Abstract
Resident macrophages (i.e., Kupffer cells) are derived from hematopoietic stem cells (HSCs) and are primarily responsible for the removal from plasma of oxidized forms of low-density lipoprotein (LDL). The therapeutic potential of Kupffer cell expression of a transgene encoding paraoxonase-1 (PON1), whose plasma activity correlates with the protection from atherosclerosis, was examined in mice rendered atherosclerosis-susceptible through genetic deletion of the LDL receptor. Mice having their bone marrow engrafted with HSCs expressing the PON1 transgene (PON1-Tg) driven by a macrophage-specific promoter were injected i.v. with saline (vehicle only) or with gadolinium chloride (GdCl3), an agent that rapidly causes Kupffer cell apoptosis. One month later, GdCl3-facilitated Kupffer cell apoptosis increased the hepatic expression of transgenic PON1 mRNA by 9-fold. After 12 weeks of being fed a cholesterol-enriched atherogenic diet, mice injected with GdCl3 exhibited 50% reductions in both aortic sinus atherosclerotic lesions (P < 0.0097) and surface lesions of the abdominal aorta (P < 0.006). In contrast, mice receiving HSCs expressing the PON1-Tg but not treated with GdCl3 showed no protection from atherosclerosis. In addition, mice engrafted with HSCs not expressing the PON1-Tg but injected with GdCl3 also showed no protection from atherosclerosis. These findings, showing that GdCl3-enhanced hepatic expression of the PON1-Tg is essential for reducing atherosclerosis, indicate that Kupffer cells play an important role in atherogenesis. GdCl3-facilated replacement of Kupffer cells may enhance the efficacy of other HSC-based gene therapies.
Keywords: gene therapy, liver, stem cells
Hematopoietic stem cells (HSCs) are attractive vehicles for the delivery of therapeutic genes to different tissues to ameliorate human disease (1-3). Because of its large size and unrestricted contact with blood (4), the liver is a key tissue target for gene therapy (5). Recent studies suggest that engraftment of hepatic parenchymal cells via HSCs occurs rarely, if at all (6). Our studies were directed to develop an alternative approach to rapidly deliver therapeutic transgenes to the liver using HSCs.
Quiescent phagocytic resident macrophages (i.e., Kupffer cells) are derived from bone marrow HSCs and make up ≈10-20% of the cells in liver (7, 8). The rate of replacement of Moma-2-positive Kupffer cells from engrafted HSCs in mice is relatively slow (several months) (9). We examined whether the rate of replacement of liver resident macrophages derived from progenitor HSCs could be enhanced sufficiently to provide a more effective delivery of therapeutic transgenes.
Gadolinium chloride (GdCl3) is an imaging contrast agent that is rapidly taken up mainly by liver resident macrophages (Kupffer cells) (10). The uptake of GdCl3 by Kupffer cells induces apoptosis followed by a rapid replacement (within a few days) from HSCs (10). Circulating and spleen macrophages are relatively resistant to GdCl3-induced apoptosis (10).
To test the hypothesis that GdCl3 would enhance the rapid expression of a therapeutic transgene by Kupffer cells and ameliorate disease, we chose to quantify atherosclerotic lesion formation in a mouse model of homozygous familial hypercholesterolemia (HFH) (11). HFH mice lack low-density lipoprotein (LDL) receptors and exhibit a severe diet-induced hypercholesterolemia that results in the formation of atherogenic oxidatively modified LDL (12), xanthomatosis, and vascular atherosclerotic lesions (11). The only effective long-term therapy for most HFH patients is liver transplantation (13). Kupffer cells are a major site for the uptake and removal from plasma of oxidatively modified forms of LDL (14, 15). Oxidatively modified forms of LDL also can be metabolized and their atherogenicity inactivated by paraoxonase-1 (PON1), an enzyme produced in liver parenchymal cells and carried in plasma on high-density lipoprotein (16-22). Human and animal studies have established inverse relationships between the plasma activity of PON1 and myocardial infarction (20-22) or atherosclerotic lesion formation (16-19, 23, 24). Thus, it is reasonable to propose that expression of PON1 by Kupffer cells, the cellular site of oxidized LDL uptake, might decrease atherosclerotic lesion formation.
Materials and Methods
All experiments were performed in compliance with the relevant laws and institutional guidelines of the Institutional Animal Care and Use Committees at San Diego State University, University of California, San Diego, and The Scripps Research Institute after review and approval of detailed animal experimental protocols. Based on preliminary studies showing that Helicobacter infection of our mouse colony affected the expression of some liver genes and atherosclerosis lesion size, all mice used in these studies were Helicobacter-free (using PCR).
PON1 Transgenic Mice. The coding region of mouse PON1 was inserted into the HindIII and EcoRV sites of the polylinker in the plasmid Fxba-A1, which contains the macrophage-specific human acetyl-LDL receptor promoter and the human growth hormone polyadenylation signal (25). Both strands of the resulting plasmid (pMacPON1) were sequenced.
PON1 transgene (PON1-Tg) mice were made by injecting the excised purified PON1-Tg into C57BL/6 embryos. Genomic DNA obtained from the tail of pups was screened for the presence of the PON1-Tg. Mice having the PON1-Tg and nontransgenic littermates were bred with C57BL/6 mice expressing GFP driven by the β-actin promoter (i.e., C57BL/6-TgN ACTbEGFP) 1Osb mice (26) obtained from The Jackson Laboratory.
Bone Marrow Transplantation. Six- to eight-week-old male C57BL/6 LDL receptor knockout mice (11) (obtained from The Jackson Laboratory) were given 1,000 rads of total body irradiation and then injected (tail vein) with 2 × 106 bone marrow HSCs (27). Mice were fed with a standard chow diet (No. 5015, Harlan Teklad, Madison, WI) for 4 weeks. Mice then received an injection (tail vein) of either saline (control) or saline containing GdCl3 (25 mg/kg) and were fed the standard chow diet for an additional 4 weeks, after which they were fed an atherogenic diet (1.25% cholesterol, 6% fat, TD96335, Harlan Teklad).
