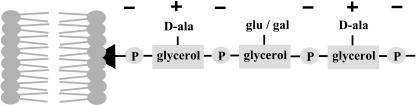
The composition of lipotechoic acid modulates the immune response.
The intestinal tract is colonized by a myriad of mainly Gram-positive microbes that show considerable spatial and temporal diversity (1–3). These intestinal microbiota reach astronomical numbers, and it has been estimated that the collective microbial genomes in our gut may contain >100 times more genes than the human genome (3). A prime function of the intestinal microbiota includes the processing of dietary components and host-produced polymers, such as mucus, leading to the production of short-chain fatty acids and a wealth of other metabolites. Moreover, several intestinal microbes develop an intimate relation with the host and modulate nutrient processing, immune function, and a variety of other host activities (4).
Lactic Acid Bacteria and Their Interactions with the Host
Notably, lactic acid bacteria, which are major components of the human upper intestinal tract, have been considered to entertain beneficial interactions with the host by producing specific metabolites or stimulating the production of specific cytokines. Their communication with the host, however, has not been addressed in detail. This interaction is specifically of interest in view of the growing application of lactic acid bacteria in dairy and other products that are marketed as probiotics (5). In a recent issue of PNAS, Annick Mercenier and her research team (6) described a novel way by which lactic acid bacteria may effect the production of inflammation-related cytokines and protect the host from intestinal disorders. They showed that the composition of lipotechoic acid (LTA; see Fig. 1), specifically the d-alanine content, modulates the immune response and determines protection in a murine colitis model. Use was made of isogenic strains of Lactobacillus plantarum, an intestinal inhabitant that is accessible to genetic modification and from which the complete genome has been determined (7). The strains of L. plantarum used have been developed into a paradigm for intestinal host–microbe interactions, and several of their >200 cell envelope-located proteins have been found to be involved in persistence in the intestinal tract (8).
Fig. 1.
Schematic representation of L. plantarum LTA anchored in the membrane with the glycolipid anchor (black arrowhead). The net charge is indicated.
It has been established that specific cell envelope structures of intestinal microbes are recognized by the host and involved in both the inflammatory response and the maintenance of intestinal epithelial homeostasis (9). The signaling is mediated by pattern recognition receptors (PRRs) that activate inflammatory pathways and hence alert the host (10). These PRRs include Toll-like receptors (TLRs) that are located in the cell membrane and may be used to discriminate between self- and non-self microbes. Notably, non-protein cell envelope components have been identified as ligands for TLRs, and a well known example is TLR4, which recognizes LPS, a pathogenic determinant present in the cell envelope of Gram-negative bacteria (10). The Gram-positive bacteria that are dominant in the intestinal tract do not contain LPS, but their cell envelope harbors a variety of other polymers, including LTAs (11). LTAs are docked in the cellular membrane by a glycolipid anchor and constitute amphiphilic polymers made up of repeating units of glycerophosphate that are decorated with d-alanine and glycosyl residues (Fig. 1). These d-alanine residues are formed from l-alanine by an alanine racemase and coupled to the LTA by the activity of the expression products of the dltABCD operon. By insertional inactivation of the dlt operon, Mercenier and collaborators (6) constructed an isogenic L. plantarum mutant that was deficient in d-alanylation as evidenced by the almost complete absence of d-alanine residues in the purified LTA, which showed somewhat higher levels of glucose residues.
Absence of d-Alanine in LTA Affects Host Interactions of L. plantarum
The Dlt- mutant was compared with the wild-type L. plantarum in a series of in vitro experiments using peripheral blood mononuclear cells and monocytes that showed a significant reduced secretion of proinflammatory cytokines, including IL-12 and TNFα, that was found to be TLR2-dependent. Similar signaling by means of TLR2 had been described previously in studies with purified or synthetic LTA from the pathogen Staphylococcus aureus, which also showed that replacing d-alanine by l-alanine reduced the proinflammatory activity of LTA 100-fold while suggesting a stereospecific effect (12). However, the studies with L. plantarum show for the first time specific signaling by intact intestinal bacteria in a way that eliminates artifacts due to purification or synthesis. Moreover, an increased production of IL-10 was observed after exposure to the Dlt- mutant as compared with the parental strain. This IL-10 production is of significant interest because this cytokine has been implicated in down-regulating inflammatory cascades. The exact mechanism by which the IL-10 induction is affected has to be established, and indirect effects on the cell envelope by the absence of d-alanylation are discussed (6). However, specific signaling by means of TLR9 that recognizes GpC motifs in genomic DNA (13) has to be considered because it is known that dlt operon inactivation weakens the cell envelope and may induce specific lysis of lactic acid bacteria (14).
