Abstract
The RNaseIII-containing enzyme Dicer is believed to be required for the processing of most, if not all, microRNAs (miRNAs) and for processing long dsRNA into small interfering RNAs. Because the complete loss of Dicer in both zebrafish and mice results in early embryonic lethality, it has been impossible to determine what role, if any, Dicer has in patterning later tissues in the developing vertebrate embryo. To bypass the early requirement of Dicer in development, we have created a conditional allele of this gene in mice. Using transgenes to drive Cre expression in discrete regions of the limb mesoderm, we find that removal of Dicer results in the loss of processed miRNAs. Phenotypically, developmental delays, in part due to massive cell death as well as disregulation of specific gene expression, lead to the formation of a much smaller limb. Thus, Dicer is required for the formation of normal mouse limbs. Strikingly, however, we did not detect defects in basic patterning or in tissue-specific differentiation of Dicer-deficient limb buds.
Keywords: microRNAs, mouse
MicroRNAs (miRNAs) are small, noncoding RNAs, ≈22 nt long. Large numbers of miRNAs are encoded in the genomes of all metazoans, including, at minimum, several hundred embedded in the genome of higher vertebrates such as mice and humans (1, 2). Animal miRNAs were first identified in Caenorhabditis elegans by mutations causing strong developmental phenotypes (3–5). Genetic and biochemical studies revealed that miRNAs act by mediating cleavage and/or inhibiting translation of specific target genes (6). These genes are targeted by virtue of regions complementary to a core sequence in the respective miRNAs. On this basis, several thousand putative targets have now been predicted for vertebrate miRNAs, and several intriguing targets have been validated in vivo and in vitro (1, 2, 7). These data, together with functional data emerging from studies in C. elegans and Drosophila (3, 8–10), support a growing belief that miRNAs may play critical roles in controlling key developmental events in vertebrates as well as invertebrates. However, direct demonstrations of the necessity of specific miRNAs for vertebrate development has been slow in coming, largely because vertebrate miRNAs exist as families of highly related or even identical sequences (11). This high level of possible redundancy suggests that simple loss-of-function approaches will be less than fruitful for studying miRNA function in vertebrate development.
An alternative, albeit heavy-handed, approach to testing the necessity of miRNAs in higher animal development is to create mutations in the upstream enzymes responsible for processing miRNAs to their mature, active form. Mature animal miRNAs are ≈22 nt in length, generated by sequential processing by a series of RNaseIII-related enzymes. Drosha produces a primary miRNA hairpin transcript of ≈70 nt from a longer precursor RNA (12). This ≈70-nt hairpin is then cleaved by a second RNaseIII enzyme, Dicer, to yield the 22-nt mature miRNA (13). Importantly, there is only a single copy of Dicer in the mouse genome. Thus, targeted deletion of Dicer should, in principle, yield mice deficient of all mature miRNAs. Dicer is also required for processing dsRNA transcripts into small interfering RNAs; hence, small interfering RNAs as well as the ability to respond to exogenously provided dsRNA would also be expected to be compromised in a Dicer mutant.
In vertebrates, Dicer-null mutations have been created in both mice and zebrafish (14–16). Mice that lack Dicer survive until embryonic day (E) 7.5, possibly because of the presence of maternal Dicer protein (15). These embryos die before axis formation, consistent with the model that Dicer products might be crucial for embryonic patterning and morphogenesis. In zebrafish, removal of zygotic Dicer does not result in loss of miRNA-processing activity in early embryogenesis (16), presumably because of the presence of maternal Dicer. These embryos have no obvious defects in their early development, although they exhibit a developmental delay before finally dying at 14–21 days after fertilization. Zebrafish mutant embryos lacking both maternal and zygotic Dicer protein show greater effects. Surprisingly, however, these embryos still survive through early embryogenesis and appear to correctly pattern and correctly specify many early cell types (14). As embryogenesis proceeds they undergo abnormal morphogenesis of the brain, heart, and other organs. Although the examination of early embryos formed in the absence of Dicer activity has not revealed any defects in cell fate specification, Dicer-deficient embryonic stem cells are defective in differentiation both in vitro and in vivo, suggesting that some aspects of differentiation are under the control of Dicer-dependent factors (17).
The inability of Dicer-null embryos to survive until later stages of development and, in particular, of Dicer-deficient mice to survive past E7.5 has inhibited study of the later functions of this unique enzyme, including the role mature miRNA may have in later development. Therefore, it is still uncertain how widespread is the requirement for Dicer and miRNAs in vertebrate development. To overcome the early lethality associated with the null mouse allele, we have created a cre-inducible conditional allele of Dicer. This conditional allele can be used to remove Dicer function in any tissue in which the site-specific cre recombinase can be expressed. Using the Dicer conditional allele and transgenic mouse lines that express cre in the limb mesoderm, we provide an initial characterization of the role that Dicer plays in vertebrate limb development.
