaire were performed at least 24 months after the end of tumor-related treatment.
Eligibility
Any patient who had histologically confirmed invasive OSCC in the anterior floor of the mouth was eligible. Only patients with cancer of the anterior floor of the mouth were included because otherwise the data would not be comparable. Computed tomography (CT) or magnetic resonance imaging (MRI) and endoscopy staging was carried out, and tumors staged T1-4, N1-3, and M0 were eligible. Exclusion criteria included previous radiotherapy or chemotherapy for previous malignancy. Patients with recurrent disease within 6 months after completion of treatment were excluded (Mücke et al. 2009).
Normal or near normal hematological and biochemical parameters were necessary for inclusion. A creatinine clearance of more than 1.0 ml/s calculated by the Gault and Cockcroft formula, or more than 0.83 ml/s by direct measurement, was required.
Postoperative radiation therapy
The radiotherapy technique has been described earlier (Mücke et al. 2011; Poulsen et al. 2001). The dose was prescribed and delivered according to the International Commission on Radiation Units and Measurement Report 50. Macroscopically, uninvolved neck node regions adjacent to the primary tumor or involved nodes received a total dose of 60 Gy and uninvolved nodes at low risk a total dose of 50 Gy, respectively. Patients received one fraction per day, five fractions per week for 7 weeks. The final dose was delivered to the tumor bed with ≥1.5 cm margin up to a total dose of 70 Gy.
Data analysis
Data for the study were prospectively collected in one department and analyzed. Descriptive statistics for quantitative variables are given as the mean ± SD. If appropriate, medians and ranges were also computed. Group-related data were compared by use of the t test. P values are two-sided and subject to a global significance level of 0.05.
The data were analyzed with the “Statistical Package for the Social Sciences” (SPSS for Windows, release 18.0.0, 2010, SPSS Inc, Chicago, IL, USA). Figures are generated with SPSS and Microsoft® Office Excel (Microsoft Excel for Windows, release 11.0, 2003, Microsoft Corporation, Redmond, WA, USA).
Results
A total of 96 patients were included in this study. The mean age was 62.79 ± 8.93 years (range 41–82). There were 58 men (60.4%) and 38 women (39.6%). Patients who underwent surgery all had immediate free flap reconstructions for comparability (Mücke et al. 2010). The patients who received postoperative radiation therapy received a mean dosage of 63.3 ± 7.9 Gy. The tumor stages of patients were equally distributed between the different groups receiving postoperative radiation therapy (P = 0.873). A total of 11 patients (11.4%) had a T1 stage, 24 patients had T2 (25%), 4 patients had T3 (4.2%), and 57 patients had T4 stage (59.4%). The nodal stage of patients was N0 in 35 patients (36.5%), N1 in 14 patients (14.6%), and N2 in 47 patients (48.9%). The grading was found to be G1 in 4 patients (4.2%), G2 in 63 patients (65.6%), and G3 in 29 patients (30.2%). All tumors were resected completely, but in 12 patients, the resection margin was <5 mm indicating postoperative radiation therapy. At the time of examination, all patients were tumor free and at least two CT or MRI were performed as part of postoperative aftercare.
Function of the head and neck
The results of the different functional examinations and percentage of changes after completion of therapy in comparison with the preoperative examination of patients are presented in Table 1.
Table 1.
Measurements of head and neck movement in absolute and relative values
| Baseline | Surgery only | Surgery and RTX | ORN after surgery and RTX | |
|---|---|---|---|---|
| Mouth opening (mm) | 45.7 ± 7.7 |
35.4 ± 7.1 (−22.5%) |
23.2 ± 9.4 (−49.2%) |
23.3 ± 8.8 (−49%) |
| Head flexion (degree) | 62.8° ± 3.9° |
56.9° ± 7.9° (−9.4%) |
48.6° ± 11.4° (−22.6%) |
47.8° ± 12.6° (−23.9%) |
| Head extension (degree) | 67.8° ± 3.9° |
59.8° ± 9.4° (−11.8%) |
42.3° ± 13.3° (−37.6%) |
51.9° ± 14° (−23.5%) |
| Head rotation (right) (degree) | 79.4° ± 3.8° |
71.7° ± 11° (−9.7%) |
50.3° ± 15.3° (−36.6%) |
58.3° ± 16.8° (−26.6%) |
| Head rotation (left) (degree) | 79.4° ± 4.1° |
71.7° ± 9.6° (−9.7%) |
50.5° ± 15° (−36.4%) |
58.6° ± 15.7° (−26.2%) |
The percentage reduction of mobility is given in brackets
RTX radiation therapy, ORN osteoradionecrosis of the mandible
Patients from group 1 had no changes of mouth opening in 14 cases (43.7%), 12 patients (37.5%) had a decrease of mouth opening of less than the half distance measured preoperatively, and 6 patients (18.8%) had a decrease of mouth opening more than the half compared with preoperative measurement. No patient was found to have a trismus defined as an opening of <5 mm.