Immunohistochemistry. Frozen liver tissue embedded in OCT compound was sectioned at a thickness of 10 μm on a Leica CM1850 cryostat. Slides with liver sections were fixed for 2 min in ice-cold acetone (-20°C) and then rehydrated for 2 min in PBS. Liver tissue was blocked for 30 min at room temperature with 10% goat serum in blocking buffer (1% BSA/0.15% Triton X-100 in PBS, pH 7.4). Fc receptors on Kupffer cells were blocked further with purified rabbit IgG (Vector Laboratories) at 0.5 mg/ml in blocking buffer for 2 h at room temperature. Tissue sections were washed with PBS and incubated at room temperature for 2 h with a rat anti-mouse Moma-2 (Serotec) antibody applied at a 1:200 dilution in blocking buffer. Sections were washed with PBS and incubated for 1 h with a 1:30 dilution of goat anti-rat IgG antibody conjugated to rhodamine (Calbiochem). Sections were washed in PBS, and 25 μl of Vectashield Mounting Medium (Vector Laboratories) was applied. Liver sections were viewed by using a Leica Confocal Microscope to view colocalization of rhodamine (indicative of macrophages) and GFP fluorescence.
Plasma Lipids and PON1 Activity. After an overnight fast (14 h), mice were anesthetized with isofluorane. Blood was collected by retroorbital puncture. The lipids contained in individual lipoprotein fractions were quantitated by standard colorimetric assay (28). Paraoxonase activity was determined as described (29, 30).
Macrophage Isolation and Characterization. Four days after mice received an i.p. injection of 1 ml of 4% thioglycollate, peritoneal macrophages were isolated, washed with 20 ml of ice-cold sterile PBS, resuspended in 10 ml of 10% FBS DMEM, and plated onto 100-mm culture dishes. After 6 h, adherent macrophages were used for the following experiments.
Total RNA was isolated from attached macrophages by using the RNeasy Minikit from Qiagen, Valencia, CA. cDNA was made from 2 μg of RNA (random primers) by using the Superscript II RT kit (Invitrogen).
Bone marrow cells were extracted from the femurs and tibias of male mice 4-6 weeks of age, both PON1-transgenic and control nontransgenic siblings. Cells were plated overnight in RPMI medium 1640 with l-glutamine, 10% FBS, and 30% L-cell conditioned media (31). Nonadherent cells were removed at 24 h, counted, and plated at a density of 500,000 cells/ml with 10 ml per 100-mm plate. After 5 days of culture in RPMI medium 1640 with l-glutamine, 10% FBS, and 30% L-cell conditioned media, adherent cells were harvested with a cell lifter and replated in a six-well plate at a density of 500,000 cells/ml, 1.5 ml/well. L-cell media were removed 24 h later, and the cells were cultured for 24 h in RPMI medium 1640 with l-glutamine and 10% FBS. The media were removed and replaced with 1.5 ml of RPMI medium 1640 with l-glutamine and 10% FBS or 1.5 ml of RPMI medium 1640 with l-glutamine, 10% FBS, and 2 μg/ml LPS.
RT-PCR was performed on the total RNA by using random hexamers, and the cDNA was used for real-time Taqman PCR. Amplification was done in an Applied Biosystems 7700 with the conditions 95°C for 10 min, then 40 cycles of 95°C for 15 sec, 55°C for 1 min. PCR primers for SRA, CD-36, and GAPDH are as described (32). PCR primers for IL-1β were (5′ to 3′): AGG CAG GCA GTA TCA CTC ATT GT (forward), GGA AGG TCC ACG GGA AAG A (reverse), and TGT GGA GAA GCT GTG GCA GCT ACC TGT (probe); PCR primers for IL-12 were (5′ to 3′): AAG GTG CGT TCC TCG TAG AGA A (forward), GAG CTT GCA CGC AGA CAT TC (reverse), and CAT CTA CCG AAG TCC AAT GCA AAG GCG (probe). Samples were run in triplicate and normalized against GAPDH.
Real-Time PCR Analysis of Liver cDNA. SyBr green real-time PCR analysis for the PON1-Tg and the endogenous mouse PON1 was performed on an IQ-Cycler (Bio-Rad). PON1-Tg PCR conditions were 95°C for 3 min, followed by 40 cycles of 95°C for 10 sec, 61°C for 20 sec, and 72°C for 20 sec. Mouse PON1 PCR conditions were 95°C for 3 min, followed by 40 cycles of 95°C for 10 sec, 57°C for 45 sec, and 72°C for 30 sec. GAPDH PCR conditions were 95°C for 3 min, followed by 40 cycles of 95°C for 10 sec, 55°C for 1 min. All PCR analyses were run in triplicate with a standard curve and melt curve analysis. Data were normalized to the internal GAPDH control. PCR primers for PON1-Tg were (5′-3′): ATA TCT CTA GAC CGC GGG GA (forward), GGG TGT CGG AAT AGA CTC TG (reverse); PCR primers for mouse PON1 were: GAT TGG CAC TGT GTT CCA C (forward), ATC ACT GTG GTA GGC ACC TT (reverse). PCR primers for GAPDH were: GTG TCC GTC GTG GAT CTG A (forward), CCT GCT TCA CCA CCT TCT TG (reverse).
Plasma Lipids. Plasma was fractionated into individual lipoprotein subclasses by size-exclusion FPLC (33). An aliquot of each fraction was used to measure protein (Bradford assay) and cholesterol. Plasma levels of very LDL, intermediate density lipoprotein, and LDL (together) and high-density lipoprotein cholesterol were determined by differential precipitation (33). Cholesterol quantitation was achieved by using the commercially available Infinity Cholesterol Reagent Kits (Thermo Electron, San Jose, CA).