Materials and Methods
Creation of a Dicer-Null Conditional Allele. The Dicer conditional allele (Dicerflox) was created by inserting loxP sites around an exon that encodes most of the second RNaseIII domain (Fig. 1). Cre-mediated recombination resulted in the removal of 90 aa. Upon cre-mediated recombination, an antibody made against a region of the Dicer protein 3′ to the deletion was still able to recognize Dicer protein, suggesting that removal of this exon does not result in a downstream frame-shift or an unstable protein (data not shown). Dicer was conditionally removed from the limb mesenchyme by using the previously described Cre lines prx1cre (18) and Shhgfpcre (19). The conditional floxed allele was genotyped by using primers DicerF1 (CCTGACAGTGACGGTCCA A AG) and DicerR1 (CATGACTCTTCAACTCAAACT). This PCR combination flanks the 5′ loxP site. The floxed allele produced a 420-bp PCR product whereas a wild-type allele resulted in a 351-bp product. The deletion allele was genotyped by using primers DicerF1 and DicerDel (CCTGAGCAAGGCAAGTCATTC). The DicerDel primer is downstream of the neomycin cassette. The deletion allele produced a 471-bp PCR product whereas a wild-type allele resulted in a 1,300-bp product. Mice homozygous for the conditional allele or containing a conditional allele and a recombined deletion allele were indistinguishable from wild-type littermates. The neomycin cassette has not been removed in the floxed Dicer allele, suggesting that it does not interfere with wild-type Dicer expression.
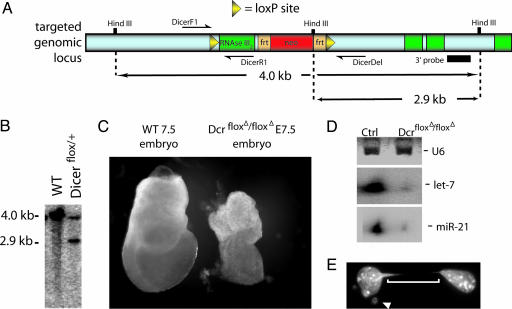
Fig. 1.
Construction of a Dicer conditional mouse allele. (A) Schematic of the Dicer conditional targeting construct. (B) Southern blot of correctly targeted embryonic stem cells. By using a 3′ probe, a 2.9-kb fragment is detected in addition to the wild-type 4.0-kb fragment in targeted embryonic stem cells. (C) Upon Cre-mediated recombination of the Dicerflox allele in the germ line, homozygous mutant embryos resemble the reported null allele (15). (D) RNA was extracted from primary fibroblasts from wild-type or Dicerflox/Dicerflox mice. Parallel experiments with GFP adenovirus indicated near 90–100% infection efficiency. The presence of a small amount of processed let-7 and miR-21 RNA in Dicer-null cells may be due to the inclusion of Dicer-positive cells in our RNA preparations or may indicate that a small amount of miRNA processing is independent of Dicer. (E) A representative example of a cell displaying defects in chromosome segregation upon removal of Dicer. The arrowhead points to a micro nucleus, and the brackets indicate an anaphase spindle. Counting the number of anaphase spindles and satellite nuclei present among four randomly chosen fields of DAPI-stained cells revealed that ≈25% of the Dicer-null cells displayed defects in chromosome segregation and accumulation of satellite nuclei despite a normal morphological appearance under the light microscope. In control adeno-GFP-treated cells, 1.4% of the cells contained satellite nuclei but no anaphase bridges.
Small RNA Northern Blots, Immunohistochemistry, and in Vitro Cell Culture. Small RNA Northern blots were performed as in ref. 35. Derivation, culture, and cre infection of mouse embryonic fibroblasts follow standard protocols. Phosphorylated extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase antibody staining was performed as described in ref. 20. Skeletal preparations were performed as in ref. 21, and whole-mount in situ hybridizations using digoxigenin-labeled RNA probes were performed as described in refs. 22 and 23. β-Galactosidase staining was performed as in ref. 19. To examine cell death in embryos in which Dicer had been removed from the limb mesoderm (Dicerflox/Dicerflox;prx1cre), we used acridine orange (24). A working stock of 5 mg/ml acridine orange was diluted 1:10,000 in PBS. Dissected embryos were transferred to the working solution of acridine orange and incubated for 30 min at 37°C in the dark. Embryos were then washed twice in PBS for 5 min and viewed with a fluorescence microscope.
Results
Recombination of the Dicer Conditional Allele Results in Loss of miRNA Processing. Because Dicer-null mouse embryos arrest development at E7.5 (15), well before the limbs and most other organ systems form, we constructed a conditional allele of this gene by flanking an exon encoding most of the second RNaseIII domain with loxP sites (see Materials and Methods and Fig. 1). This deletion would be predicted to abolish the enzymatic activity of Dicer. To confirm that the removal of the Dicer floxed exon produced a null allele, we removed this exon in the germ line by crossing the floxed allele to a germ-line cre (β-actin cre). Homozygous DicerfloxΔ/DicerfloxΔ embryos arrested at E7.5 and were similar in appearance to the reported Dicer-null mutant (Fig. 1C) (15).
