Abstract
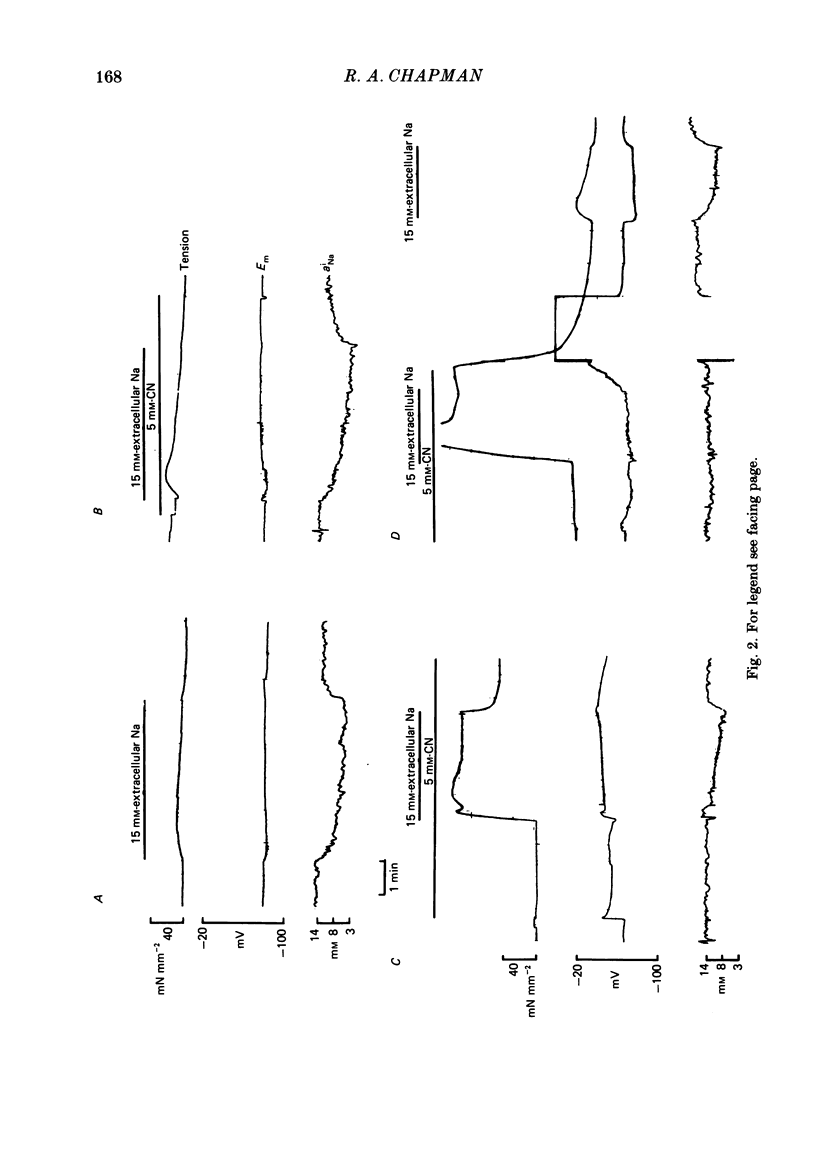
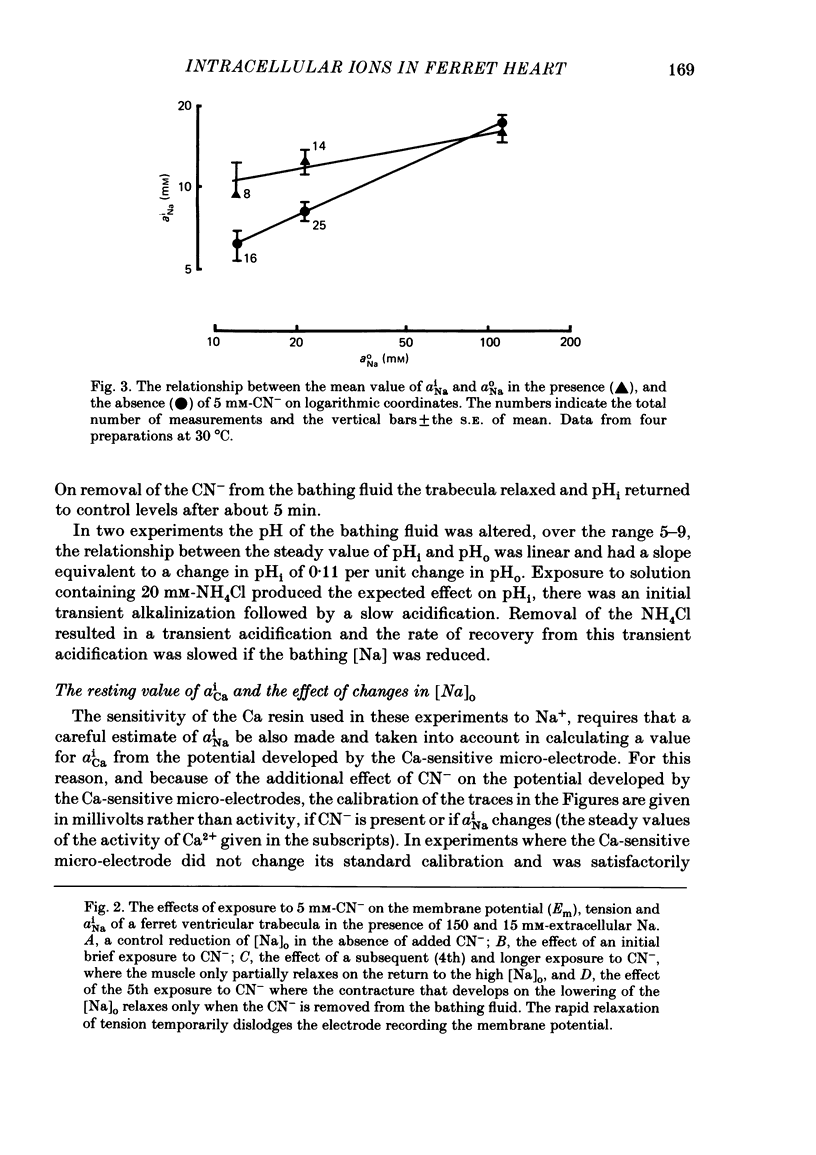
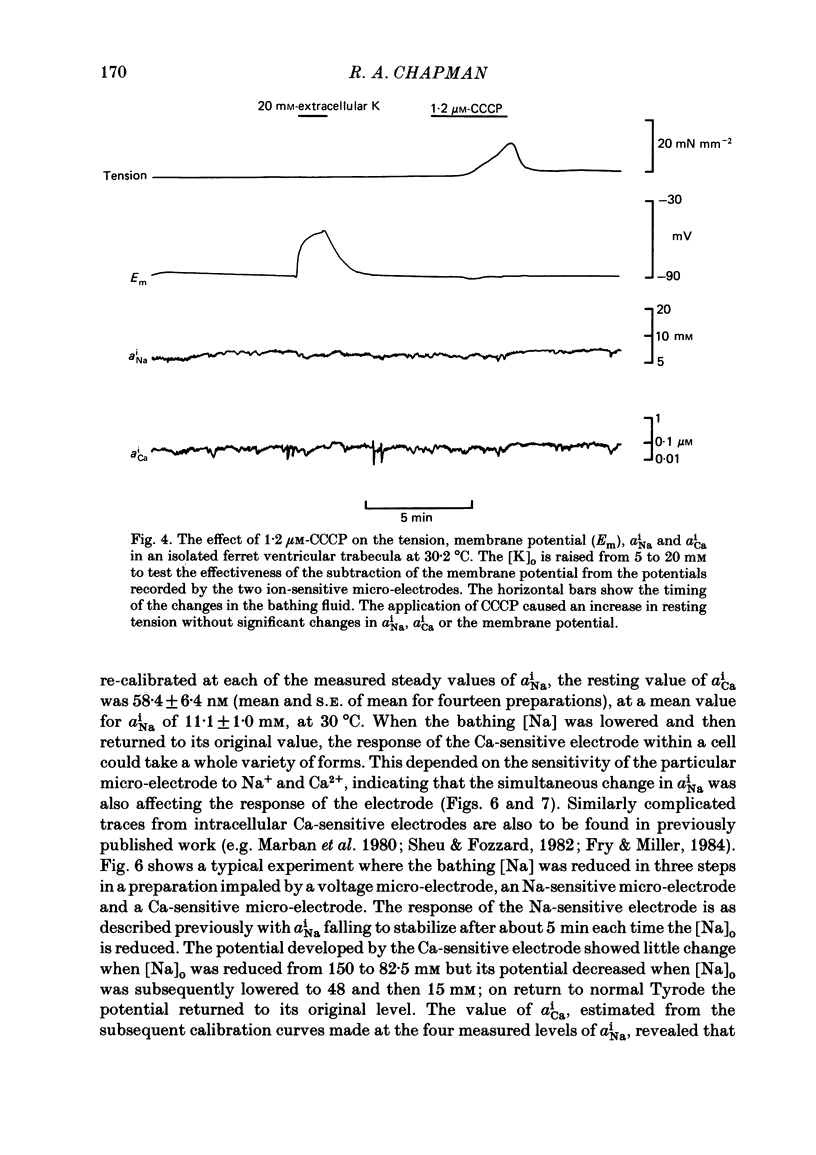
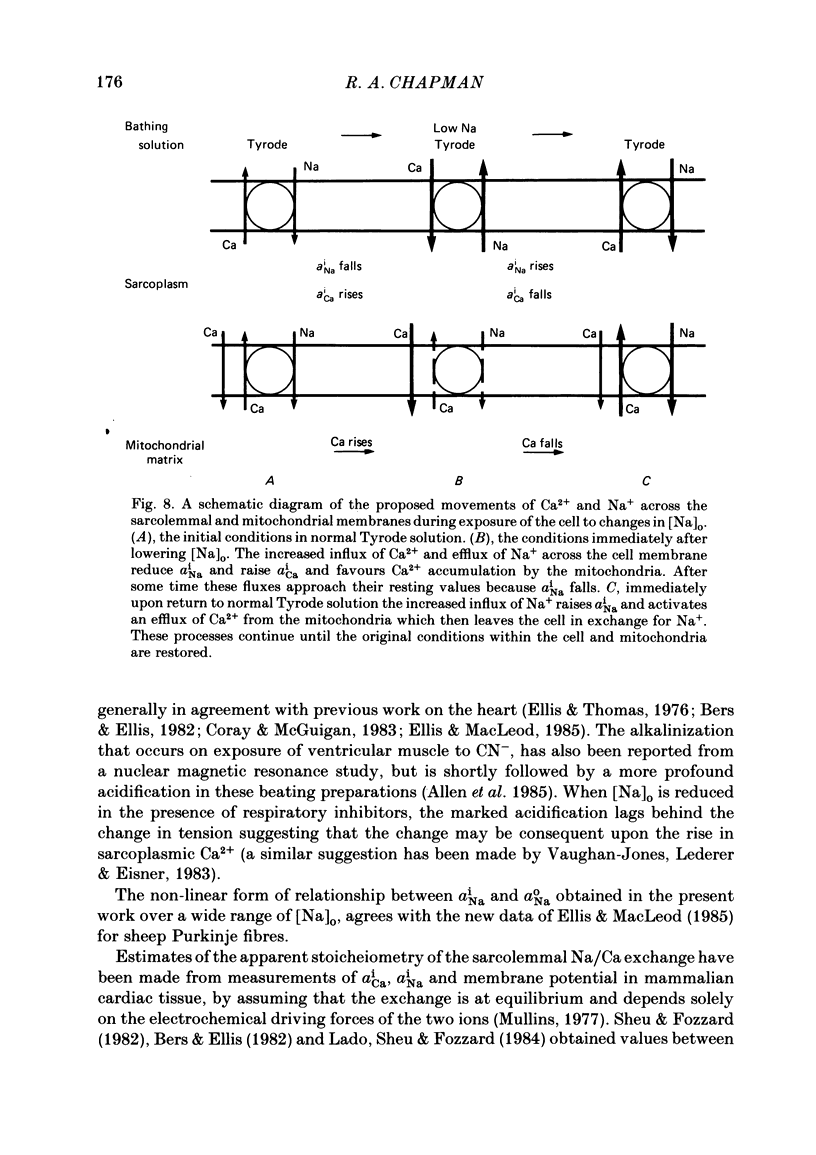
Measurements of the intracellular activity of Ca (aiCa), Na (aiNa) and H (pHi) ions have been made with resin-filled ion-sensitive micro-electrodes in ferret ventricular trabeculae. The mean values in quiescent muscle at 30 degrees C were: aiNa, 11.1 +/- 1.0 mM; aiCa, 58.4 +/- 6.4 nM, and pHi, 7.20 +/- 0.11. The relation between aiNa and extracellular Na activity (aoNa) is not linear and is sensitive to temperature: the Q10 for the change in aiNa in normal Tyrode solution is 1.3 +/- 0.5 and rises to 3.5 +/- 0.5 when aoNa is reduced to 1.1 mM. The addition of CN to the bathing fluid causes little or no change in aiNa or aiCa but pHi rises to 7.38 +/- 0.10, yet in some preparations resting tension increases. Similar results are seen with carbonyl cyanide m-chlorophenyl hydrazone. On lowering [Na]o, the fall in aiNa is very much greater than the rise in aiCa and the pHi is generally unchanged. When [Na]o is lowered in the presence of a respiratory inhibitor, the fall in aiNa is reduced, the rise in aiCa and the contracture tension are increased while pHi falls. The apparent coupling ratio for the Na/Ca exchange varies between 3 and 4 depending on the experimental conditions. These results suggest that an intracellular process, with a high Q10 and which depends upon respiration and aiNa, is able to remove Ca2+ from the sarcoplasm and thereby interact with the sarcolemmal Na/Ca exchange. This process could be the increase in the energy-dependent accumulation of Ca2+ within mitochondria that will occur when the Ca efflux from these organelles is progressively inhibited as aiNa falls.