FACS Analysis. FACS was performed according to the protocol described by Pharmigen. Briefly, 1 μl of FcBlock (Pharmigen no. 01241D), 1 μl of anti-Gr-1(CD-11b) (Pharmigen no. 553129), and 1 μl of anti-Mac3 (Pharmigen no. 553324) were added to 100 μl of whole blood, mixed, and incubated for 30 min on ice in the dark. The mixture was then washed with 2% FBS in PBS, and the pellet was incubated with Pharmlyse Ammonium Chloride Lysing Reagent (Pharmigen no. 555899) in the dark for 30 min. The pellet was washed twice with PBS and resuspended in 1 ml of 2% FBS in PBS for FACS analysis.
Analysis of Atherosclerosis. The heart was dissected out, cleaned of fascia, and stored at -80°C. OCT compound-embedded hearts were sectioned in a cryostat until all three leaflets were visible within the aortic valve. From this point, 10-μm sections were collected for the next 300 μm of the valve region, and each section was collected on a Superfrost slide (Fisher Scientific). The lipid-rich lesions were visualized by staining the sections with oil red O followed by counterstaining with hematoxylin (27). A total of four sections taken every 40 μm were used to quantitate individual mouse lesion areas by using a computer-assisted video imaging system. The lesion areas of the four sections were used to calculate the mean lesion area per mouse.
After excision of the heart, the aorta was stripped of its adventitial fat and dissected out from the right common carotid artery to the superior mesenteric artery. The aortas were opened longitudinally from the common iliac arteries to the aortic valve and subsequently detached and pinned flat onto the smooth black wax surface of a dissecting pan and stained with Sudan IV. The percent surface area stained red and indicative of fatty lesions was calculated by using a computer-assisted video imaging system.
Statistical Analysis. Statistical analysis of atherosclerotic lesion areas was performed by using a Mann-Whitney unpaired test. All other statistical analyses between the groups of mice were performed by using Student's t test (double tailed, unpaired).
Results
Development and Characterization of HSCs Expressing a Macrophage-Specific Transgene. To use HSCs as vehicles for transgenic expression by Kupffer cells, we developed mice that express a mouse PON1-Tg driven by a macrophage-specific promoter element (25). This transgene was constructed and used to produce PON1 transgenic C57BL/6 mice (i.e., PON1-Tg mice). Analysis of mRNA from PON1 transgenic C57BL/6 mice showed that the PON1-Tg was expressed only in circulating monocyte/macrophages and tissues containing resident macrophages (i.e., liver, lung, spleen, but not in heart, kidney, brain, or skeletal muscle; data not shown). Thus, expression of the PON1-Tg exhibited the expected macrophage specificity (25). PON1-Tg mice displayed no detectable abnormalities: body weight gain, size of litters, longevity, and gross appearance were indistinguishable from nontransgenic littermates.
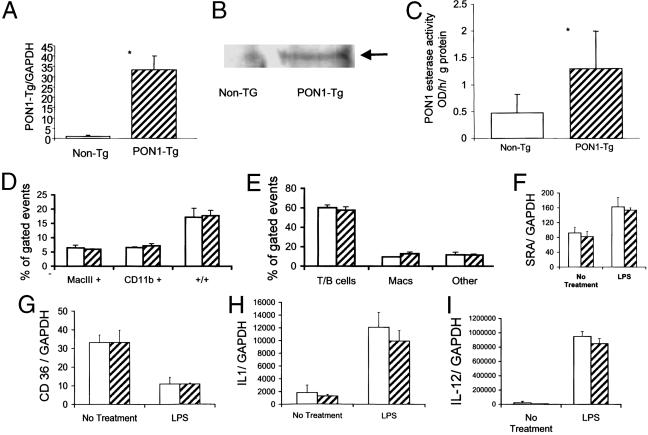
The functional characteristics of the PON1-Tg were examined by using peritoneal macrophages obtained from PON1-Tg and nontransgenic mice 4 days after thioglycolate treatment. Peritoneal macrophages obtained from the PON1 transgenic mice expressed PON1 mRNA (Fig. 1A), protein (Fig. 1B), and enzyme activity (Fig. 1C), whereas macrophages from nontransgenic littermates exhibited no detectable expression of transgenic PON1 mRNA (Fig. 1A), no immunodetectable PON1 protein (Fig. 1B), and ≈60% less esterase activity (Fig. 1C). The level of transgenic PON1 mRNA expressed by thioglycolate-elicited peritoneal macrophages was ≈10% of the level of endogenous PON1 mRNA expressed by liver (comparison made relative to GAPDH by using Northern blot analyses).
Fig. 1.
Characterization of PON1 transgenic mice. (A) Expression of the PON1-Tg in peritoneal macrophages. C57BL/6 mice expressing a transgene constructed from the cDNA encoding PON1 driven by the macrophage-specific promoter elements obtained from the human SRA gene (25) or their nontransgenic littermates were injected i.p. with 1 ml of 4% thioglycolate. Four days later, abdominal macrophages were obtained (25) by flushing the peritoneum with PBS and plating the cells onto plastic culture dishes. Cells adhering to plastic culture dishes after 6 h were harvested, and mRNA was extracted and used to make cDNA. Transgenic PON1 mRNA relative to GAPDH was quantitated by SyBr green real-time PCR. Each value represents the mean ± SD of six separate plates of cells. *, Significant differences, P < 0.001. (B) Presence of immunoreactive PON1 protein. Macrophages were homogenized and separated into microsomes (100,000 × g pellet) and supernatant. Western blotting of cell membrane fraction (100,000 × g pellet) separated by SDS/PAGE by using antibody specific for mouse PON1 (39) showed an immunoreactive band having the same molecular weight as mouse PON1 in plasma (arrow). No PON1 immunoreactivity was present in the 100,000 × g supernatant. (C) PON1 enzyme activity by using phenylacetate as the substrate and the membrane fraction of peritoneal macrophages. Each value represents the mean ± SD of six separate plates of cells. *, Significant differences, P < 0.02. No PON1 enzyme activity was present in the 100,000 × g supernatant. (D and E) FACS analysis of peripheral blood cells. Blood was collected through retroorbital eye bleed from four PON transgenic mice (hatched bars) and four nontransgenic littermates (open bars). The red blood cells were lysed, and the remaining cells were incubated with APC-mac3 and PE-CD11b mouse antibodies. The cells were quantitated by FACS analysis. Data were derived from 20,000 gated events. (D) Analysis obtained from cells separated by fluorescent markers for: Mac3 (macrophages and not lymphocytes), CD11b antigen (mature and immature macrophages and granulocytes), and +/+ cells positive for both markers. (E) Separation of cells based on size and granularity; “others” are cells in blood that do not fit into the gates denoted for T/B cells or macrophages based on size and granularity. (F-I) Response of bone marrow-derived monocyte/macrophages to LPS. Bone marrow cells were extracted from the femurs and tibias of PON1-Tg and nontransgenic littermates. Cells were cultured in medium containing 10% FBS and 30% L-cell conditioned media. Differentiated monocytes were replated in medium containing 10% FBS with and without 2 mg/ml LPS. Cells were harvested 24 h later, and the indicated mRNAs were quantitated by SyBr real-time PCR. Each value represents the mean ± SD of six individual plates of cells.