Because Dicer has been postulated to process miRNAs, we investigated in vitro whether mature miRNAs were produced in cells that lacked the Dicer conditional allele. Primary fibroblasts were prepared from embryos homozygous for the floxed Dicer allele. Cre was introduced into these cells by using an adenovirus vector (a gift from Beverly Davidson, University of Iowa, Iowa City). Using PCR, we detected no wild-type alleles of Dicer after adenovirus infection (data not shown), suggesting that the infection of the cell culture by the adenovirus was complete. To determine whether miRNAs were processed in Dicer-minus fibroblasts, we examined the processing of two miRNAs, let-7 and miR-21. Whereas both of these miRNAs were easily seen as 22-nt processed forms in control primary cells, the cultures infected with the Cre adenovirus showed barely detectable levels of processed let-7 and miR-21 (Fig. 1D). The remaining processed miRNAs can be attributable to perdurance of Dicer protein or processed miRNAs after genomic recombination of the Dicer locus or may suggest that low levels of miRNA processing can occur in the absence of Dicer. After removal of Dicer activity in these primary fibroblasts, no overt differences in cell morphology were observed, although the cells exhibited a reduction in growth rate. By the third passage, dividing cells could be observed to contain nuclear bridges and microsatellites in up to 25% of the cells in the culture (Fig. 1E), consistent with previous reports of the effect of loss of Dicer after extended cell passage (24–26).
Dicer Is Required for Regulating Limb Size. To investigate the in vivo role of Dicer in limb development, we removed this gene specifically from the entire limb mesoderm using the prx1cre transgene (18). The prx1cre allele is a transgene that expresses Cre throughout the limb mesoderm in both the mouse forelimb and hindlimb. In the forelimb of prx1cre mice, Cre expression is observed in the limb mesoderm beginning at E9.5, whereas expression in the hindlimb commences ≈1 day later (18). No Cre expression is observed in the limb ectoderm using this allele.
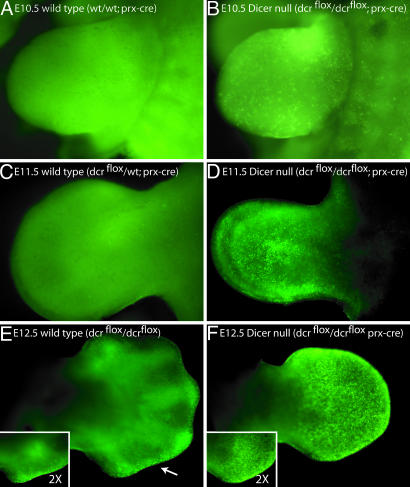
Upon prx1cre-dependent removal of Dicer in the entire limb mesoderm, consistent with our in vitro data, the vast majority of miRNAs were not processed (Fig. 2). Moreover, morphologically, we observed a striking decrease in limb size. In the forelimb, the limb was visually indistinguishable from those of age-matched littermates from E9.5 (when a limb bud forms) until E11. At E11, Dicer-minus forelimbs were smaller than those of age-matched littermates, and by E12.5 mutant limbs resembled the size of E11.5 limbs. Limbs that lacked Dicer remained smaller than age-matched controls from E11 onward. This phenotype is also observed in the hindlimb but is not as severe, possibly because of the relative delay of prx1cre expression in the hindlimb. Consistent with these results, a reduction in the size of multiple organs upon removal of Dicer has also been reported in zebrafish (16).
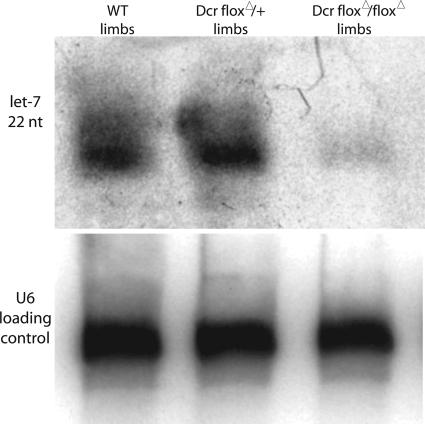
Fig. 2.
let-7 is not correctly processed in limbs that lack Dicer. RNA was extracted from wild-type, heterozygous, or Dicer-null E12.5 forelimbs. The small amount of mature let-7 present in Dicerflox/Dicerflox;prx1cre forelimbs may result from the correct processing of let-7 in the limb ectoderm [let-7 is known to be expressed in the limb ectoderm (35), and the prx1cre allele removes Dicer activity only from the limb mesoderm].
Loss of Dicer Results in Ectopic Cell Death but Not Defects in Pattering of Differentiation of the Vertebrate Limb. To understand the decrease in size of the Dicer-mutant limb buds in Dicer flox/Dicer flox;prx1cre embryos, we examined various cellular parameters. No overt change in cell proliferation was seen in vitro after removal of Dicer activity (data not shown). We therefore turned our attention to the possibility that the decrease in limb size was due to an increase in programmed cell death. We stained the limbs of live embryos with acridine orange, a marker of cells undergoing apoptosis (27). In the forelimbs of E10 Dicer-minus embryos, no increase in cell death was observed (data not shown). Similarly, in the hindlimb, no ectopic cell death in E10, E10.5, or E11 limbs was found. However, starting at E10.5 in the forelimbs and E12.5 in the hindlimbs, significant cell death was observed throughout the limb mesoderm in limbs that lacked Dicer (Fig. 3).
