Abstract
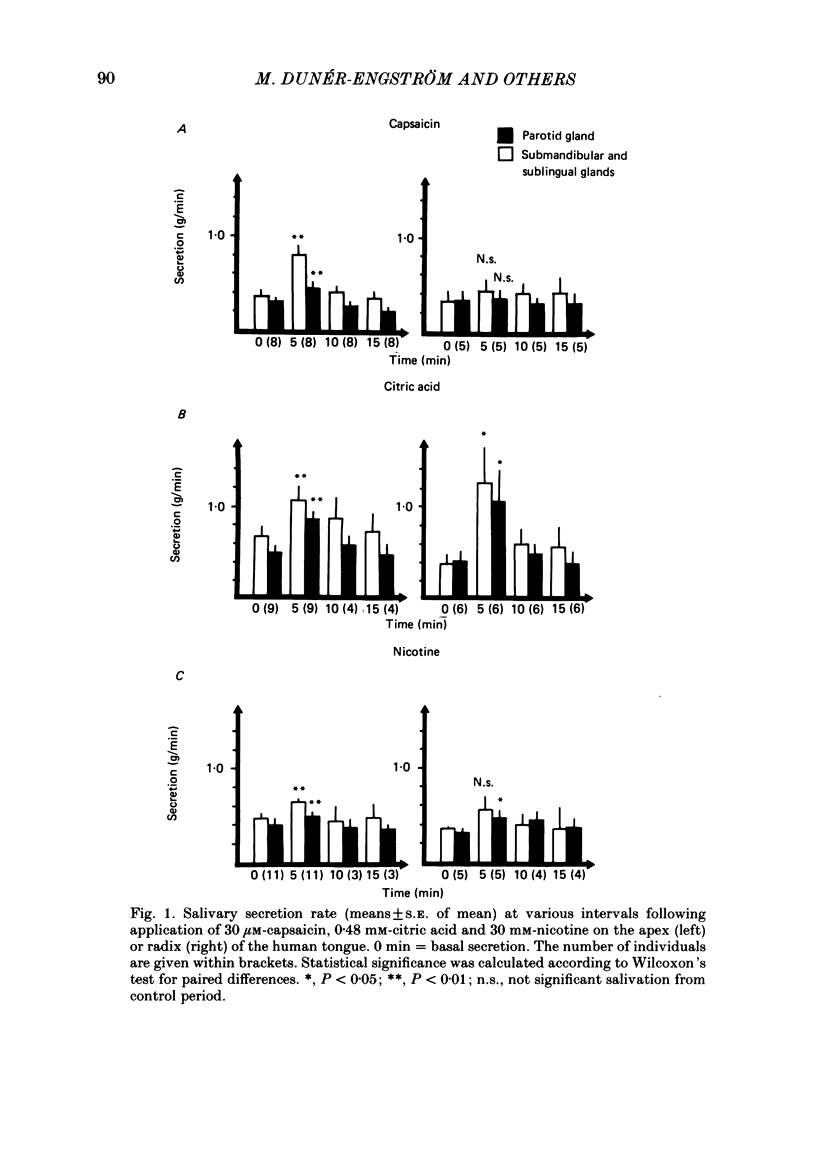
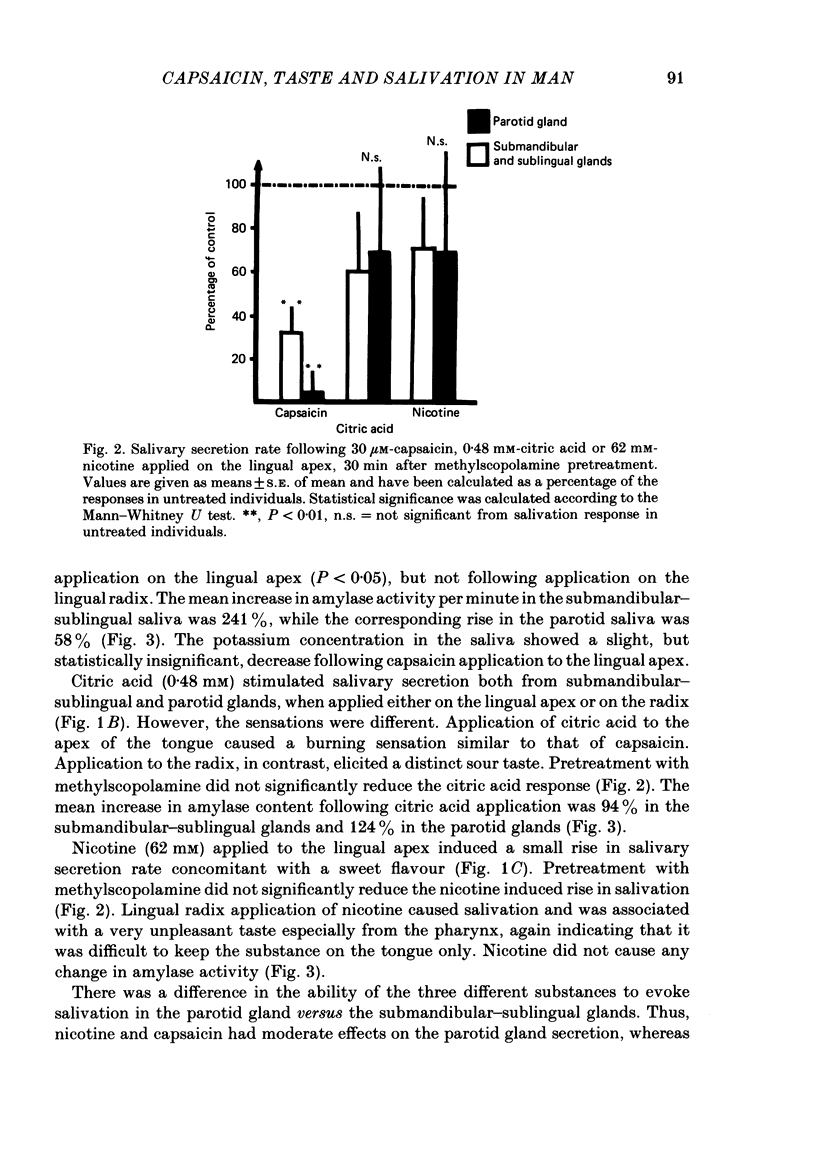
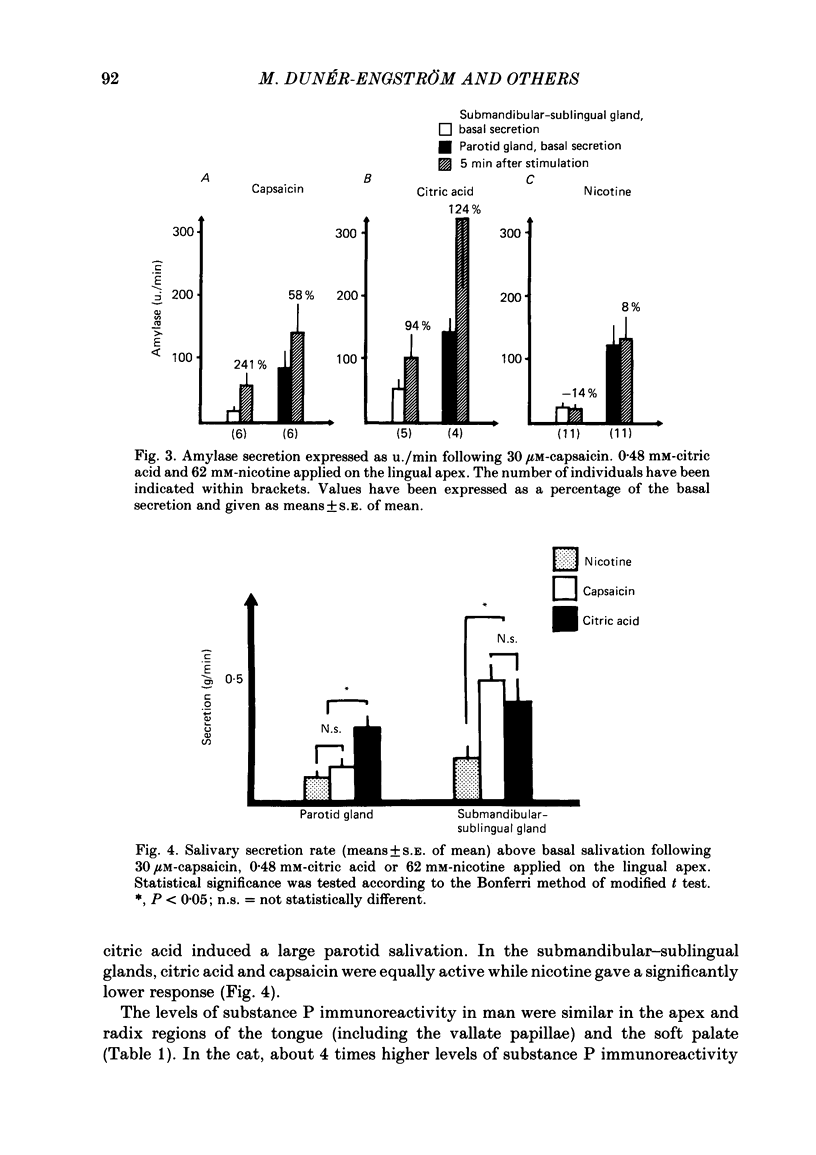
The effects of capsaicin, citric acid and nicotine applied to the apex or radix of the tongue on taste sensations and salivation were studied in relation to the presence of substance P immunoreactive neurones in man. Application of capsaicin (30 micron) to the apex of the tongue or to the palatinal mucosa, but not to the radix of the tongue, caused a reproducible burning sensation and salivation from the submandibular-sublingual and parotid glands. The salivation response to capsaicin was reduced by methylscopolamine pretreatment. Similar levels of substance P immunoreactivity were present in the lingual apex and radix area (including vallate papillae) of man, while in the cat about 4 times higher levels of substance P immunoreactivity were present in the vallate papillae than in the lingual apex. Immunohistochemistry showed that in the cat many substance P immunoreactive nerves were associated with the taste buds of the vallate papillae, while in man substance P immunoreactive fibres were only seen penetrating into the epithelium of the lingual apex. In addition some subepithelial blood vessels in all regions were surrounded by substance P immunoreactive nerves in both cat and man. Citric acid application to the tongue apex caused both submandibular-sublingual and parotid salivary secretion concomitant with a burning sensation. Salivary secretion was also seen after citric acid application to the radix of the tongue. This response was associated with a sour taste. The salivation response to citric acid was not significantly reduced by methylscopolamine pretreatment. Lingual apex application of