Essentially all of the PON1 protein and esterase activity detected in peritoneal macrophages obtained from PON1-Tg mice was retained in the 100,000 × g membrane pellet, and none was in the supernatant (Fig. 1 B and C). These findings are consistent with previous studies showing that PON1 expressed by Chinese hamster ovary cells is retained within the plasma membrane and inefficiently secreted (30). The lack of cleavage of the N-terminal signal sequence of endogenous PON1 may be responsible for its inefficient secretion (34-36). Additional studies showed that after an 18-h incubation of peritoneal macrophages obtained from PON1-Tg mice, no PON1 protein or enzyme activity was secreted (i.e., detected in culture medium).
The expression of the PON1-Tg did not affect circulating levels of individual types of leukocytes (Fig. 1 D and E). Furthermore, the presence of the PON1-Tg did not affect the ability of bone marrow HSCs to differentiate into monocyte/macrophages ex vivo in culture medium (bone marrow from both groups of mice yielded identical numbers of differentiated monocyte/macrophages (Fig. 1 F-I). In addition, bone marrow-derived monocyte/macrophages obtained from control and PON1 transgenic mice expressed similar levels of mRNA encoding SRA, CD36, IL-1, and IL-12 both before and after stimulation with LPS (Fig. 1 F-I). These data show that the expression of the PON1-Tg did not impair HSC differentiation or the general monocyte/macrophage function.
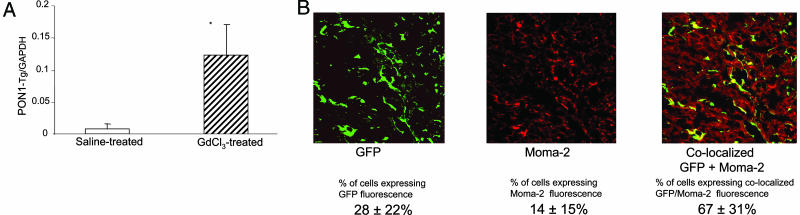
Engraftment and Expression of Kupffer Cells Expressing PON1-Tg. To have a fluorescent marker for engrafted HSCs, mice expressing the PON1-Tg were bred with mice expressing a GFP transgene driven by the β-actin promoter [i.e., C57BL/6-TgN (ACTbEGFP) 1Osb mice (26)]. Bone marrow from mice expressing both the GFP marker transgene and the macrophage-specific PON1-Tg was engrafted into lethally irradiated LDL-receptor-deficient mice. One month later, mice were injected with either saline (control) or GdCl3 (25 mg/kg in saline). Livers from mice killed 4 weeks after treatment with GdCl3 contained markedly more (i.e., 9-fold, P < 0.02) PON1-Tg mRNA compared with the livers injected with saline only (Fig. 2A). Confocal fluorescence microscopy of serial sections obtained from GdCl3-treated mice stained with a rhodamine-conjugated macrophage-specific antibody Moma-2 (37) showed that the majority (67 ± 31%) (Fig. 2B) of hepatic macrophages (presence of Moma-2 antigen) displayed GFP fluorescence and thus were derived from engrafted HSCs (expressing the PON1-Tg and GFP transgene). The 9-fold increase in the expression of the PON1-Tg mRNA indicates that GdCl3-facilitated apoptosis markedly accelerated the rate of Kupffer cell replacement and liver expression of the PON1-Tg.
Fig. 2.
Effect of GdCl3 on hepatic engraftment of macrophages derived from HSCs. Lethally irradiated LDL receptor-deficient mice (11) were injected with bone marrow HSCs expressing the PON1-Tg and GFP [derived from C57BL/6-TgN(ACTbEGFP) 1Osb mice (40)]. After 14 days of recovery, mice were injected with either GdCl3 (25 mg/kg in saline) or saline only and killed after 4 weeks. (A) Hepatic expression of transgenic PON1 mRNA relative to GAPDH. Livers from mice treated without (saline control, open bars) or with GdCl3 (hatched bars) obtained 4 weeks after treatment were used to make cDNA. The hepatic content of transgenic PON1 mRNA relative to GAPDH was quantitated by SyBr green real-time PCR. Each value represents the mean ± SD of six separate mice per group. *, Significant differences, P < 0.02. (B) Confocal fluorescent microscopic colocalization of GFP (green) and the macrophage Moma-2 antigen (detected with rhodamine red-conjugated antibody) in frozen thin sections of liver from mice 4 weeks after treatment with GdCl3. Six serial sections from three separate mice per group were used to estimate fluorescence attributed to GFP (green), Moma-2 (red), and colocalization of GFP and Moma-2 (yellow) and are indicated ± SD below A and B.