Fig. 3.
Loss of Dicer in the mouse limb leads to cell death. A, C, and E show wild-type limbs, and B, D, and F show limbs in which Dicer has been removed from the limb mesoderm using the prx1cre allele. An increase in cell death in Dicer-minus limbs compared with age-matched littermates was observed in E10.5 limbs (compare A and B). By E11.5, Dicer-minus limbs were narrower than wild-type limbs and exhibited an increase in cell death throughout the limb mesoderm. Note that loss of a single copy of Dicer does not lead to an increase in cell death (compare C and D). By E12.5, a wild-type pattern of cell death was observed in the apical ectodermal ridge (AER) of wild-type limbs (arrow in E). Dicer-minus limbs exhibited cell death in the AER in addition to ectopic cell death throughout the limb mesoderm (F). Dicer-minus limbs were also smaller and digit condensations were not visible in E12.5 mutant limbs. The ×2 magnification insets in E and F are of the distal, posterior region of the respective limbs. All pictures are of forelimbs from littermates. Images in A–D were taken at the same magnification.
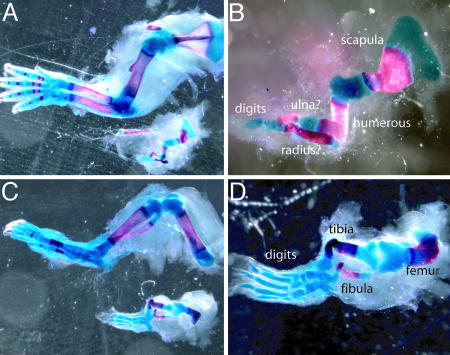
Strikingly, however, when postnatal limbs were examined, all of the differentiated cell types normally found in the mature limb bud appeared to be present in the Dicer-deficient limbs. Moreover, although morphological malformations were apparent, these did not appear to be due to patterning defects. The long bones of the arm and leg were twisted in appearance, and development from cartilage into bone was delayed, both features consistent with the decrease in size of the developing limb bud (28, 29). Importantly, however, analysis of skeletal preparations of Dicer-minus mouse forelimbs and hindlimbs revealed that all proximodistal segments were present (Fig. 4). The Dicer-minus forelimbs did show a notable reduction in the number of digits as well as some digit fusions. These could have resulted from the decrease in the width of the handplate during limb development but alternatively could have represented a forelimb-patterning defect. To distinguish between these possibilities, we used an Shhgfpcre allele to remove Dicer at E9.75, at the onset of Shh expression, specifically from the cells of the zone of polarizing activity (ZPA), a known signaling center responsible for patterning the anterior/posterior limb axis. During normal limb development, cells that have at one time expressed Shh (cells of the ZPA) form digits 5 and 4 and part of digit 3 (19). An increase in apoptosis of cells that lacked Dicer was observed in a subset of cells expressing the Shhgfpcre allele, consistent with our observation of an increase in cell death throughout the entire limb mesoderm after global removal of Dicer (data not shown). Importantly, limbs in which Dicer was removed from the ZPA did not contain defects in anterior/posterior patterning, and Dicer-minus cells that did not die were still found in the distal/posterior region of the autopod and formed digits 5 and 4 and part of digit 3 of both the forelimbs and hindlimbs (Fig. 5 and data not shown) demonstrating that, at least for cells that have at one time expressed Shh in the limb, Dicer is not required for their patterning.
Fig. 4.
Skeletal preparations of E20 Dicer-minus limbs. (A) The upper image shows a wild-type E20 forelimb, and the lower image shows a Dicerflox/Dicerflox;prx1cre forelimb from an age-matched littermate. (B) Magnification of the mutant limb shown in A. Notice that despite its small size the mutant limb contains most of the bones associated with normal limb development. (C) The upper image shows a wild-type E20 hindlimb, and the lower image shows a Dicerflox/Dicerflox;prx1cre hindlimb from an age-matched littermate. (D) Magnification of the mutant limb shown in C.
Fig. 5.
Loss of Dicer in cells that express Shh does not alter Shh signaling or cell migration. Removal of Dicer in Shh cells of E18.5 embryos was accomplished by mating Dicerflox/+;Shhgfpcre/+ males with Dicerflox/Dicerflox;R26R/+ females. Using the R26R allele, we were able to identify and follow the fate of cells that expressed the Shhgfpcre allele, had undergone a recombination event activating the LacZ gene, and lacked Dicer. Previously, we had demonstrated that cells that express Shh in the ZPA migrate to the distal limb and form digits 5 and 4 and part of digit 3 (19). In limbs in which Dicer was removed in Shh-expressing cells, abnormal cell migrations were not observed. No blue cells (Dicer-minus) were observed in abnormal locations compared with wild-type age-matched littermates.