For patients in group 2, the mouth opening after completion of surgery was normal in 3 cases (9.4%), those with opening of less than half the pretreatment value 14 (43.8%) and more than half in 14 patients (43.8%), and 1 patient had a trismus with less than 5-mm opening. The assessment after adjuvant radiation therapy in the same patients revealed 2 patients (6.3%) with normal mouth opening, 12 patients (37.5%) with a decrease of mouth opening less than half and 12 patients more than the half compared to the preoperative examination (37.5%), and 6 patients (18.7%) had a trismus.
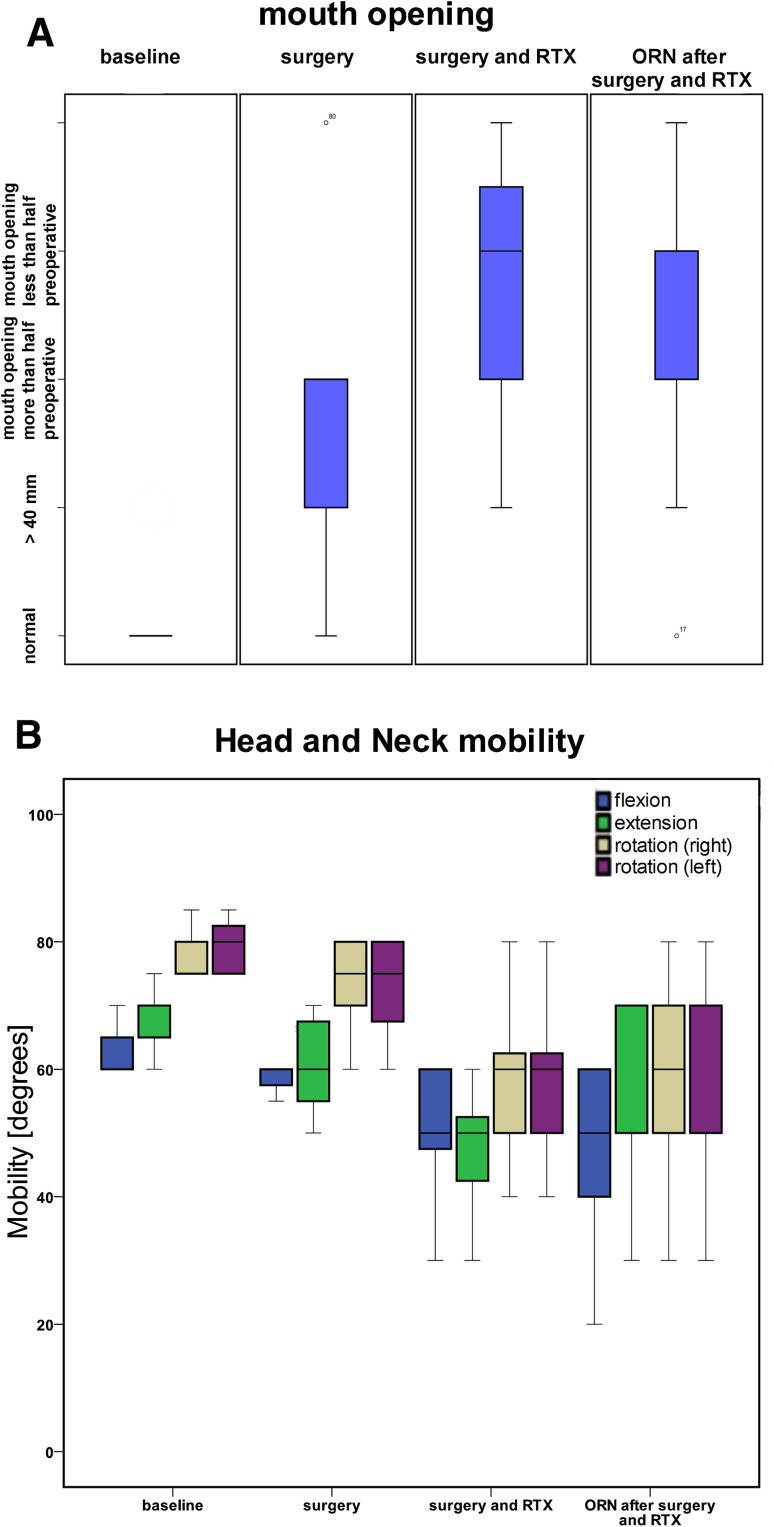
In patients from group 3, the mouth opening after completion of surgery was normal in 3 cases (9.4%), a decrease of mouth opening less than half the pretreatment value was found in 22 patients (68.8%), of more than half was found in 6 patients (18.8%), and 1 patient (3.1%) had a trismus. After adjuvant radiation therapy in the same patients, there was 1 patient (3.1%) with normal mouth opening, 15 patients (46.9%) with a decrease of mouth opening less than half and 10 patients (31.3%) more than half of mouth opening compared to the preoperative examination, and 6 patients (18.7%) had a trismus. One year after completion of therapy for osteoradionecrosis, 27 patients (84.4%) had a severe decreasing of mouth opening more than the half of the preoperative measurement. Only 1 patient (3.1%) had a nearly normal mouth opening of ≥40 mm, and 4 patients (12.5%) had trismus. The distribution of mouth opening is presented in Fig. 1a.
Fig. 1.
Boxplots illustrating the functional results of baseline values and values obtained after different type of treatments. a Mouth opening assessed in the different groups. b Head and neck mobility of patients indicated by flexion, extension, and rotation of the head
Head and neck mobility measured in patients is presented in Fig. 1b. Compared to the baseline, a significant reduction of mouth opening and head and neck mobility was noted in all groups (Table 2). Comparing the different groups with the different treatment modalities, a significant difference was detected between group 1 and group 2 and group 1 and group 3 (Table 3). Between group 2 and group 3, significances were noted in head extension and rotation only (Table 3).