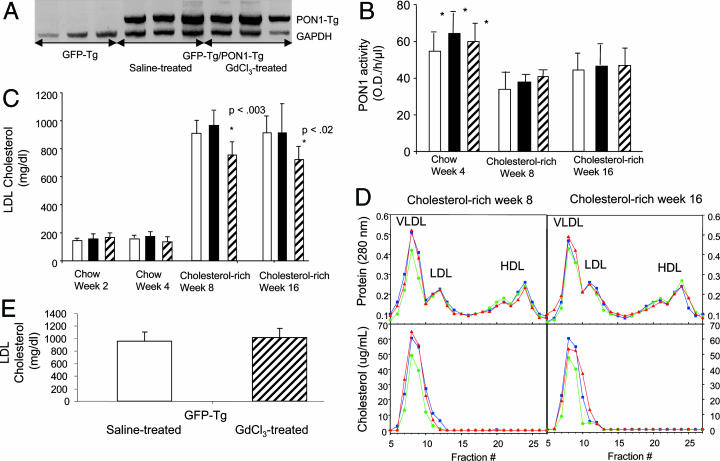
Effect of Kupffer Cell Gene Expression of PON1 on Atherosclerosis Lesion Formation. To examine whether GdCl3-facilitated transgenesis of PON1 exhibited a therapeutic amelioration of atherosclerosis, lethally irradiated LDL receptor-deficient mice were injected with bone marrow from mice expressing either the GFP transgene only (control) or both the GFP transgene and PON1-Tg. One month later, mice were treated with either GdCl3 or saline vehicle. Mice from all three groups were fed a chow diet for 4 weeks and then a cholesterol-enriched atherogenic diet (1.25% cholesterol, 6% fat) for the remaining 12 weeks. Leukocytes obtained from the blood of recipients 4 weeks after receiving PON1 bone marrow displayed the PON1-Tg mRNA, whereas leukocytes from mice receiving nontransgenic bone marrow did not contain detectable transgenic PON1 mRNA (Fig. 3A). PON1 esterase activity in plasma was similar among all groups (Fig. 3B). These findings support experiments showing that PON1 protein and enzyme activity are retained in the microsomal membrane fraction of peritoneal macrophages (Fig. 1 B and C) (34-36). Consistent with the results of others (23, 24), consumption of the cholesterol-enriched atherogenic diet caused a ≈30% decrease in plasma activities of PON1 (Fig. 3B).
Fig. 3.
GdCl3 treatment decreases atherogenic LDL cholesterol in cholesterol-fed LDL-receptor-deficient mice engrafted with bone marrow expressing the PON1-Tg. (A) RT-PCR for PON1-Tg mRNA verified expression of PON1 in peripheral blood leukocytes of recipient LDL-receptor-deficient mice 4 weeks after injection of bone marrow obtained from mice expressing both the PON1-Tg and the GFP transgene. The migration of PON1-Tg and GAPDH cDNA standards are indicated. (B) Plasma PON1 enzyme activity by using paraoxon as the substrate is shown as the mean ± SD for 12 separate mice per group. There were no significant differences in the plasma activity of PON1 in the three groups of mice. *, Significant difference between the activities in mice fed chow compared with their activities when fed the cholesterol-enriched diet (P < 0.01). Open bars represent mice receiving bone marrow expressing only the GFP transgene; filled bars represent mice receiving bone marrow expressing both the PON1-Tg and the GFP transgene and treated subsequently with saline vehicle only; and hatched bars represent mice receiving bone marrow expressing both the PON1-Tg and the GFP transgene and subsequently treated with GdCl3 (25 mg/kg in saline). Time in weeks corresponds to time after treatment with GdCl3 or saline vehicle only. Mice were fed a chow diet until the end of week 4. After week 4, mice were fed an atherogenic cholesterol-enriched diet for 12 weeks. (C) GdCl3 treatment reduced plasma LDL cholesterol levels in cholesterol-fed LDL receptor-negative mice engrafted with HSCs expressing the PON1-Tg. Plasma LDL cholesterol levels are shown as the mean ± SD for 12 separate mice in each group. *, Significant differences between mice treated with GdCl3 and the other two groups (not receiving GdCl3), P < 0.02. Columns represent the same groups of mice designated in B.(D) GdCl3 treatment reduced cholesterol content of the LDL fraction of plasma obtained from cholesterol-fed LDL receptor-negative mice engrafted with HSCs expressing the PON1-Tg. Red lines represent mice receiving bone marrow expressing only the GFP transgene; blue lines represent mice receiving bone marrow expressing both the PON1-Tg and the GFP transgene and treated subsequently with saline vehicle only; and green lines represent mice receiving bone marrow expressing both the PON1-Tg and the GFP transgene and subsequently treated with GdCl3 (25 mg/kg in saline). Time in weeks corresponds to time after treatment with GdCl3 or saline vehicle only. Mice were fed a chow diet until the end of week 4. After week 4, mice were fed an atherogenic cholesterol-enriched diet for 12 weeks. Blood was obtained at 4 and 8 weeks after beginning the cholesterol-rich diet. Plasma from all mice in the same group were pooled and subjected to FPLC separation (33). (E) GdCl3 treatment did not reduce plasma LDL cholesterol levels in cholesterol-fed LDL receptor-negative mice engrafted with HSCs not expressing the PON1-Tg. Mice treated identically as in C (except they received bone marrow expressing the GFP transgene but not the PON1-Tg) were killed after being fed for 12 weeks the cholesterol-rich atherogenic diet. Plasma LDL levels are shown as the mean ± SD for 11 separate mice in each group. There were no significant differences among groups.
When mice were fed the chow diet, plasma cholesterol levels were unchanged by the expression of the PON1-Tg or treatment with GdCl3 (Fig. 3C). However, when mice were fed the cholesterol-enriched atherogenic diet, GdCl3-treated mice exhibited a significant reduction (≈20%, P < 0.05) in plasma LDL cholesterol (Fig. 3 C and D). Plasma high-density lipoprotein cholesterol levels were unaffected by GdCl3 treatment. Additional data showed that GdCl3 treatment of cholesterol-fed mice receiving bone marrow expressing GFP, but not PON1, had no effect on plasma LDL levels (Fig. 3E). Thus, the reduction in plasma LDL cholesterol required GdCl3 treatment, the PON1-Tg, and the cholesterol-rich atherogenic diet.