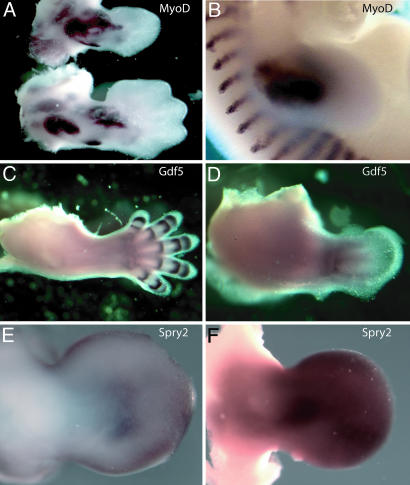
Developmental Delay in the Expression of a Subset of Genes in Dicer-Deficient Vertebrate Limbs. Consistent with the lack of morphological patterning defects, we saw no alteration in the spatial expression patterns of genes known to play critical roles in limb patterning, including Shh, Fgf8, Bmp-4, Bmp-7, MyoD, and Gdf5, although at later stages these displayed a developmental delay such that they were expressed in patterns that were consistent with an earlier stage of limb development compared with age-matched littermates (Fig. 6 and data not shown). One gene family displaying this property was potentially of particular relevance to the morphological phenotype. In wild-type limbs, Spry genes, a family of receptor tyrosine kinase (RTK) signaling antagonists, are initially expressed broadly but then become restricted in their expression to the distal mesenchyme during midlimb bud stages, reflecting their normal dependence on Fgf signaling (30). In contrast, in age-matched Dicerflox/Dicerflox;prx1cre littermates we observed a significant increase in Spry2 expression throughout the midlimb bud mesenchyme (compare Fig. 6 E and F). Intriguingly, both the decrease in size of the early limb bud and the subsequent normally patterned limb skeleton with reduced and twisted skeletal elements observed in Dicer-mutant limbs are very reminiscent of the phenotype obtained upon viral overexpression of Spry genes in the chick limb (30).
Fig. 6.
Gene expression in a Dicer-minus limb. (A) RNA in situ hybridizations for MyoD (muscle) staining. The upper image shows a Dicerflox/Dicerflox;prx1cre E12.5 forelimb, and the lower image shows a Dicerflox/+;prx1cre control limb. (B) RNA in situ hybridizations for MyoD staining in an E11.5 wild-type forelimb. Notice that the staining pattern in an E12.5 Dicerflox/Dicerflox;prx1cre E12.5 forelimb is similar to MyoD expression in an E11.5 limb. (C) RNA in situ hybridizations for Gdf5, a marker of joint formation. Staining is in an E13.0 wild-type forelimb. (D) Gdf5 staining in a Dicerflox/Dicerflox;prx1cre forelimb (×2 magnified compared with C). All control limbs are from age-matched littermates. (E and F) Spry2 whole-mount RNA in situ hybridizations on Dicerflox/Dicerflox (E) and Dicerflox/Dicerflox;prx1cre (F) E11.75 littermates, respectively. Notice that Spry2 is expressed at much higher levels in mesoderm that lacks Dicer (F). For all probes, more than four mutant and wild-type embryos were examined.
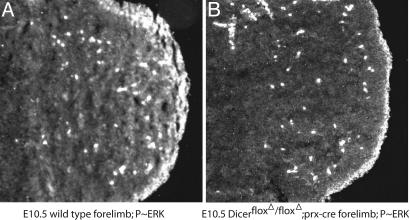
If the increase we observe in Spry gene expression contributes to the cell death observed at E10.5 in the forelimb of conditional Dicer mutants, this overexpression should be reflected in a decrease in FgfR and other RTK signaling in the limb bud at E10.5 when cell death is first observed. To test this hypothesis, we made use of an antibody specific for the phosphorylated form of ERK, a downstream component of RTK signaling (31). In immunohistochemistry on sectioned E10.5 wild-type forelimb buds, there is a high level of phospho-ERK-specific staining in the distal limb directly under the ectoderm and a clearly evident graded staining at a lower level throughout the rest of the mesenchyme (Fig. 7A). In contrast, parallel sections of Dicer-deficient limb buds show the same level of intense staining at the distal margin but a complete loss of detectable phospho-ERK in the rest of the mesenchyme, correlating with elevated levels of Spry gene products throughout the mesenchyme (Fig. 7). These results suggest that the elevation of Spry genes in the developmentally delayed Dicer-deficient limbs results in decreased RTK signaling. These observations are consistent with the observed increase in mesenchymal cell death upon removal of Dicer (see Fig. 3).
Fig. 7.
FGF signaling is reduced in limb buds lacking Dicer activity. Frontal sections through E10.5 control (A) and Dicerflox/Dicerflox;prx1cre (B) limbs were immunostained with an antibody against phosphorylated ERK/mitogen-activated protein kinase (MAPK), an indicator of active FGF and other RTK signal transduction as described in ref. 20. Phosphorylated ERK/MAPK is detectable at high levels in the mesoderm directly apposed to the apical ectodermal ridge (AER), a potent source of FGF signals, and is present in a gradient extending proximally through the mesoderm from the distal tip (A) (31). In contrast, in limb buds lacking Dicer activity (B), this graded signal is absent, indicating a reduction in FGF/RTK signaling. High levels of phosphorylated ERK/MAPK are still detected beneath the AER, consistent with maintained FGF signaling in the limb ectoderm.