Table 2.
Comparison of baseline values between the three different treatment groups
| P value | ||||
|---|---|---|---|---|
| Baseline | Surgery | Surgery and RTX | ORN after surgery and RTX | |
| Mouth opening | Reference | <0.0001* | <0.0001* | <0.0001* |
| Head flexion | Reference | 0.002* | <0.0001* | <0.0001* |
| Head extension | Reference | 0.001* | <0.0001* | <0.0001* |
| Head rotation | Reference | 0.003* | <0.0001* | <0.0001* |
| Head rotation | Reference | 0.001* | <0.0001* | <0.0001* |
RTX radiation therapy, ORN osteoradionecrosis of the mandible
* P value < 0.05
Table 3.
Comparison of values between the three different treatment groups
| Groups to be compared P value |
|||
|---|---|---|---|
| Surgery only versus surgery and RTX | Surgery only versus ORN after surgery and RTX | Surgery and RTX versus ORN after surgery and RTX | |
| Mouth opening | <0.0001* | <0.0001* | 0.987 |
| Head flexion | 0.004* | 0.002* | 0.809 |
| Head extension | <0.0001* | 0.014* | 0.007* |
| Head rotation | <0.0001* | 0.001* | 0.054 |
| Head rotation | <0.0001* | 0.001* | 0.040* |
RTX radiation therapy, ORN osteoradionecrosis of the mandible
* P value < 0.05
Patients’ assessment of therapy severity
The performances of surgery and radiation therapy in the two groups were assessed by the patients as presented in Table 4. Comparing both treatments, the patients of group 2 estimated the surgical treatment as more severe in 3 (9.4%) cases, whereas 19 patients (59.4%) felt more impaired by radiation therapy. Ten patients (31.3%) assessed both treatments equally. Patients of group 3 assessed the surgical therapy as more severe in 5 cases (15.6%), radiation therapy was felt to be more severe in 19 cases (59.4%), and 8 patients (15%) assessed both treatments equally. A significant difference between the assessed treatments was found (Table 4).