The combined data suggested that GdCl3 treatment decreased LDL cholesterol in diet-induced hypercholesterolemic mice through a mechanism dependent on the expression of the PON1-Tg by Kupffer cells. Moreover, because plasma activity of PON1 (Fig. 3B) was unaffected by engraftment of Kupffer cells expressing PON1 (Fig. 3B), and the PON1-Tg protein and enzyme activity were not secreted and were retained within macrophages (Fig. 1), the PON1-Tg reduced plasma LDL cholesterol levels via a mechanism occurring predominantly within Kupffer cells.
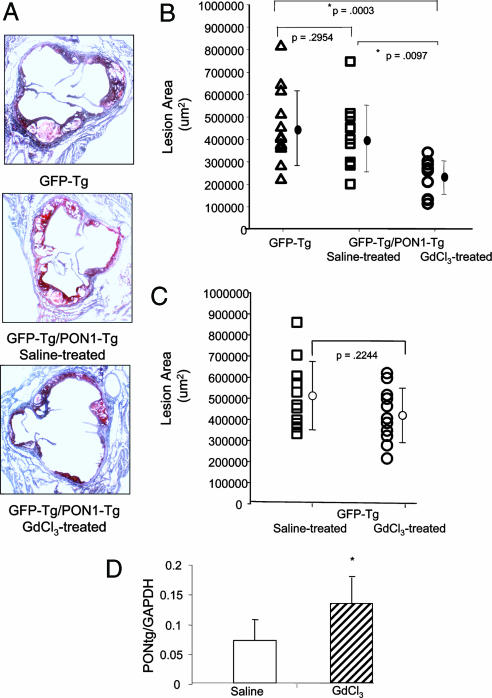
Mice receiving bone marrow from PON1 transgenic mice treated with GdCl3 exhibited a 50% reduction (P < 0.0003) in aortic sinus atherosclerotic lesions compared with mice receiving bone marrow not containing the PON1-Tg (Fig. 4 A and B). Whereas atherosclerotic lesions appeared to be slightly less in mice receiving PON1 bone marrow without GdCl3 treatment compared with mice receiving bone marrow lacking PON1-Tg expression, this difference was not statistically significant (Fig. 4 A and B). Similar findings were obtained with regard to the surface area of atherosclerotic lesions in abdominal aortae; mice receiving bone marrow from PON1 transgenic mice treated with GdCl3 exhibited a 45% reduction in abdominal aorta atherosclerotic surface lesions compared with mice receiving bone marrow not containing PON1 [in percent surface area, 1.51 ± 0.84 (control) vs. 0.84 ± 0.7 (GdCl3-treated), P < 0.006].
Fig. 4.
GdCl3 treatment decreased atherosclerotic lesions in LDL receptor-deficient mice engrafted with HSCs expressing PON1. (A) The atherosclerotic lesion area (27, 33) was quantitated by using aortic sinus frozen thin sections stained with oil red O obtained from mice killed after 12 weeks of being fed an atherogenic cholesterol-enriched diet. Representative micrographs are shown. (B) The mean lesion area ± SD for 12 separate mice per group is shown. Statistical differences (P values) among the groups are indicated. GFP-Tg represents mice receiving bone marrow expressing the GFP transgene; GFP-Tg/PON1-Tg saline treated represents mice receiving bone marrow expressing both the GFP transgene and the PON1-Tg and subsequently treated with saline only; and GFP-Tg/PON1-Tg GdCl3-treated represents mice receiving bone marrow expressing both the GFP transgene and the PON1-Tg and subsequently treated with GdCl3 (25 mg/kg in saline). (C) GdCl3 treatment did not reduce atherosclerotic lesions in LDL-receptor-deficient mice engrafted with HSCs not expressing the PON1-Tg. Mice were treated identically as in A (except they received bone marrow expressing the GFP transgene but not the PON1-Tg), and the aortic sinus lesions were analyzed. There were 11 mice per group. There was no significant difference between groups. (D) Hepatic expression of transgenic PON1 mRNA relative to GAPDH. Livers from LDL receptor-deficient mice engrafted with bone marrow expressing the PON1-Tg were treated without (saline control, open bars) or with GdCl3 (hatched bars), killed 12 weeks after being fed the cholesterol-rich diet and used to make cDNA. The relative hepatic content of transgenic PON1 mRNA relative to GAPDH was quantitated by SyBr green real-time PCR. Each value represents the mean ± SD of 12 separate mice in each group. *, Significant differences, P < 0.02.
To further examine the role of GdCl3 treatment in the 50% reduction in atherosclerosis lesions, LDL receptor-deficient mice were engrafted with bone marrow expressing the GFP transgene but not the PON1-Tg and then treated with and without GdCl3 (Fig. 4C). In the absence of the PON1-Tg, GdCl3 treatment had no significant effect on atherosclerosis lesion formation (Fig. 4C). These data clearly show that HSCs expressing the PON1-Tg are essential for GdCl3 treatment to significantly reduce atherosclerosis.
After 16 weeks of cholesterol feeding, hepatic expression of transgenic PON1 mRNA was still 2-fold greater in GdCl3-treated mice than in the livers of mice receiving bone marrow cells expressing the PON1-Tg but not GdCl3 (Fig. 4D). This 2-fold difference in the expression of the PON1 transgenic mRNA observed 16 weeks after GdCl3 treatment (Fig. 4D) is less than the 9-fold difference observed 4 weeks after GdCl3 treatment (Fig. 2A). The smaller difference between the two groups of mice was due to an increased expression of the PON1 transgenic mRNA by the saline (control mice). These data suggest that Kupffer cells expressing the PON1-Tg were replaced in the absence of GdCl3 treatment (albeit at a rate slower than with GdCl3 treatment). Mice receiving HSCs expressing the PON1-Tg showed no significant reduction in atherosclerotic lesions compared with mice receiving HSCs lacking the PON1-Tg (Fig. 4 A and B). Thus, GdCl3-facilitated engraftment of Kupffer cells enhanced the rapid expression of the therapeutic PON1-Tg throughout the 16-week experiment and was responsible for both the reduction in LDL cholesterol (Fig. 3 C and D) and the decrease in atherosclerosis (Fig. 4 A and B).