Discussion
Constructing a unique mouse conditional allele of Dicer has allowed us to examine the role played by Dicer in vertebrate limb development. We demonstrate that Dicer is required for proper morphogenesis of the vertebrate limb, although the nature of the defects we observe are not as severe as might have been expected.
All of the morphological defects we observe can be explained on the basis of a global decrease in the number of cells in the developing limb bud. Limb buds of Dicer-deficient limbs were notably smaller in size from E11 onward. The skeletal elements that formed in these limbs were smaller than normal, were developmentally delayed, and formed a reduced number of digits in a narrower-than-normal hand plate. The reduction in number of digits could, in principle, also be explained by a decrease in signaling from the ZPA or other disruptions in anterior/posterior patterning. However, early patterning gene expression is normal in these limbs. Moreover, removal of Dicer activity specifically in the ZPA does not affect patterning of posterior limb structures, arguing that the digit reductions are indeed related to the decrease in size of the limb bud and not a patterning defect. In addition, the skeletal elements that form in Dicer-deficient limbs displayed fusions and were strikingly twisted and bent. The twisted bone phenotype is most likely not due to defects in patterning the early limb, because the twisting occurs after condensation of the primordia of the skeletal elements. This class of skeletal defect has been previously seen in limb buds displaying a reduction in size, e.g., due to Goosecoid misexpression in chicks (28) and in compound mutations in Prx1 and Prx2 mice (29).This phenotype can, in fact, be mimicked by the blocking of cell proliferation in chick limb buds (32), indicating that a decrease in the size of the limb primordia is sufficient to lead to the twisting of the skeletal elements.
The cause of the decrease in size of the limb bud, and consequent developmental delay and morphological defects, appears to be an increase in apoptosis throughout the limb mesenchyme. We observed no overt change in cell proliferation after Dicer removal. In contrast, a cell death study revealed a dramatic increase in apoptosis starting at E10.5 in the forelimb.
The increased cell death observed in limbs lacking Dicer activity is potentially due to a requirement for the enzyme at two levels: for specific modulation of Spry gene expression and possibly for assuring proper chromatin segregation, although our experiments do not address the extent to which the cell death observed upon loss of Dicer is due to one or the other of these factors. The chromatin segregation defects we observed in vitro are consistent with prior studies of cells deficient in Dicer activity in fission yeast (26, 33, 34). It is extremely likely that in the absence of Dicer activity similar defects occur in vivo as well. It is also likely that, as in cell culture, these defects manifest only after the Dicer products critical for this process have been diluted through multiple cell divisions or degraded sufficiently. Based on our in vitro work, such defects in chromatin specification would not be expected for six to eight cell divisions. In contrast, we observed an increase in cell death in the forelimb bud, with a time frame allowing for only three to four cell divisions after expression of the Cre transgene. This finding suggests that additional Dicer-regulated factors, not found in Dicer-minus cell lines studied to date, may be functioning in the limb mesenchyme.
Increased apoptosis in Dicer-minus limbs may, in part, result from the up-regulation of sprouty genes in Dicer-deficient limb buds. Spry2 expression is significantly increased throughout the midlimb bud. Moreover, as an RTK antagonist, this increase is correlated with the expected decrease in detectable phospho-ERK throughout the limb bud. During limb development, RTK-mediated Fgf signaling is required in the distal limb to block apoptosis, and total loss of the source of Fgf protein in the distal ectoderm results in distal cell death and skeletal truncation (31). More proximal regions of the limb mesenchyme do not depend on Fgf signaling but may require other factors signaling through RTK-mediated pathways for their survival. The observed increase in Spry2 would result in a decrease in RTK signaling throughout the limb bud but not a complete loss of such signaling. Hence, the level of cell death is not high enough to cause truncation or amelia but rather results in a decrease in the amount of mesenchyme in the limb bud.
To test whether the observed increase in spry expression is, in itself, sufficient to induce the level of cell death we observed in Dicer-deficient limb buds, one would want to increase the level of Spry expression in the absence of Dicer-related changes, e.g., by viral misexpression in chick embryos. Such an experiment has been done previously (30). When Spry2 is misexpressed throughout the limb mesenchyme, there is indeed a resultant decrease in the size of the early limb bud very similar to that observed in the mouse Dicer mutant (see Fig. 3 and ref. 30). Such limbs also develop shortened, twisted skeletal elements as observed in the absence of Dicer. Additionally, the limbs in which Spry has been misexpressed show a block or delay in ossification that we do not see in Dicer-deficient embryos. This difference can be explained by the continued high level of Spry activity present throughout late limb development in the case of viral misexpression whereas Dicer-deficient limb buds display elevated Spry expression only in the early limb mesenchyme. Nonetheless, at early bud stages when the expression of Spry is equivalent in the two cases, the viral misexpression experiment shows that high levels of Spry are sufficient to induce the pattern of limb defects one observes in Dicer-deficient Spry overexpressing mouse limb buds.