nicotine was associated with a sweet taste and a small rise in salivary secretion rate. This response was not significantly reduced by methylscopolamine. In conclusion, the sensitivity to capsaicin of the human tongue is restricted to the apex portion. This is in parallel with the occurrence of intraepithelial substance P immunoreactive nerve fibres. Capsaicin induced salivary secretion seems mainly to be mediated via parasympathetic, cholinergic reflex mechanisms. Citric acid and nicotine induced salivation responses are comparatively more resistant to methylscopolamine pretreatment.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuello A. C., Galfre G., Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. A method for the determination of amylase in intestinal content. Scand J Clin Lab Invest. 1962;14:145–151. doi: 10.3109/00365516209079686. [DOI] [PubMed] [Google Scholar]
- DIAMANT H., ENFORS B., HOLMSTEDT B. Salivary secretion in man elicited by means of stimulation of the chorda tympani. Acta Physiol Scand. 1959 Apr 22;45(4):293–299. doi: 10.1111/j.1748-1716.1959.tb01701.x. [DOI] [PubMed] [Google Scholar]
- Davis B., Roberts A. M., Coleridge H. M., Coleridge J. C. Reflex tracheal gland secretion evoked by stimulation of bronchial C-fibers in dogs. J Appl Physiol Respir Environ Exerc Physiol. 1982 Oct;53(4):985–991. doi: 10.1152/jappl.1982.53.4.985. [DOI] [PubMed] [Google Scholar]
- Emmelin N. Nervous control of mammalian salivary glands. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):27–35. doi: 10.1098/rstb.1981.0168. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. Capsaicin and sensory neurones--a review. Pain. 1983 Feb;15(2):109–130. doi: 10.1016/0304-3959(83)90012-x. [DOI] [PubMed] [Google Scholar]