Discussion
In this report, we demonstrate a simple method for rapidly replacing a significant fraction of liver macrophages with Kupffer cells derived from bone marrow HSCs and for permanently expressing therapeutic transgenes. This method is based on the i.v. injection of gadolinium chloride, an inorganic contrast material commonly used in humans, which is rapidly and specifically taken up by Kupffer cells. The uptake of GdCl3 causes Kupffer cell apoptosis, allowing for transplanted HSCs to repopulate the liver. Using HSCs with normal expression of endogenous genes or HSCs carrying therapeutic transgenes, Kupffer cell precursors can thus act as “Trojan Horses” and provide rapid nontoxic physiologically relevant expression of therapeutic transgenes.
Our combined data supported two conclusions: (i) that GdCl3-facilitated apoptosis was an effective method to accelerate the engraftment of Kupffer cells derived from bone marrow HSCs in mice; and (ii) that engrafted Kupffer cells provided a large tissue reservoir for expression of a therapeutic transgene (i.e., PON1) via the human SRA macrophage-specific promoter element (25) at levels sufficient to significantly reduce plasma LDL cholesterol levels and decrease aortic atherosclerotic lesions. This PON1-based gene therapy provided an effective treatment for reducing atherosclerosis in a murine model of homozygous familial hypercholesterolemia, a disease in humans for which the only long-term effective treatment is liver transplantation (13).
The additional finding that the transgenic PON1 protein and enzyme activity were retained within macrophages demonstrates that the therapeutic transgene acted within Kupffer cells. Kupffer cells play a critical role in removal from plasma of oxidized and chemically modified forms of LDL (14, 15). Kupffer cell-specific ablation immediately after GdCl3 injection blocks hepatic uptake of oxidized [125I]-LDL, causing a ≈2- to 3-fold increased uptake by peripheral tissues including aorta, heart, lungs, and kidney (38). Our findings showing that Kupffer cell expression of PON1 reduced LDL cholesterol levels and atherosclerosis were predicted by the proposal that PON1 prevents atherosclerosis by metabolizing and inactivating the proatherogenic, inflammatory lipids produced by oxidative modification of LDL (16-24). Kupffer cell/PON1 transgenic disposal of proatherogenic lipid mediators associated with oxidatively modified LDL presumably prevented them from entering the arterial wall and promoting atherosclerotic lesion formation.
Our findings clearly show that GdCl3-facilated Kupffer cell replacement was essential to show a significant amelioration of atherosclerosis lesions [i.e., mice receiving HSCs expressing the PON1-Tg but not treated with GdCl3 showed no protection from atherosclerosis (Fig. 4C)]. Considering that GdCl3 is relatively nontoxic and is commonly used as a contrast reagent in humans, GdCl3-facilitated gene transfer may provide an important enhancement of macrophage-based gene therapies.
Acknowledgments
We thank Aldons J. Lusis, Diana Shih, Joseph Witztum, and Alan Attie for valuable discussions and editorial suggestions. This work was supported by Grant HL-57974 from the National Institutes of Health.
Author contributions: G.B., A.G., J.H.M., C.K.G., L.K.C., and R.A.D. designed research; G.B., A.G., J.H.M., K.R.D., and A.C.L. performed research; C.K.G. contributed new reagents/analytic tools; G.B., A.G., J.H.M., K.R.D., A.C.L., C.K.G., L.K.C., and R.A.D. analyzed data; and G.B., A.G., J.H.M., A.C.L., C.K.G., L.K.C., and R.A.D. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HSC, hematopoietic stem cell; PON1, paraoxonase-1; LDL, low-density lipoproteins; PON1-Tg, PON1 transgene; GdCl3, gadolinium chloride.
References
- 1.Weissman, I. L. (2000) Science 287, 1442-1446. [DOI] [PubMed] [Google Scholar]
- 2.Spangrude, G. J. (2003) BioTechniques 35, 1273-1279. [DOI] [PubMed] [Google Scholar]
- 3.Nabel, G. J. (2004) Nat. Med. 10, 135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCuskey, R. S. (2000) Liver 20, 3-7. [DOI] [PubMed] [Google Scholar]
- 5.Wu, G. Y. & Grove, R. I. (1998) Adv. Drug Delivery Rev. 30, 199-204. [DOI] [PubMed] [Google Scholar]
- 6.Wagers, A. J., Sherwood, R. I., Christensen, J. L. & Weissman, I. L. (2002) Science 297, 2256-2259. [DOI] [PubMed] [Google Scholar]
- 7.Bouwens, L., De, B. P., Vanderkerken, K., Geerts, B. & Wisse, E. (1992) Enzyme 46, 155-168. [DOI] [PubMed] [Google Scholar]
- 8.Naito, M., Hasegawa, G., Ebe, Y. & Yamamoto, T. (2004) Med. Electron Microsc. 37, 16-28. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy, D. W. & Abkowitz, J. L. (1997) Blood 90, 986-993. [PubMed] [Google Scholar]
- 10.Hardonk, M. J., Dijkhuis, F. W., Hulstaert, C. E. & Koudstaal, J. (1992) J Leukocyte Biol. 52, 296-302. [DOI] [PubMed] [Google Scholar]