Table 4.
Questionnaire about willingness to repeat the treatment
| Group | Question | Willing to repeat | P value | ||
|---|---|---|---|---|---|
| Yes (%) | No (%) | Not sure (%) | |||
| Surgery and RTX | |||||
| Repetition of surgery | 19 (59.4) | 10 (31.3) | 3 (9.4) | <0.001* | |
| Repetition of RTX | 11 (34.4) | 18 (56.3) | 3 (9.4) | <0.001* | |
| ORN after surgery and RTX | |||||
| Repetition of surgery | 17 (53.1) | 9 (28.1) | 6 (18.8) | <0.001* | |
| Repetition of RTX | 17 (53.1) | 12 (37.5) | 3 (9.4) | <0.001* | |
Data are given as absolute numbers and percentages in brackets
* P value < 0.05
Discussion
To the best of our knowledge, this is the first study to objectively assess the different influence of treatment modalities on the head and neck mobility and mouth opening of patients undergoing different therapies for OSCC. The three studied groups were prospectively examined and had different results in overall functional outcome. Although both treatment options (surgery and surgery with radiotherapy) were performed according to the tumor stage of patients in common with most oncologic centers treating head and neck carcinomas (Shah and Gil 2009; Eich et al. 2008; Mohr et al. 1994; Wanebo et al. 1997; Wanebo et al. 2001), there are significant differences in the functional outcome of these patients as observed in this study. Adjuvant radiation therapy is generally performed over a longer time period with several adverse side effects as well described in the literature (Cooper et al. 2004; Airoldi et al. 2011). The effect of radiotherapy on the head and neck has been well investigated and becomes obvious in the clinical situation by scarring and tissue fibrosis accompanied by vessel depletion (Bengtson et al. 1993; Jones et al. 2007). These circumstances lead to a decrease in head and neck mobility and mouth opening, which are strongly correlated with the quality of life of patients, especially if the mandible is affected also (Chambers et al. 2004; Tschiesner et al. 2011; Airoldi et al. 2011; Mücke et al.2011). As already indicated in the present study, patients are seriously affected by the side effects of both therapy modalities, surgery and radiotherapy, as a high percentage of patients would be not willing to repeat the performed treatments (30 vs. 28 patients, Table 4). Interestingly, the majority of patients developing osteoradionecrosis, a severe complication of radiotherapy and a condition treated surgically, would be willing to repeat the treatments (34 vs. 21 patients, Table 4). This effect may be due to the long-lasting and difficult therapies for patients developing osteoradionecrosis, and their intense contact with the surgical and radiation units treating for cure of the condition. For that reason, these patients might be more able to process their disease and the performed treatments, although slightly better functional ability in the head and neck area was also noted for these patients compared to group 2 (Tables 1, 3) which explain their assessments.
Preexisting knowledge of likely post-treatment problems might play an important role. If information for patients on their oral abilities after oncologic interventions is given pretreatment in appropriate fashion, this information may be used to inform patients about their disabilities and improve the process of care. The present results suggest that patients are less informed about the role of radiation therapy and the effects on their functional outcome in the head and neck region. In contrast, surgical therapies are supposed to be more “radical” and are thought to influence functional outcome severely, even with reconstruction. This is part of the “organ preservation” concept of radical, curative-intent non-surgical oncological treatment. Explanation of toxic side effects and especially long-term effects of radiation therapy might address these differences of assessment between both groups.
The present study also confirms findings supporting the role of quality of life and functional outcome of patients with head and neck cancer (Karvonen-Gutierrez et al. 2008; Tschiesner et al. 2011). A study by Karvonen-Gutierrez et al. (2008) found that patients with good general physical health, absence of pain, ability to eat, and speech were highly associated with survival. Pain has been shown to be predictive particularly for cancer risk and survival (Karvonen-Gutierrez et al. 2008; Macfarlane et al. 2001; McBeth et al. 2003). Efforts to reduce oral pain and increase functional ability may have implications for overall welfare. Eating problems resulting in weight loss is known to increase morbidity and mortality, (Airoldi et al. 2011; van der van Bokhorst-de et al. 1999) whereas speech problems can result in social isolation and depression with an impact on general health and outcome (Karvonen-Gutierrez et al. 2008; Airoldi et al. 2011; Macfarlane et al. 2001; Hassanein et al. 2001). These factors are critically assessed by the patients and play a important role for planning complex tumor therapies (Mücke et al. 2010; Chambers et al. 2004; Tschiesner et al. 2011; Airoldi et al. 2011).