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Lab M. J., Orchard C. H. The effects of low sodium solutions on intracellular calcium concentration and tension in ferret ventricular muscle. J Physiol. 1983 Dec;345:391–407. doi: 10.1113/jphysiol.1983.sp014984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen P. H., Bridge J. H. Electrochemical ion gradients and the Na/Ca exchange stoichiometry. Measurements of these gradients are thermodynamically consistent with a stoichiometric coefficient greater than or equal to 3. J Gen Physiol. 1985 Mar;85(3):471–475. doi: 10.1085/jgp.85.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra S. The effects of drugs on calcium uptake and calcium release by mitochondria and sarcoplasmic reticulum of frog skeletal muscle. Biochem Pharmacol. 1974 Jan 1;23(1):89–101. doi: 10.1016/0006-2952(74)90316-5. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Bridge J. H., Cabeen W. R., Jr, Langer G. A., Reeder S. Sodium efflux in rabbit myocardium: relationship to sodium-calcium exchange. J Physiol. 1981 Jul;316:555–574. doi: 10.1113/jphysiol.1981.sp013806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A. Control of cardiac contractility at the cellular level. Am J Physiol. 1983 Oct;245(4):H535–H552. doi: 10.1152/ajpheart.1983.245.4.H535. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Coray A., McGuigan J. A. Sodium/calcium exchange in mammalian ventricular muscle: a study with sodium-sensitive micro-electrodes. J Physiol. 1983 Oct;343:253–276. doi: 10.1113/jphysiol.1983.sp014891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Léoty C. The time-dependent and dose-dependent effects of caffeine on the contraction of the ferret heart. J Physiol. 