- 11.Ishibashi, S., Goldstein, J. L., Brown, M. S., Herz, J. & Burns, D. K. (1994) J. Clin. Invest. 93, 1885-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palinski, W., Tangirala, R. K., Miller, E., Young, S. G. & Witztum, J. L. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 1569-1576. [DOI] [PubMed] [Google Scholar]
- 13.Bilheimer, D. W., Goldstein, J. L., Grundy, S. M., Starzl, T. E. & Brown, M. S. (1984) N. Engl. J. Med. 311, 1658-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Berkel, T., De Rijke, Y. & Kruijt, J. K. (1991) J. Biol. Chem. 266, 2282-2289. [PubMed] [Google Scholar]
- 15.Nenseter, M. S., Gudmundsen, O., Roos, N., Maelandsmo, G., Drevon, C. A. & Berg, T. (1992) J. Lipid Res. 33, 867-877. [PubMed] [Google Scholar]
- 16.La Du, B. N., Adkins, S., Kuo, C. L. & Lipsig, D. (1993) Chem. Biol. Interact. 87, 25-34. [DOI] [PubMed] [Google Scholar]
- 17.Mackness, M. I., Arrol, S., Abbott, C. & Durrington, P. N. (1993) Atherosclerosis 104, 129-135. [DOI] [PubMed] [Google Scholar]
- 18.Mackness, M. I., Arrol, S. & Durrington, P. N. (1991) FEBS Lett. 286, 152-154. [DOI] [PubMed] [Google Scholar]
- 19.Watson, A. D., Berliner, J. A., Hama, S. Y., La, D. B., Faull, K. F., Fogelman, A. M. & Navab, M. (1995) J. Clin. Invest. 96, 2882-2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackness, M. I., Mackness, B., Durrington, P. N., Fogelman, A. M., Berliner, J., Lusis, A. J., Navab, M., Shih, D. & Fonarow, G. C. (1998) Curr. Opin. Lipidol. 9, 319-324. [DOI] [PubMed] [Google Scholar]
- 21.Leitinger, N., Tyner, T. R., Oslund, L., Rizza, C., Subbanagounder, G., Lee, H., Shih, P. T., Mackman, N., Tigyi, G., Territo, M. C., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 12010-12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackness, B., Davies, G. K., Turkie, W., Lee, E., Roberts, D. H., Hill, E., Roberts, C., Durrington, P. N. & Mackness, M. I. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1451-1457. [DOI] [PubMed] [Google Scholar]
- 23.Shih, D. M., Gu, L., Xia, Y. R., Navab, M., Li, W. F., Hama, S., Castellani, L. W., Furlong, C. E., Costa, L. G., Fogelman, A. M. & Lusis, A. J. (1998) Nature 394, 284-287. [DOI] [PubMed] [Google Scholar]
- 24.Tward, A., Xia, Y. R., Wang, X. P., Shi, Y. S., Park, C., Castellani, L. W., Lusis, A. J. & Shih, D. M. (2002) Circulation 106, 484-490. [DOI] [PubMed] [Google Scholar]
- 25.Horvai, A., Palinski, W., Wu, H., Moulton, K. S., Kalla, K. & Glass, C. A. (1995) Proc. Natl. Acad. Sci. USA 92, 5391-5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okabe, M., Ikawa, M., Kominami, K., Nakanishi, T. & Nishimune, Y. (1997) FEBS Lett. 407, 313-319. [DOI] [PubMed] [Google Scholar]
- 27.Boisvert, W. A., Spangenberg, J. & Curtiss, L. K. (1995) J. Clin. Invest. 96, 1118-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi, W., Wang, N. J., Shih, D. M., Sun, V. Z., Wang, X. & Lusis, A. J. (2000) Circ. Res. 86, 1078-1084. [DOI] [PubMed] [Google Scholar]
- 29.Shih, D. M., Xia, Y. R., Wang, X. P., Miller, E., Castellani, L. W., Subbanagounder, G., Cheroutre, H., Faull, K. F., Berliner, J. A., Witztum, J. L., et al. (2000) J. Biol. Chem. 275, 17527-17535. [DOI] [PubMed] [Google Scholar]
- 30.Deakin, S., Leviev, I., Gomaraschi, M., Calabresi, L., Franceschini, G. & James, R. W. (2002) J. Biol. Chem. 277, 4301-4308. [DOI] [PubMed] [Google Scholar]
- 31.Hundal, R. S., Salh, B. S., Schrader, J. W., Gomez-Munoz, A., Duronio, V. & Steinbrecher, U. P. (2001) J. Lipid Res. 42, 1483-1491. [PubMed] [Google Scholar]
- 32.Li, A. C., Brown, K. K., Silvestre, M. J., Willson, T. M., Palinski, W. & Glass, C. K. (2000) J. Clin. Invest. 106, 523-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyake, J. H., Duong-Polk, X. T., Taylor, J. M., Du, E. Z., Castellani, L. W., Lusis, A. J. & Davis, R. A. (2002) Arterioscler. Thromb. Vasc. Biol. 22, 121-126. [DOI] [PubMed] [Google Scholar]
- 34.Furlong, C. E., Richter, R. J., Chapline, C. & Crabb, J. W. (1991) Biochemistry 30, 10133-10140. [DOI] [PubMed] [Google Scholar]
- 35.Hassett, C., Richter, R. J., Humbert, R., Chapline, C., Crabb, J. W., Omiecinski, C. J. & Furlong, C. E. (1991) Biochemistry 30, 10141-10149. [DOI] [PubMed] [Google Scholar]
- 36.Sorenson, R. C., Aviram, M., Bisgaier, C. L., Billecke, S., Hsu, C. & La Du, B. N. (1999) Chem. Biol. Interact. 119-120, 243-249. [DOI] [PubMed] [Google Scholar]
- 37.Kraal, G., Rep, M. & Janse, M. (1987) Scand. J. Immunol. 26, 653-661. [DOI] [PubMed] [Google Scholar]
- 38.Usynin, I. F., Khar'kovsky, A. V., Balitskaya, N. I. & Panin, L. E. (1999) Biochemistry 64, 620-624. [PubMed] [Google Scholar]
- 39.Oda, M. N., Bielicki, J. K., Ho, T. T., Berger, T., Rubin, E. M. & Forte, T. M. (2002) Biochem. Biophys. Res. Commun. 290, 921-927. [DOI] [PubMed] [Google Scholar]
- 40.Schiller, N. K., Black, A. S., Bradshaw, G. P., Bonnet, D. J. & Curtiss, L. K. (2004) J. Lipid Res. 45, 1398-1409. [DOI] [PubMed] [Google Scholar]