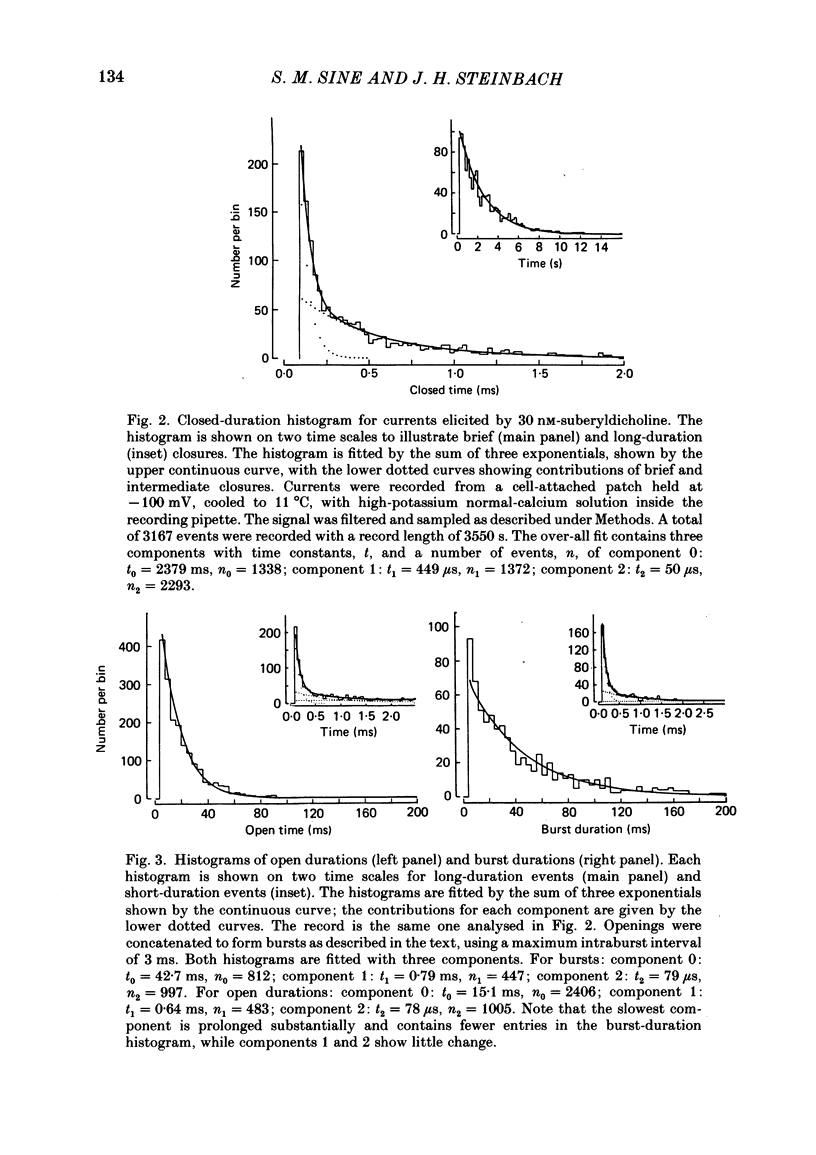
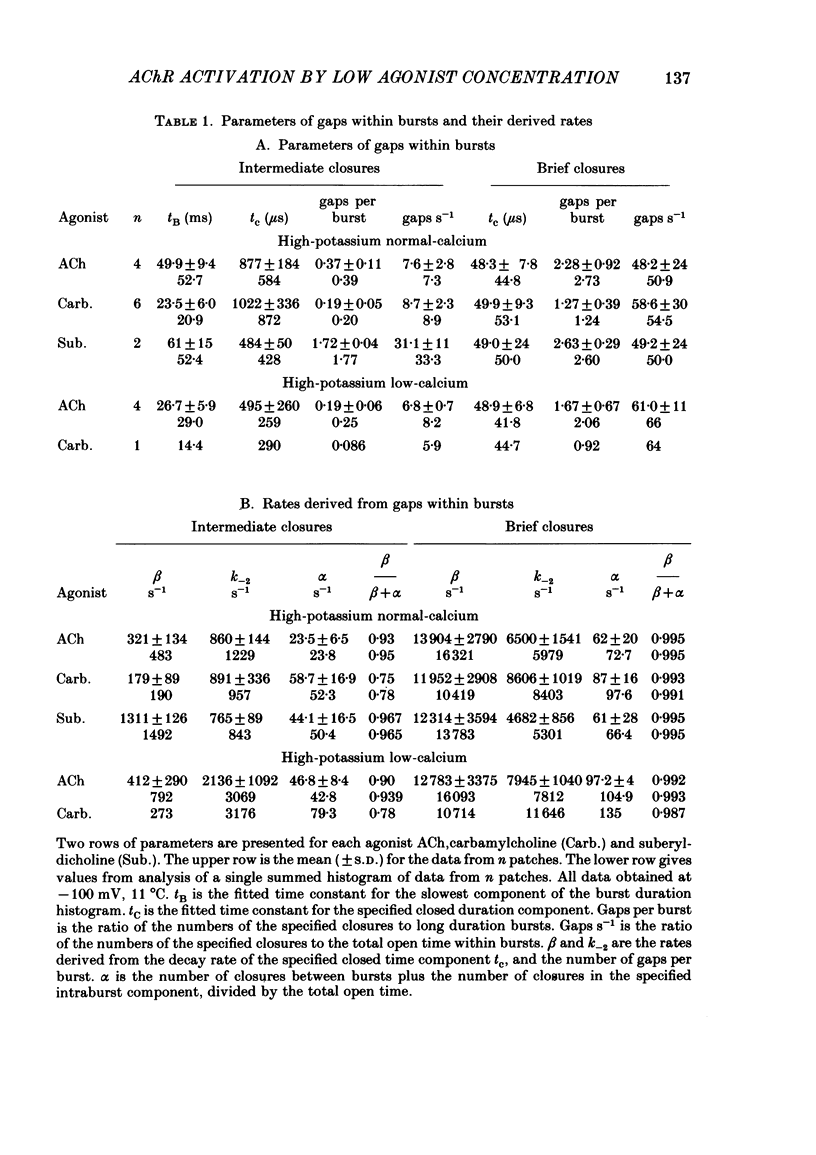
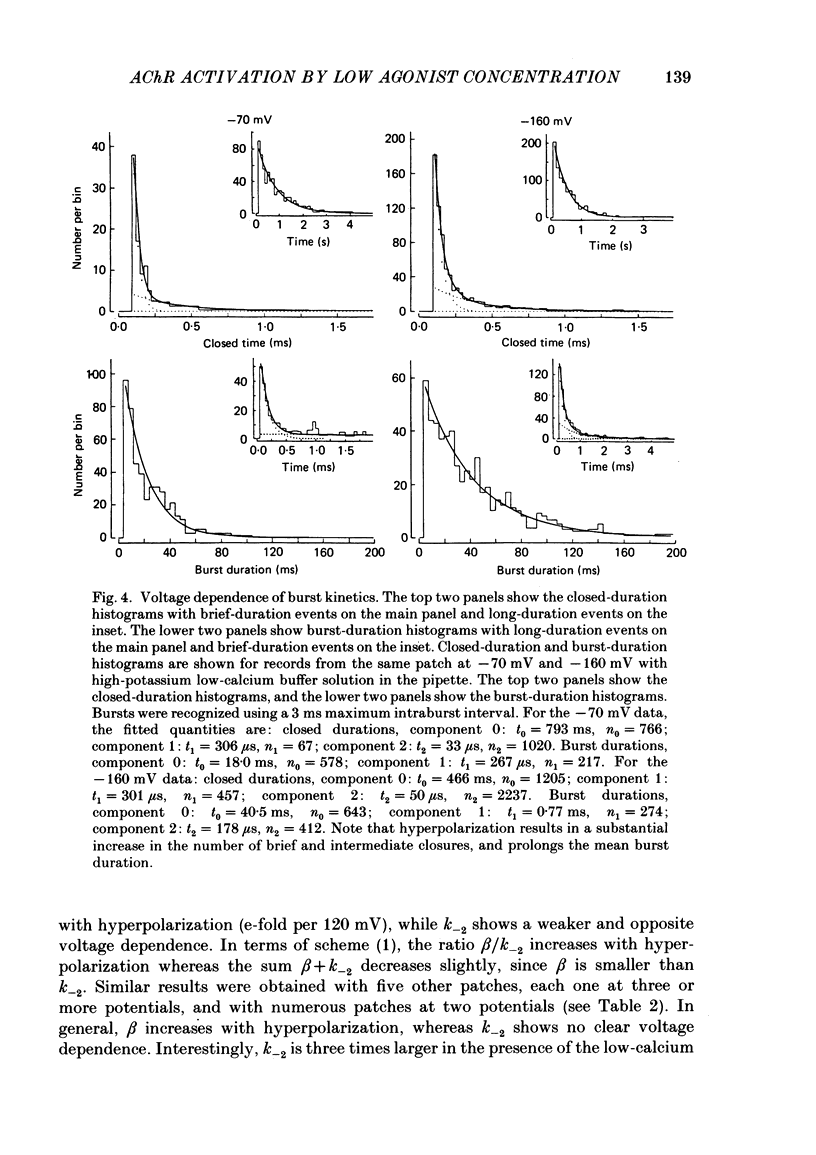
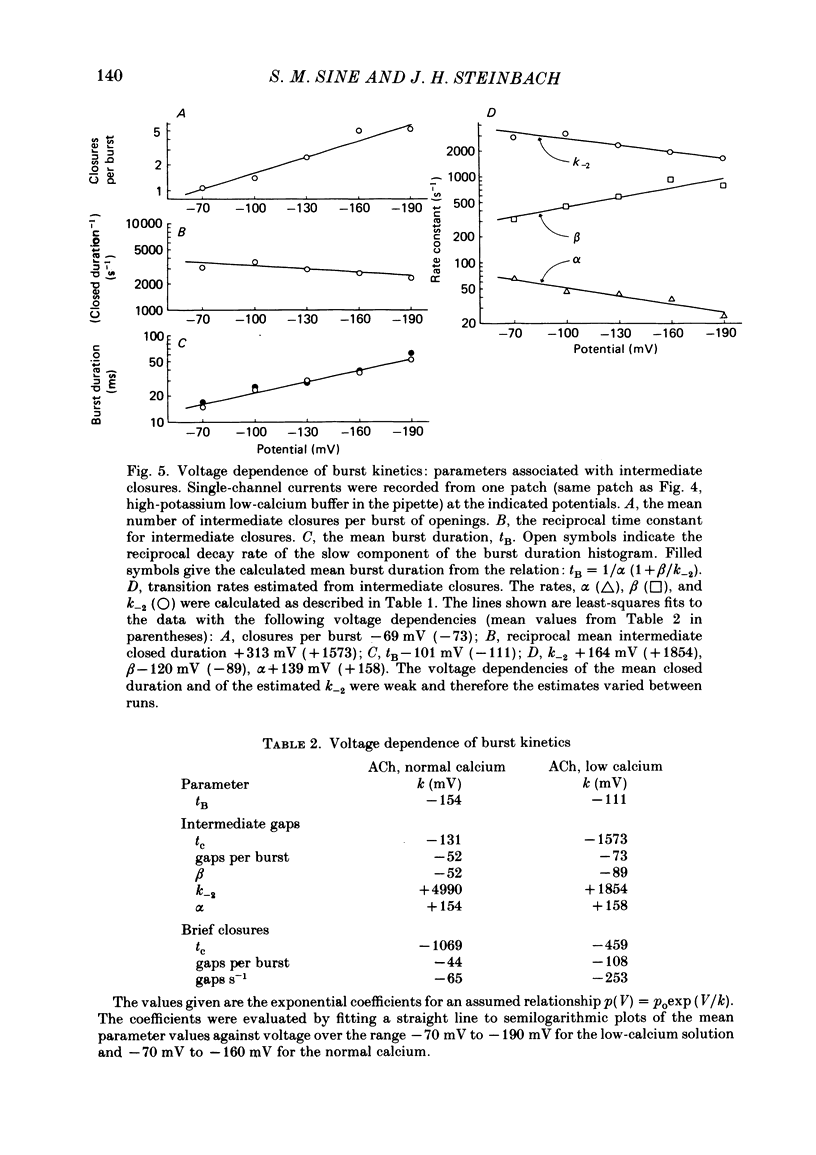
Abstract
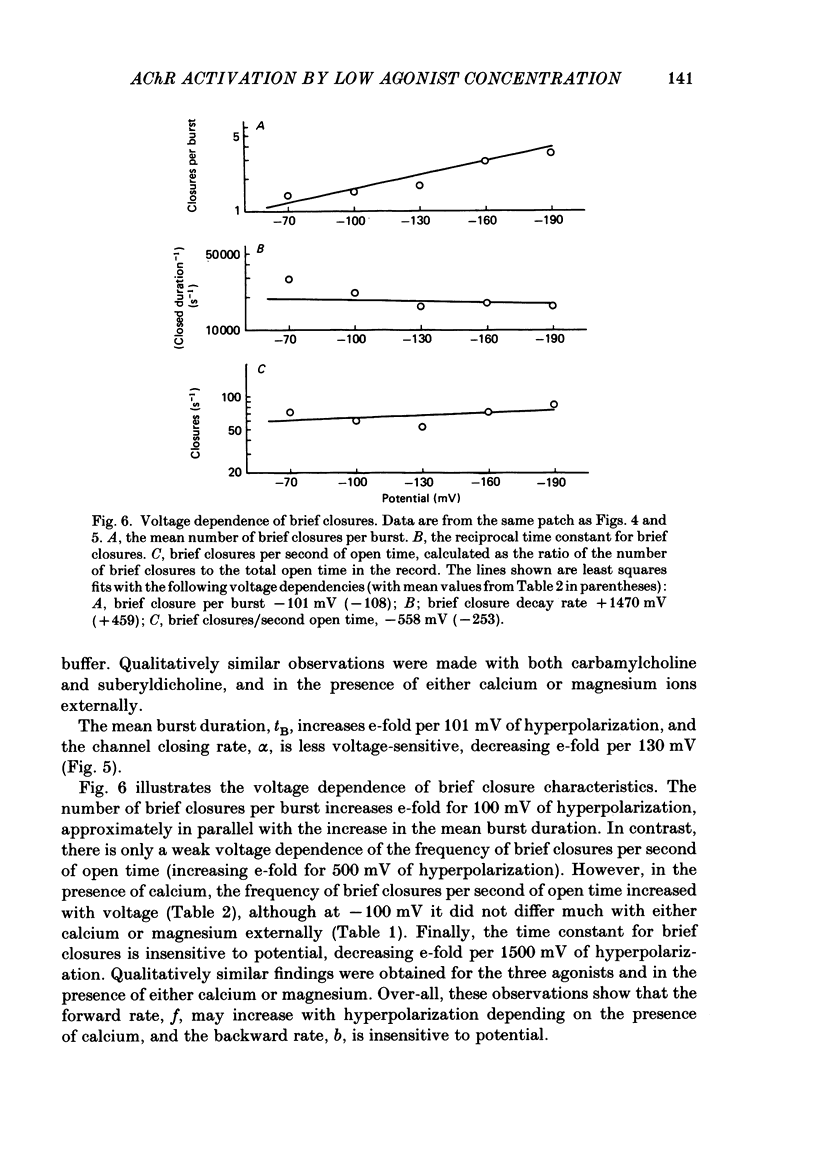
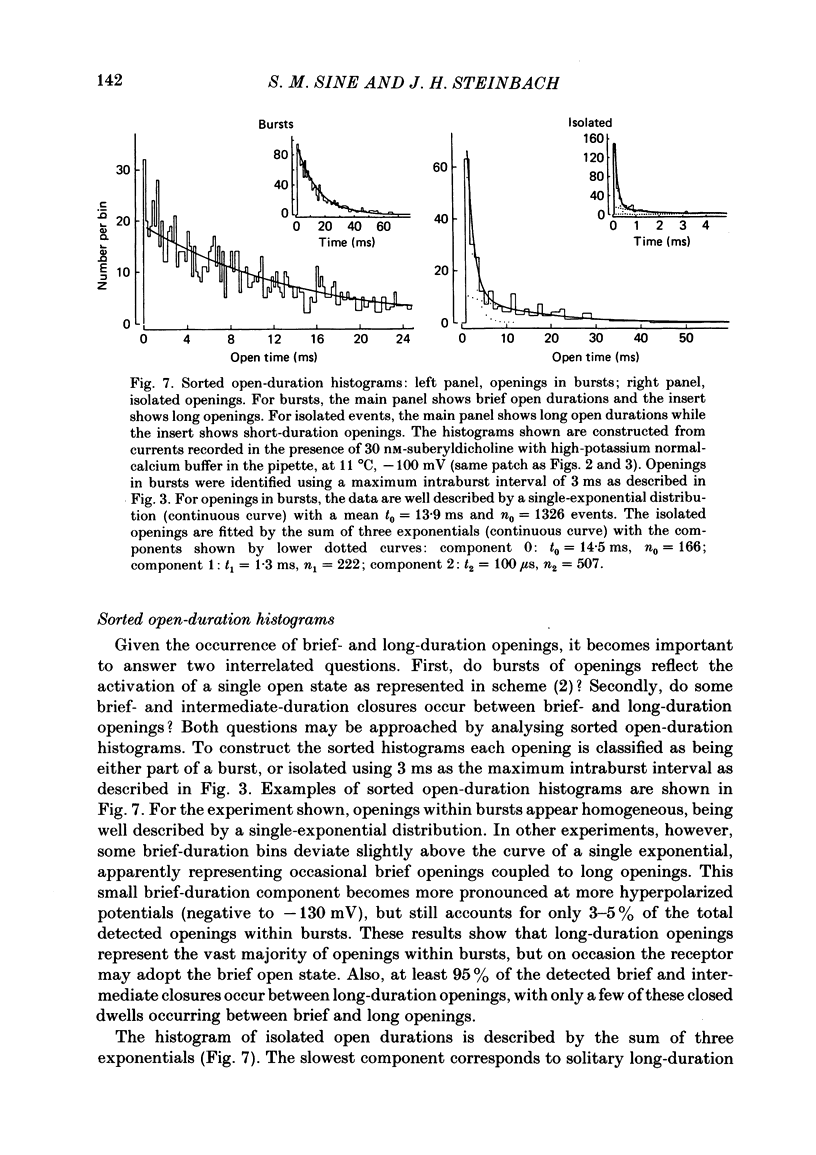
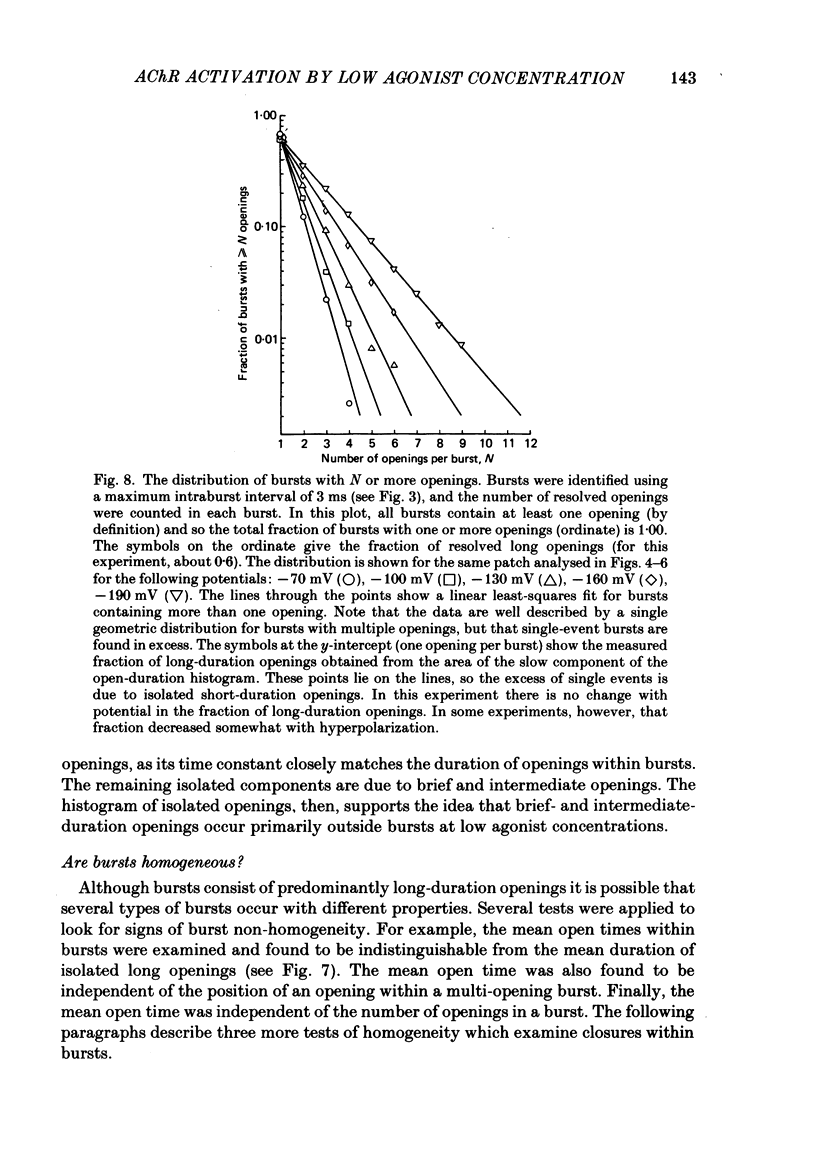