1976 Apr;256(2):287–314. doi: 10.1113/jphysiol.1976.sp011326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The measurement of intracellular sodium activity and its relationship to the action of calcium ions upon the low-sodium contracture in frog atrial trabeculae. Q J Exp Physiol. 1984 Jul;69(3):559–572. doi: 10.1113/expphysiol.1984.sp002842. [DOI] [PubMed] [Google Scholar]
- Cobbold P. H., Bourne P. K. Aequorin measurements of free calcium in single heart cells. 1984 Nov 29-Dec 5Nature. 312(5993):444–446. doi: 10.1038/312444a0. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Orchard C. H., Allen D. G. Control of intracellular ionized calcium concentration by sarcolemmal and intracellular mechanisms. J Mol Cell Cardiol. 1984 Feb;16(2):137–146. doi: 10.1016/s0022-2828(84)80702-6. [DOI] [PubMed] [Google Scholar]
- Ellis D., MacLeod K. T. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. J Physiol. 1985 Feb;359:81–105. doi: 10.1113/jphysiol.1985.sp015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D., Thomas R. C. Direct measurement of the intracellular pH of mammalian cardiac muscle. J Physiol. 1976 Nov;262(3):755–771. doi: 10.1113/jphysiol.1976.sp011619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A., Chapman R. A., Friedlander I. R. Measurement of intracellular calcium ion activity with neutral exchanger ion sensitive microelectrodes. Cell Calcium. 1985 Apr;6(1-2):57–68. doi: 10.1016/0143-4160(85)90034-x. [DOI] [PubMed] [Google Scholar]
- Glitsch H. G., Pott L. Spontaneous tension oscillations in guinea-pig atrial trabeculae. Pflugers Arch. 1975 Jul 9;358(1):11–25. doi: 10.1007/BF00584566. [DOI] [PubMed] [Google Scholar]