- Gamse R., Wax A., Zigmond R. E., Leeman S. E. Immunoreactive substance P in sympathetic ganglia: distribution and sensitivity towards capsaicin. Neuroscience. 1981;6(3):437–441. doi: 10.1016/0306-4522(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Hoyes A. D., Barber P. Degeneration of axons in the ureteric and duodenal nerve plexuses of the adult rat following in vivo treatment with capsaicin. Neurosci Lett. 1981 Aug 7;25(1):19–24. doi: 10.1016/0304-3940(81)90094-x. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br J Pharmacol Chemother. 1968 May;33(1):32–41. doi: 10.1111/j.1476-5381.1968.tb00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Iversen L. L., Cuello A. C. Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res. 1978 Aug 18;152(1):183–188. doi: 10.1016/0006-8993(78)90146-4. [DOI] [PubMed] [Google Scholar]
- LAAGE-HELLMAN J. E., STROMBLAD B. C. Secretion from human submaxillary gland after section of the chorda tympani. J Appl Physiol. 1960 Mar;15:295–297. doi: 10.1152/jappl.1960.15.2.295. [DOI] [PubMed] [Google Scholar]
- LEE T. S. Physiological gustatory sweating in a warm climate. J Physiol. 1954 Jun 28;124(3):528–542. doi: 10.1113/jphysiol.1954.sp005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Anggård A., Pernow B., Emson P. Immunohistochemical evidence for substance P immunoreactive nerve fibres in the taste buds of the cat. Acta Physiol Scand. 1979 Dec;107(4):389–391. doi: 10.1111/j.1748-1716.1979.tb06490.x. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Anggård A., Terenius L., Elde R., Markey K., Goldstein M., Kimmel J. Organizational principles in the peripheral sympathetic nervous system: subdivision by coexisting peptides (somatostatin-, avian pancreatic polypeptide-, and vasoactive intestinal polypeptide-like immunoreactive materials). Proc Natl Acad Sci U S A. 1982 Feb;79(4):1303–1307. doi: 10.1073/pnas.79.