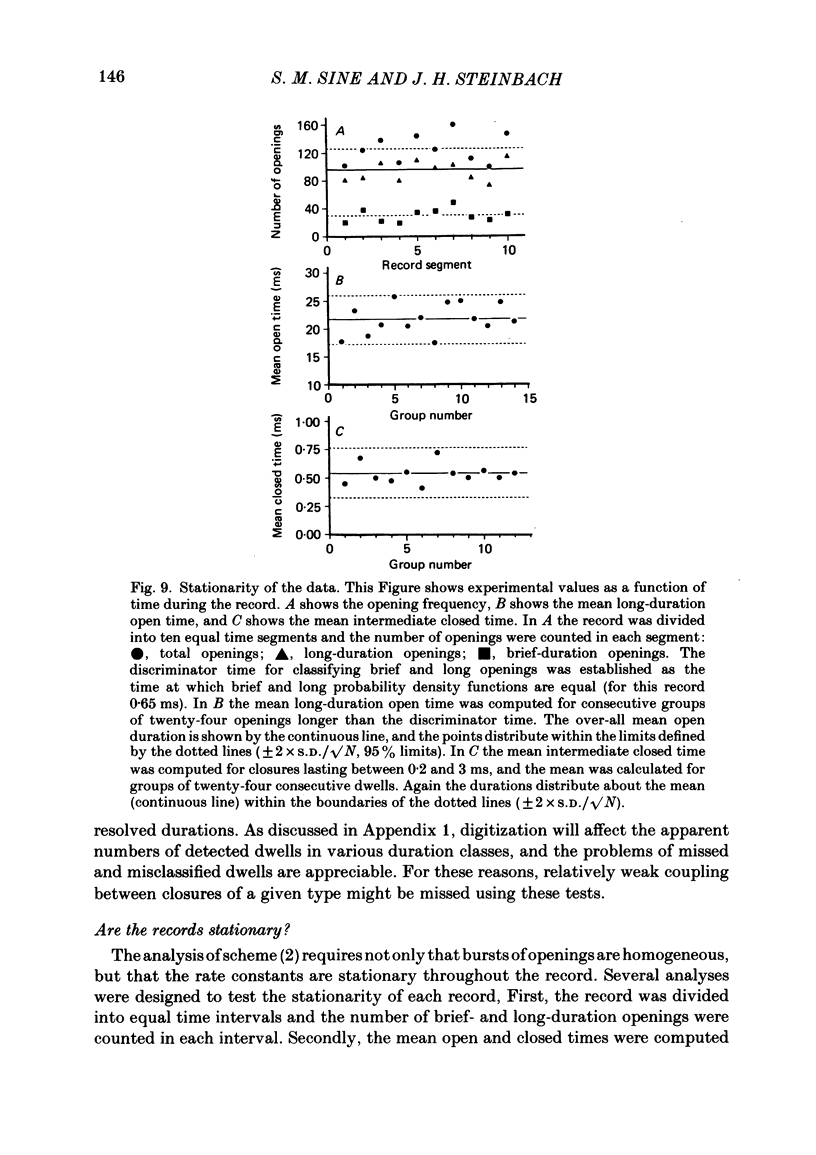
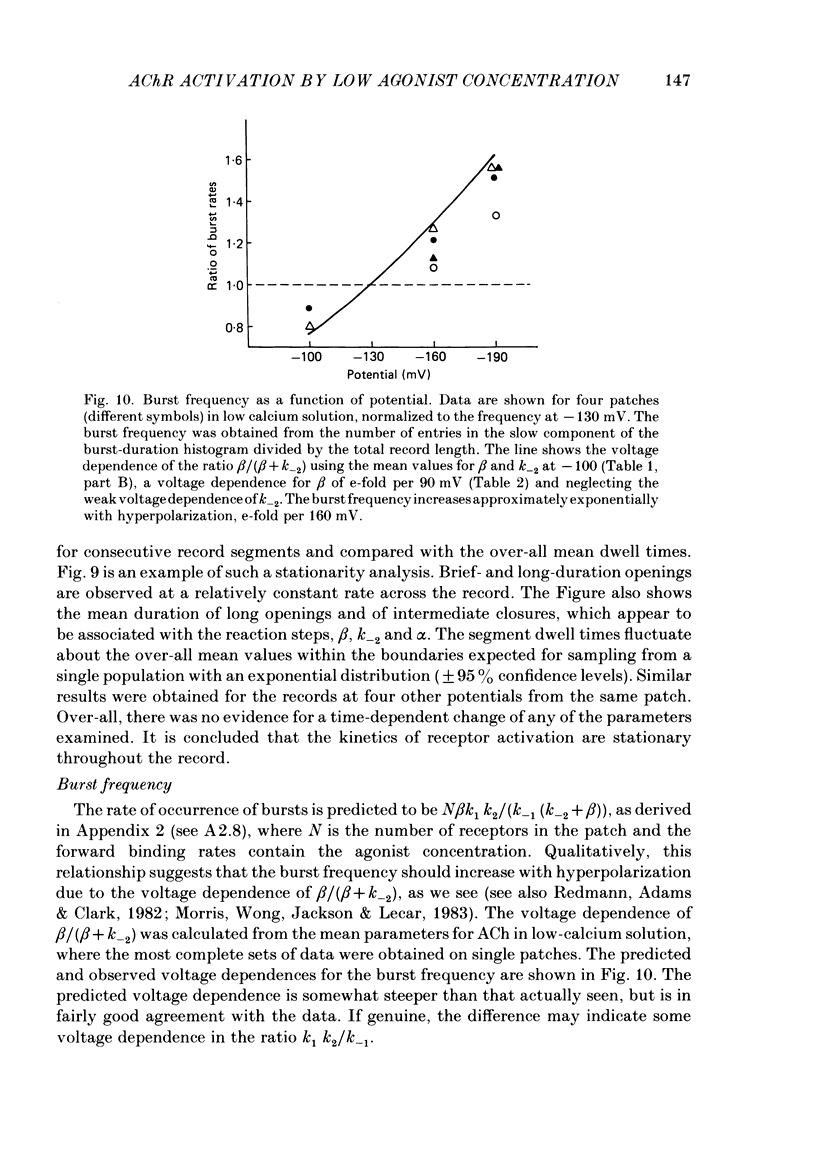
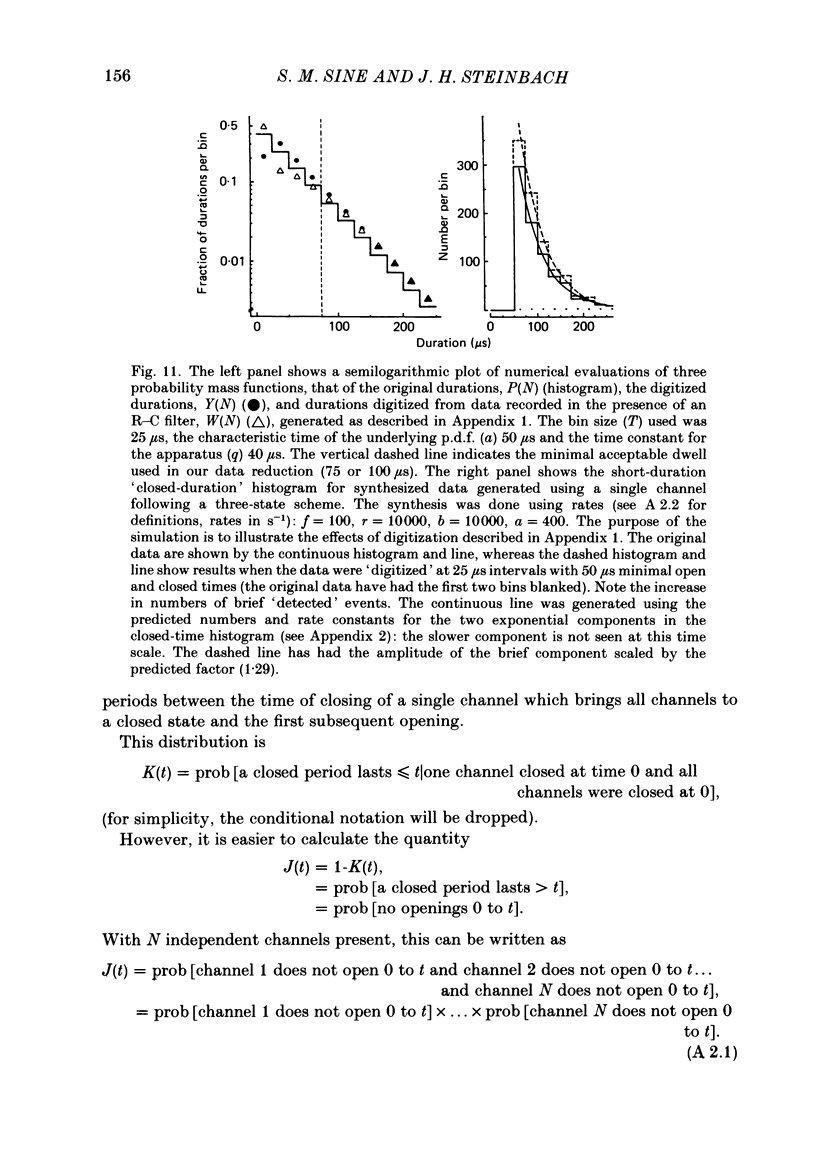
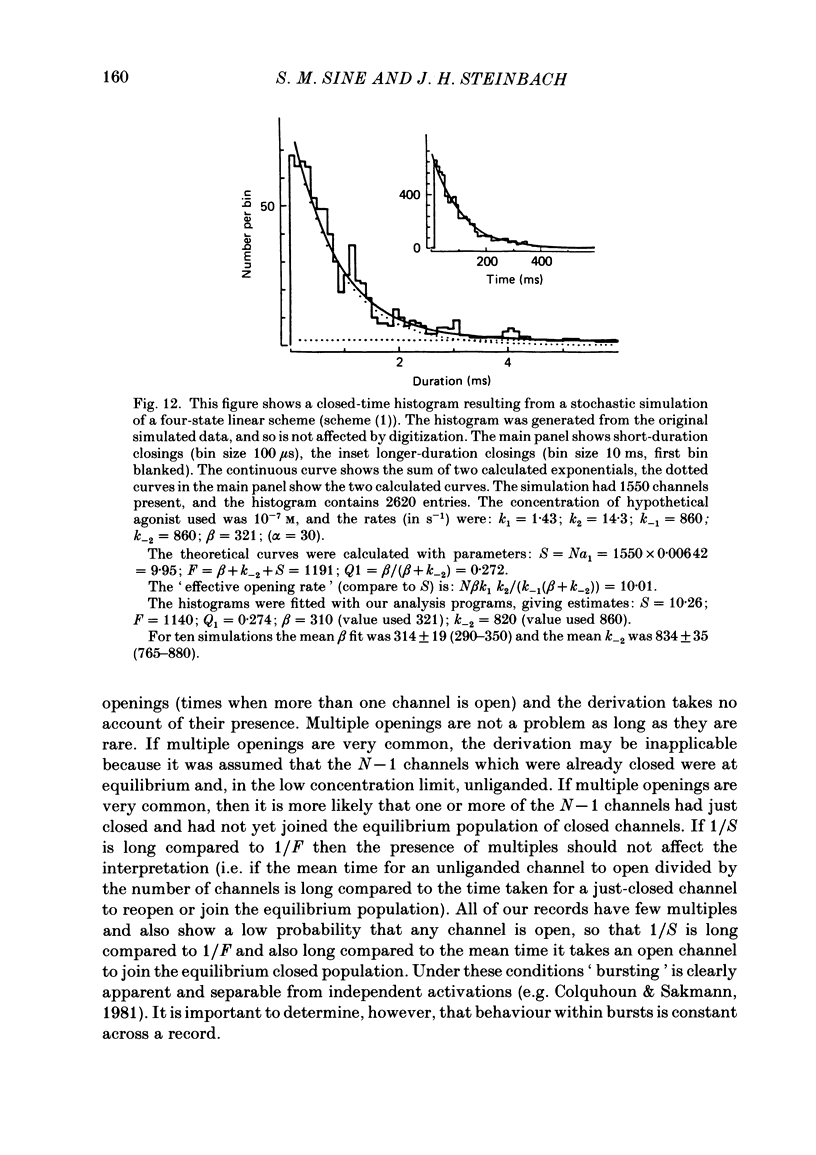
The patch-clamp technique was used to examine the activation of single acetylcholine receptor channels of clonal BC3H-1 mouse muscle cells. Single-channel currents