- January C. T., Fozzard H. A. The effects of membrane potential, extracellular potassium, and tetrodotoxin on the intracellular sodium ion activity of sheep cardiac muscle. Circ Res. 1984 Jun;54(6):652–665. doi: 10.1161/01.res.54.6.652. [DOI] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lado M. G., Sheu S. S., Fozzard H. A. Effects of tonicity on tension and intracellular sodium and calcium activities in sheep heart. Circ Res. 1984 May;54(5):576–585. doi: 10.1161/01.res.54.5.576. [DOI] [PubMed] [Google Scholar]
- Lee C. O. Ionic activities in cardiac muscle cells and application of ion-selective microelectrodes. Am J Physiol. 1981 Oct;241(4):H459–H478. doi: 10.1152/ajpheart.1981.241.4.H459. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Uhm D. Y., Dresdner K. Sodium-calcium exchange in rabbit heart muscle cells: direct measurement of sarcoplasmic Ca2+ activity. Science. 1980 Aug 8;209(4457):699–701. doi: 10.1126/science.7394527. [DOI] [PubMed] [Google Scholar]
- Marban E., Rink T. J., Tsien R. W., Tsien R. Y. Free calcium in heart muscle at rest and during contraction measured with Ca2+ -sensitive microelectrodes. Nature. 1980 Aug 28;286(5776):845–850. doi: 10.1038/286845a0. [DOI] [PubMed] [Google Scholar]
- Mullins L. J. A mechanism for Na/Ca transport. J Gen Physiol. 1977 Dec;70(6):681–695. doi: 10.1085/jgp.70.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J. The thermostatics and thermodynamics of cotransport. Biochim Biophys Acta. 1984 Nov 21;778(1):155–175. doi: 10.1016/0005-2736(84)90459-0. [DOI] [PubMed] [Google Scholar]