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Saria A., Martling C. R. Capsaicin pretreatment abolishes cigarette smoke-induced oedema in rat tracheo-bronchial mucosa. Eur J Pharmacol. 1982 Dec 24;86(2):317–318. doi: 10.1016/0014-2999(82)90338-7. [DOI] [PubMed] [Google Scholar]
- Mózsik G., Jávor T., Dobi S., Petrássy K., Szabó A. Development of 'pharmacological denervation phenomenon' in patients treated with atropine. Eur J Pharmacol. 1967 Sep;1(5):391–395. doi: 10.1016/0014-2999(67)90100-8. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Goedert M., Hunt S. P., Bond A. The nature of the substance P-containing nerve fibres in taste papillae of the rat tongue. Neuroscience. 1982;7(12):3137–3151. doi: 10.1016/0306-4522(82)90236-6. [DOI] [PubMed] [Google Scholar]
- Papka R. E., Furness J. B., Della N. G., Murphy R., Costa M. Time course of effect of capsaicin on ultrastructure and histochemistry of substance P-immunoreactive nerves associated with the cardiovascular system of the guinea-pig. Neuroscience. 1984 Aug;12(4):1277–1292. doi: 10.1016/0306-4522(84)90021-6. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J. A pharmacological approach to elucidation of the role of different nerve fibres and receptor endings in mediation of pain. J Physiol (Paris) 1977 Sep;73(3):251–259. [PubMed] [Google Scholar]
- Yamasaki H., Kubota Y., Takagi H., Tohyama M. Immunoelectron-microscopic study on the fine structure of substance-P-containing fibers in the taste buds of the rat. J Comp Neurol. 1984 Aug 10;227(3):380–392. doi: 10.1002/cne.902270308. [DOI] [PubMed] [Google Scholar]