were activated by low concentrations of the strong agonists acetylcholine (ACh, 50-100 nM), carbamylcholine (1-2 microM), and suberyldicholine (30-50 nM). At low agonist concentrations channel openings occur as isolated short-duration openings and as bursts of longer duration openings separated by brief closed periods. Two distinct types of brief closed periods separate long duration openings: brief closures (mean duration, 50 microseconds) and intermediate closures (mean duration, 0.5-1.0 ms). The kinetic properties of intermediate closures depend on the agonist, suggesting that they reflect receptor reopening from the closed state leading to the open state. Properties of brief closures, in contrast, are independent of the agonist, indicating that they result from an additional closed state leading away from the pathway producing the open state. A receptor activation scheme is proposed which accounts for the observed closed states, and transition rate estimates are presented for steps within the proposed scheme. The channel opening rate, beta, differs several-fold for the agonists studied (200-1400 s-1) and is comparable to the dissociation rate, k-2 (900 s-1). The dissociation rate is similar for the three agonists studied. The channel closing rate, alpha, is much slower than the opening rate (20-60 s-1). The probability is high that a doubly liganded channel is in the open state and depends on the agonist (0.75-0.97). Beta increases and alpha decreases at more negative membrane potentials, whereas k-2 shows little potential dependence.