The Spry up-regulation we observed in Dicer-deficient limb buds could be a reflection of the general developmental delay in these limbs and hence an indirect consequence of the loss of Dicer activity. Indeed, in the earliest limb buds Spry2 is expressed throughout the limb mesenchyme. However, it is also possible that the Spry2 gene may be a direct target of miRNAs. In support of this hypothesis, a recent report (7) has identified eight miRNAs that have the potential to interact directly with the 3′ UTR of Spry2. Using miRNA arrays we have confirmed that five of eight of these miRNAs are expressed in the vertebrate limb (unpublished results).
Dicer has been implicated in a number of processes during development, including processing of miRNAs, chromatin remodeling, and gene silencing. Our conditional allele removes the second Dicer RNaseIII domain but leaves the rest of the protein intact. This allele drastically reduces or possibly eliminates all miRNA processing in the limb mesoderm and is clearly important for cell division. In addition, Cre-mediated recombination of the conditional allele in the germ-line phenocopies the reported Dicer-null allele. However, we cannot rule out the possibility that the conditional allele is not null for all aspects of Dicer function in the vertebrate limb.
Surprisingly, given the large number of miRNAs identified in vertebrates and their known ability to modulate gene activity, we did not observe differentiation or patterning defects upon loss of Dicer activity. These data do not rule out early patterning roles for Dicer, because many critical aspects of limb patterning occur in the earliest stages of limb development when Dicer protein or processed miRNAs might still be present in the limb cells in our conditional mice. However, from mid-bud stage on we see a dramatic decrease in miRNA processing. Moreover, at later stages all Dicer activity should be absent. Thus, it is striking that later patterning events, such as segmentation of cartilage, and differentiation of the various lineages in the limb, such as the sequential cellular transitions of endochondrial ossification, proceed normally. These data suggest that Dicer (and, as a consequence, we conclude miRNAs) are not involved in the specification of individual tissue types in the vertebrate limb. These data are consistent with the recent observation that zebrafish (14) also specify individual tissues types in embryos lacking both maternal and zygotic Dicer.
Acknowledgments
We thank Gail Martin (University of California, San Francisco) and Xin Sun (University of Wisconsin, Madison) for helpful discussions and probes, Kate Harfe for critically reading the manuscript, Ava Brent for help with figures, Susan Ellor for performing in situ hybridizations, and Phil Sharp for supporting aspects of this project in his laboratory. C.J.T. was supported by March of Dimes Grant FY03-83, and B.D.H. was supported by a grant from Oak Ridge Associated Universities.
Author contributions: B.D.H. and M.T.M. designed research; B.D.H., M.T.M., and J.H.M. performed research; B.D.H., M.T.M., and C.J.T. analyzed data; E.H. contributed new reagents/analytic tools; and B.D.H. wrote the paper.
Abbreviations: En, embryonic day n; ERK, extracellular signal-regulated kinase; miRNA, microRNA; RTK, receptor tyrosine kinase; ZPA, zone of polarizing activity.
References
- 1.Bartel, D. P. (2004) Cell 116, 281-297. [DOI] [PubMed] [Google Scholar]
- 2.Ambros, V. (2004) Nature 431, 350-355. [DOI] [PubMed] [Google Scholar]
- 3.Lee, R. C., Feinbaum, R. L. & Ambros, V. (1993) Cell 75, 843-854. [DOI] [PubMed] [Google Scholar]
- 4.Reinhart, B. J., Slack, F. J., Basson, M., Pasquinelli, A. E., Bettinger, J. C., Rougvie, A. E., Horvitz, H. R. & Ruvkun, G. (2000) Nature 403, 901-906. [DOI] [PubMed] [Google Scholar]
- 5.Wightman, B., Ha, I. & Ruvkun, G. (1993) Cell 75, 855-862. [DOI] [PubMed] [Google Scholar]
- 6.McManus, M. T. & Sharp, P. A. (2002) Nat. Rev. Genet. 3, 737-747. [DOI] [PubMed] [Google Scholar]
- 7.Lewis, B. P., Burge, C. B. & Bartel, D. P. (2005) Cell 120, 15-20. [DOI] [PubMed] [Google Scholar]
- 8.Brennecke, J., Hipfner, D. R., Stark, A., Russell, R. B. & Cohen, S. M. (2003) Cell 113, 25-36. [DOI] [PubMed] [Google Scholar]