- Nicholls D., Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta. 1982 Sep 1;683(1):57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- Orchard C. H., Eisner D. A., Allen D. G. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983 Aug 25;304(5928):735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Powell T., Tatham P. E., Twist V. W. Cytoplasmic free calcium measured by quin2 fluorescence in isolated ventricular myocytes at rest and during potassium-depolarization. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1012–1020. doi: 10.1016/0006-291x(84)91192-6. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Na/Ca exchange in the intact cardiac cell. J Gen Physiol. 1985 Mar;85(3):476–478. [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu S. S., Sharma V. K., Banerjee S. P. Measurement of cytosolic free calcium concentration in isolated rat ventricular myocytes with quin 2. Circ Res. 1984 Dec;55(6):830–834. doi: 10.1161/01.res.55.6.830. [DOI] [PubMed] [Google Scholar]
- Snowdowne K. W., Borle A. B. Measurement of cytosolic free calcium in mammalian cells with aequorin. Am J Physiol. 1984 Nov;247(5 Pt 1):C396–C408. doi: 10.1152/ajpcell.1984.247.5.C396. [DOI] [PubMed] [Google Scholar]
- Thorson J., White D. C. Role of cross-bridge distortion in the small-signal mechanical dynamics of insect and rabbit striated muscle. J Physiol. 1983 Oct;343:59–84. doi: 10.1113/jphysiol.1983.sp014881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Wendt I. R., Langer G. A. The sodium-calcium relationship in mammalian myocardium: effect of sodium deficient perfusion on calcium fluxes. J Mol Cell Cardiol. 1977 Jul;9(7):551–564. doi: 10.1016/s0022-2828(77)80370-2. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Kort A. A., Stern M. D., Lakatta E. G., Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]