Full text
PDF






















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Acetylcholine receptor kinetics. J Membr Biol. 1981 Feb 28;58(3):161–174. doi: 10.1007/BF01870902. [DOI] [PubMed] [Google Scholar]
- Auerbach A., Sachs F. Single-channel currents from acetylcholine receptors in embryonic chick muscle. Kinetic and conductance properties of gaps within bursts. Biophys J. 1984 Jan;45(1):187–198. doi: 10.1016/S0006-3495(84)84147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Sakmann B. Neurotrophic control of channel properties at neuromuscular synapses of rat muscle. J Physiol. 1983 Apr;337:159–171. doi: 10.1113/jphysiol.1983.sp014617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash D. J., Aoshima H., Hess G. P. Acetylcholine-induced cation translocation across cell membranes and inactivation of the acetylcholine receptor: chemical kinetic measurements in the millisecond time region. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3318–3322. doi: 10.1073/pnas.78.6.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Hawkes A. G. On the stochastic properties of single ion channels. Proc R Soc Lond B Biol Sci. 1981 Mar 6;211(1183):205–235. doi: 10.1098/rspb.1981.0003. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Leibowitz M. D. Acetylcholine receptor kinetics. A description from single-channel currents at snake neuromuscular junctions. Biophys J. 1982 Sep;39(3):253–261. doi: 10.1016/S0006-3495(82)84515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jackson M. B. Stochastic behavior of a many-channel membrane system. Biophys J. 1985 Feb;47(2 Pt 1):129–137. doi: 10.1016/s0006-3495(85)83886-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Wong B. S., Morris C. E., Lecar H., Christian C. N. Successive openings of the same acetylcholine receptor channel are correlated in open time. Biophys J. 1983 Apr;42(1):109–114. doi: 10.1016/S0006-3495(83)84375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz M. D., Dionne V. E. Single-channel acetylcholine receptor kinetics. Biophys J. 1984 Jan;45(1):153–163. doi: 10.1016/S0006-3495(84)84144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder T. M., Pennefather P., Quastel D. M. The time course of miniature endplate currents and its modification by receptor blockade and ethanol. J Gen Physiol. 1984 Mar;83(3):435–468. doi: 10.1085/jgp.83.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. E., Wong B. S., Jackson M. B., Lecar H. Single-channel currents activated by curare in cultured embryonic rat muscle. J Neurosci. 1983 Dec;3(12):2525–2531. doi: 10.1523/JNEUROSCI.03-12-02525.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Voltage-dependence of drug-induced conductance in frog neuromuscular junction. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2140–2144. doi: 10.1073/pnas.72.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J., McMillan J., Wolfson H., O'Brien J. C. Acetylcholine receptor metabolism in a nonfusing muscle cell line. J Biol Chem. 1977 Mar 25;252(6):2143–2153. [PubMed] [Google Scholar]
- Quast U., Schimerlik M., Lee T., Witzemann T. L., Blanchard S., Raftery M. A. Ligand-induced conformation changes in Torpedo californica membrane-bound acetylcholine receptor. Biochemistry. 1978 Jun 13;17(12):2405–2414. doi: 10.1021/bi00605a024. [DOI] [PubMed] [Google Scholar]
- Siegelbaum S. A., Trautmann A., Koenig J. Single acetylcholine-activated channel currents in developing muscle cells. Dev Biol. 1984 Aug;104(2):366–379. doi: 10.1016/0012-1606(84)90092-7. [DOI] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Acetylcholine receptor activation by a site-selective ligand: nature of brief open and closed states in BC3H-1 cells. J Physiol. 1986 Jan;370:357–379. doi: 10.1113/jphysiol.1986.sp015939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Activation of a nicotinic acetylcholine receptor. Biophys J. 1984 Jan;45(1):175–185. doi: 10.1016/S0006-3495(84)84146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Steinbach J. H. Agonists block currents through acetylcholine receptor channels. Biophys J. 1984 Aug;46(2):277–283. doi: 10.1016/S0006-3495(84)84022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine S. M., Taylor P. Relationship between reversible antagonist occupancy and the functional capacity of the acetylcholine receptor. J Biol Chem. 1981 Jul 10;256(13):6692–6699. [PubMed] [Google Scholar]
- Sine S. M., Taylor P. The relationship between agonist occupation and the permeability response of the cholinergic receptor revealed by bound cobra alpha-toxin. J Biol Chem. 1980 Nov 10;255(21):10144–10156. [PubMed] [Google Scholar]
- Sine S., Taylor P. Functional consequences of agonist-mediated state transitions in the cholinergic receptor. Studies in cultured muscle cells. J Biol Chem. 1979 May 10;254(9):3315–3325. [PubMed] [Google Scholar]
- Weiland G., Taylor P. Ligand specificity of state transitions in the cholinergic receptor: behavior of agonists and antagonists. Mol Pharmacol. 1979 Mar;15(2):197–212. [PubMed] [Google Scholar]