- 9.Lin, S. Y., Johnson, S. M., Abraham, M., Vella, M. C., Pasquinelli, A., Gamberi, C., Gottlieb, E. & Slack, F. J. (2003) Dev. Cell 4, 639-650. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, S. M., Grosshans, H., Shingara, J., Byrom, M., Jarvis, R., Cheng, A., Labourier, E., Reinert, K. L., Brown, D. & Slack, F. J. (2005) Cell 120, 635-647. [DOI] [PubMed] [Google Scholar]
- 11.Lagos-Quintana, M., Rauhut, R., Yalcin, A., Meyer, J., Lendeckel, W. & Tuschl, T. (2002) Curr. Biol. 12, 735-739. [DOI] [PubMed] [Google Scholar]
- 12.Lee, Y., Ahn, C., Han, J., Choi, H., Kim, J., Yim, J., Lee, J., Provost, P., Radmark, O., Kim, S. & Kim, V. N. (2003) Nature 425, 415-419. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein, E., Caudy, A. A., Hammond, S. M. & Hannon, G. J. (2001) Nature 409, 363-366. [DOI] [PubMed] [Google Scholar]
- 14.Giraldez, A. J., Cinalli, R. M., Glasner, M. E., Enright, A. J., Thomson, M. J., Baskerville, S., Hammond, S. M., Bartel, D. P. & Schier, A. F. (2005) Science 308, 833-838. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein, E., Kim, S. Y., Carmell, M. A., Murchison, E. P., Alcorn, H., Li, M. Z., Mills, A. A., Elledge, S. J., Anderson, K. V. & Hannon, G. J. (2003) Nat. Genet. 35, 215-217. [DOI] [PubMed] [Google Scholar]
- 16.Wienholds, E., Koudijs, M. J., van Eeden, F. J., Cuppen, E. & Plasterk, R. H. (2003) Nat. Genet. 35, 217-218. [DOI] [PubMed] [Google Scholar]
- 17.Kanellopoulou, C., Muljo, S. A., Kung, A. L., Ganesan, S., Drapkin, R., Jenuwein, T., Livingston, D. M. & Rajewsky, K. (2005) Genes Dev. 19, 489-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logan, M., Martin, J. F., Nagy, A., Lobe, C., Olson, E. N. & Tabin, C. J. (2002) Genesis 33, 77-80. [DOI] [PubMed] [Google Scholar]
- 19.Harfe, B. D., Scherz, P. J., Nissim, S., Tian, H., McMahon, A. P. & Tabin, C. J. (2004) Cell 118, 517-528. [DOI] [PubMed] [Google Scholar]
- 20.Brent, A. E. & Tabin, C. J. (2004) Development 131, 3885-3896. [DOI] [PubMed] [Google Scholar]
- 21.Karp, S. J., Schipani, E., St-Jacques, B., Hunzelman, J., Kronenberg, H. & McMahon, A. P. (2000) Development 127, 543-548. [DOI] [PubMed] [Google Scholar]
- 22.Murtaugh, L. C., Chyung, J. H. & Lassar, A. B. (1999) Genes Dev. 13, 225-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson, D. G. (1992) in In Situ Hybridization: A Practical Approach, ed. Wilkinson, D. G. (IRL, Oxford), pp. 75-83.
- 24.Fukagawa, T., Nogami, M., Yoshikawa, M., Ikeno, M., Okazaki, T., Takami, Y., Nakayama, T. & Oshimura, M. (2004) Nat. Cell Biol. 6, 784-791. [DOI] [PubMed] [Google Scholar]
- 25.Volpe, T., Schramke, V., Hamilton, G. L., White, S. A., Teng, G., Martienssen, R. A. & Allshire, R. C. (2003) Chromosome Res. 11, 137-146. [DOI] [PubMed] [Google Scholar]
- 26.Provost, P., Silverstein, R. A., Dishart, D., Walfridsson, J., Djupedal, I., Kniola, B., Wright, A., Samuelsson, B., Radmark, O. & Ekwall, K. (2002) Proc. Natl. Acad. Sci. USA 99, 16648-16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abrams, J. M., White, K., Fessler, L. I. & Steller, H. (1993) Development 117, 29-43. [DOI] [PubMed] [Google Scholar]
- 28.Heanue, T. A., Johnson, R. L., Izpisua-Belmonte, J. C., Stern, C. D., De Robertis, E. M. & Tabin, C. J. (1997) Mech. Dev. 69, 31-37. [DOI] [PubMed] [Google Scholar]
- 29.ten Berge, D., Brouwer, A., Korving, J., Reijnen, M. J., van Raaij, E. J., Verbeek, F., Gaffield, W. & Meijlink, F. (2001) Development 128, 2929-2938. [DOI] [PubMed] [Google Scholar]
- 30.Minowada, G., Jarvis, L. A., Chi, C. L., Neubuser, A., Sun, X., Hacohen, N., Krasnow, M. A. & Martin, G. R. (1999) Development 126, 4465-4475. [DOI] [PubMed] [Google Scholar]
- 31.Corson, L. B., Yamanaka, Y., Lai, K. M. & Rossant, J. (2003) Development 130, 4527-4537. [DOI] [PubMed] [Google Scholar]
- 32.Singh, J. D. & Singh, S. (1976) Acta Orthop. Scand. 47, 509-514. [DOI] [PubMed] [Google Scholar]
- 33.Volpe, T. A., Kidner, C., Hall, I. M., Teng, G., Grewal, S. I. & Martienssen, R. A. (2002) Science 297, 1833-1837. [DOI] [PubMed] [Google Scholar]
- 34.Hall, I. M., Noma, K. & Grewal, S. I. (2003) Proc. Natl. Acad. Sci. USA 100, 193-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansfield, J. H., Harfe, B. D., Nissen, R., Obenauer, J., Srineel, J., Chaudhuri, A., Farzan-Kashani, R., Zuker, M., Pasquinelli, A. E., Ruvkun, G., et al. (2004) Nat. Genet. 36, 1079-1083. [DOI] [PubMed] [Google Scholar]