The cohort of patients observed in this study is subject to some limitations. The patients were examined shortly after surgical treatment and at least 24 months (range 24–28) after completion of treatments. The literature suggests differences in functional outcome after about 5 years because problems related to radiotherapy, like osteoradionecrosis, tooth decay, and dry mouth are aggravated over time (Tschiesner et al. 2011; Nordgren et al. 2008). In contrast, surgical reconstructions are assessed more positively by patients after about 6–12 months after reconstruction, probably due to improved swallowing and adaption of patients to the restoration of functions by the surgical procedure (Dassonville et al. 2008; Wong and Wei 2010). It is planned to perform further prospective studies to investigate the influence of physiological therapies for patients undergoing surgical and radiation therapies to try to improve oral function, the overall mobility in the head and neck area, and the overall quality of life.
Conflict of interest
None.
References
- Airoldi M, Garzaro M, Raimondo L, Pecorari G, Giordano C, Varetto A et al (2011) Functional and psychological evaluation after flap reconstruction plus radiotherapy in oral cancer. Head Neck 33(4):458–468 [DOI] [PubMed] [Google Scholar]
- Bengtson BP, Schusterman MA, Baldwin BJ, Miller MJ, Reece GP, Kroll SS et al (1993) Influence of prior radiotherapy on the development of postoperative complications and success of free tissue transfers in head and neck cancer reconstruction. Am J Surg 166(4):326–330 [DOI] [PubMed] [Google Scholar]
- Chambers MS, Garden AS, Kies MS, Martin JW (2004) Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management. Head Neck 26(9):796–807 [DOI] [PubMed] [Google Scholar]
- Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB et al (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350(19):1937–1944 [DOI] [PubMed] [Google Scholar]
- Dassonville O, Poissonnet G, Chamorey E, Vallicioni J, Demard F, Santini J et al (2008) Head and neck reconstruction with free flaps: a report on 213 cases. Eur Arch Otorhinolaryngol 265(1):85–95 [DOI] [PubMed] [Google Scholar]
- Eich HT, Loschcke M, Scheer M, Kocher M, Bongartz R, Wacker S et al (2008) Neoadjuvant radiochemotherapy and radical resection for advanced squamous cell carcinoma of the oral cavity. Outcome of 134 patients. Strahlenther Onkol 184(1):23–29 [DOI] [PubMed] [Google Scholar]
- Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W et al (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349(22):2091–2098 [DOI] [PubMed] [Google Scholar]
- Hassanein KA, Musgrove BT, Bradbury E (2001) Functional status of patients with oral cancer and its relation to style of coping, social support and psychological status. Br J Oral Maxillofac Surg 39(5):340–345 [DOI] [PubMed] [Google Scholar]
- Jones NF, Jarrahy R, Song JI, Kaufman MR, Markowitz B (2007) Postoperative medical complications–not microsurgical complications–negatively influence the morbidity, mortality, and true costs after microsurgical reconstruction for head and neck cancer. Plast Reconstr Surg 119(7):2053–2060 [DOI] [PubMed] [Google Scholar]
- Karvonen-Gutierrez CA, Ronis DL, Fowler KE, Terrell JE, Gruber SB, Duffy SA (2008) Quality of life scores predict survival among patients with head and neck cancer. J Clin Oncol 26(16):2754–2760 [DOI] [PubMed] [Google Scholar]
- Macfarlane GJ, McBeth J, Silman AJ (2001) Widespread body pain and mortality: prospective population based study. BMJ 323(7314):662–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBeth J, Silman AJ, Macfarlane GJ (2003) Association of widespread body pain with an increased risk of cancer and reduced cancer survival: a prospective, population-based study. Arthritis Rheum 48(6):1686–1692 [DOI] [PubMed] [Google Scholar]