Remarkably, the induction of IL-10 was also observed in vivo by using a colitis murine model (6). Moreover, it was shown that administration of mutant cells was significantly more protective than cells of the parental L. plantarum. These findings indicate that the LTA composition of complete cells of lactic acid bacteria can modulate the inflammatory response and change it to an antiinflammatory equilibrium. This is a significant observation because it paves the way for the development of rational therapies using mutant lactic acid bacteria for intestinal disorders, such as intestinal bowel disease, which are increasing in the Western world (10). A genetically modified Lactococcus lactis strain overproducing IL-10 has successfully been used to deliver IL-10 in the intestine and has provided protection in murine colitis models (15). Further research has to show whether the level of IL-10 production induced by cells of L. plantarum Dlt- mutant is comparable with this dedicated delivery and whether these are effective in human trials. The present study of Mercenier and colleagues (6), however, shows that the immunomodulatory properties of L. plantarum can be improved by directed mutation rather than by genetic modification and opens the way for further exploiting the genomes of probiotic and other lactic acid bacteria (16).
Author contributions: W.M.d.V. wrote the paper.
See companion article on page 10321 in issue 29 of volume 102.
References
- 1.Eckburg, P. B., Bik, E. M., Bernstein, C. N., Purdom, E., Dethlefsen, L., Sargent, M., Gill, S. R., Nelson, K. E. & Relman, D. A. (2005) Science 308, 1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoetendal, E. G., Ben-Amor, K., Harmsen, H. J., Schut, F., Akkermans, A. D. & de Vos, W. M. (2002) Appl. Environ. Microbiol 68, 4225-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. (2005) Science 307, 1915-1919. [DOI] [PubMed] [Google Scholar]
- 4.Hooper, L. V., Midtvedt, T. & Gordon, J. I. (2002) Annu. Rev. Nutr. 22, 283-307. [DOI] [PubMed] [Google Scholar]
- 5.Saxelin, M., Tynkkynen, S., Mattila-Sandholm, T. & de Vos, W. M. (2005) Curr. Opin. Biotechnol. 16, 204-211. [DOI] [PubMed] [Google Scholar]
- 6.Grangette, C., Nutten, S., Palumbo, E., Morath, S., Hermann, C., Dewulf, J., Pot, B., Hartung, T., Hols, P. & Mercenier, A. (2005) Proc. Natl. Acad. Sci. USA 102, 10321-10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleerebezem, M., Boekhorst, J., van Kranenburg, R., Molenaar, D., Kuipers, O. P., Leer, R., Tarchini, R., Peters, S. A., Sandbrink, H. M., Fiers, M. W., et al. Proc. Natl. Acad. Sci. USA 100, 1990-1995. [DOI] [PMC free article] [PubMed]
- 8.Bron, P. A., Gangrette, C., Mercenier, A., de Vos, W. M. & Kleerebezem, M. (2004) J. Bacteriol. 186, 5721-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rakoff-Nahoun, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. (2004) Cell 118, 229-241. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald, T. T. & Monteleone, G. (2005) Science 307, 1920-1925. [DOI] [PubMed] [Google Scholar]
- 11.Delcour, J., Ferain, T., Deghorain, M., Palumbo, E. & Hols, P. (1999) Antonie Leeuwenhoek 76, 159-184. [PubMed] [Google Scholar]
- 12.Morath, S., Stadelmaier, A., Geyer, A., Schmidt, R. R. & Hartung, T. (2002) J. Exp. Med. 195, 1635-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rachmilewitz, D., Katakura, K., Karmeli, F., Hayashi, T., Reinus, C., Rudensky, B., Akira, S., Takeda, K., Lee, J., Takabayashi, K. & Raz, E. (2004) Gastroenterology 126, 520-528. [DOI] [PubMed] [Google Scholar]
- 14.Steen, A., Palumbo, E., Deghorain, M., Cocconcelli, P. S., Delcour, J., Kuipers, O. P., Kok, J., Buist, G. & Hols, P. (2005) J. Bacteriol. 187, 114-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steidler, L., Schotte, H. W., Neirynck, S., Obermeier, F., Falk, W., Fiers, W. & Remaut, E. (2000) Science 289, 1311-1312. [DOI] [PubMed] [Google Scholar]
- 16.de Vos, W. M., Bron, P. A. & Kleerebezem, M. (2004) Curr. Opin. Biotechnol. 15, 86-93. [DOI] [PubMed] [Google Scholar]