- Mohr C, Bohndorf W, Carstens J, Harle F, Hausamen JE, Hirche H et al (1994) Preoperative radiochemotherapy and radical surgery in comparison with radical surgery alone. A prospective, multicentric, randomized DOSAK study of advanced squamous cell carcinoma of the oral cavity and the oropharynx (a 3-year follow-up). Int J Oral Maxillofac Surg 23(3):140–148 [DOI] [PubMed] [Google Scholar]
- Mücke T, Wagenpfeil S, Kesting MR, Hölzle F, Wolff KD (2009) Recurrence interval affects survival after local relapse of oral cancer. Oral Oncol 45(8):687–691 [DOI] [PubMed] [Google Scholar]
- Mücke T, Wolff KD, Wagenpfeil S, Mitchell DA, Hölzle F (2010) Immediate microsurgical reconstruction after tumor ablation predicts survival among patients with head and neck carcinoma. Ann Surg Oncol 17(1):287–295 [DOI] [PubMed] [Google Scholar]
- Mücke T, Konen M, Wagenpfeil S, Kesting MR, Wolff KD, Hölzle F (2011a) Low-dose preoperative chemoradiation therapy compared with surgery alone with or without postoperative radiotherapy in patients with head and neck carcinoma. Ann Surg Oncol 18:2739–2747 [DOI] [PubMed] [Google Scholar]
- Mücke T, Hölzle F, Wagenpfeil S, Wolff KD, Kesting M (2011b) The role of tumor invasion into the mandible of oral squamous cell carcinoma. J Cancer Res Clin Oncol 137(1):165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordgren M, Hammerlid E, Bjordal K, Ahlner-Elmqvist M, Boysen M, Jannert M (2008) Quality of life in oral carcinoma: a 5-year prospective study. Head Neck 30(4):461–470 [DOI] [PubMed] [Google Scholar]
- Pignon JP, Bourhis J, Domenge C, Designe L (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 355(9208):949–955 [PubMed] [Google Scholar]
- Poulsen MG, Denham JW, Peters LJ, Lamb DS, Spry NA, Hindley A et al (2001) A randomised trial of accelerated and conventional radiotherapy for stage III and IV squamous carcinoma of the head and neck: a Trans-Tasman Radiation Oncology Group Study. Radiother Oncol 60(2):113–122 [DOI] [PubMed] [Google Scholar]
- Rogers SN, Lowe D, Patel M, Brown JS, Vaughan ED (2002) Clinical function after primary surgery for oral and oropharyngeal cancer: an 11-item examination. B J Oral Maxillofac Surg 40(1):1–10 [DOI] [PubMed] [Google Scholar]
- Shah JP, Gil Z (2009) Current concepts in management of oral cancer–surgery. Oral Oncol 45(4–5):394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschiesner U, Schuster L, Strieth S, Harreus U (2011) Functional outcome in patients with advanced head and neck cancer: surgery and reconstruction with free flaps versus primary radiochemotherapy. Eur Arch Otorhinolaryngol. doi:10.1007/s00405-011-1642-7 [DOI] [PubMed]
- van der van Bokhorst-de S, van Leeuwen PA, Kuik DJ, Klop WM, Snow GB et al (1999) The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer 86(3):519–527 [PubMed] [Google Scholar]
- Wanebo HJ, Chougule P, Akerley WL 3rd, Koness RJ, McRae R, Nigri P et al (1997) Preoperative chemoradiation coupled with aggressive resection as needed ensures near total control in advanced head and neck cancer. Am J Surg 174(5):518–522 [DOI] [PubMed] [Google Scholar]
- Wanebo H, Chougule P, Ready N, Safran H, Ackerley W, Koness RJ et al (2001) Surgical resection is necessary to maximize tumor control in function-preserving, aggressive chemoradiation protocols for advanced squamous cancer of the head and neck (stage III and IV). Ann Surg Oncol 8(8):644–650 [DOI] [PubMed] [Google Scholar]
- Wong CH, Wei FC (2010) Microsurgical free flap in head and neck reconstruction. Head Neck 32(9):1236–1245 [DOI] [PubMed] [Google Scholar]