Abstract
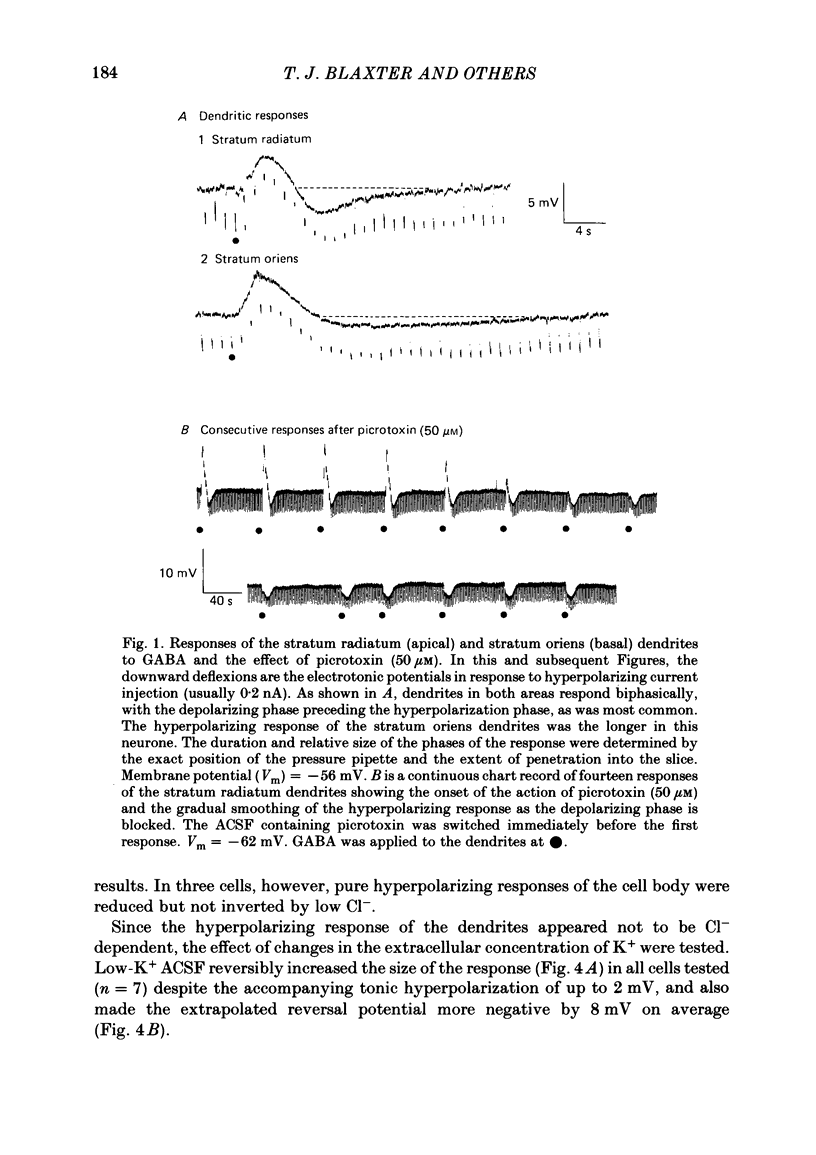
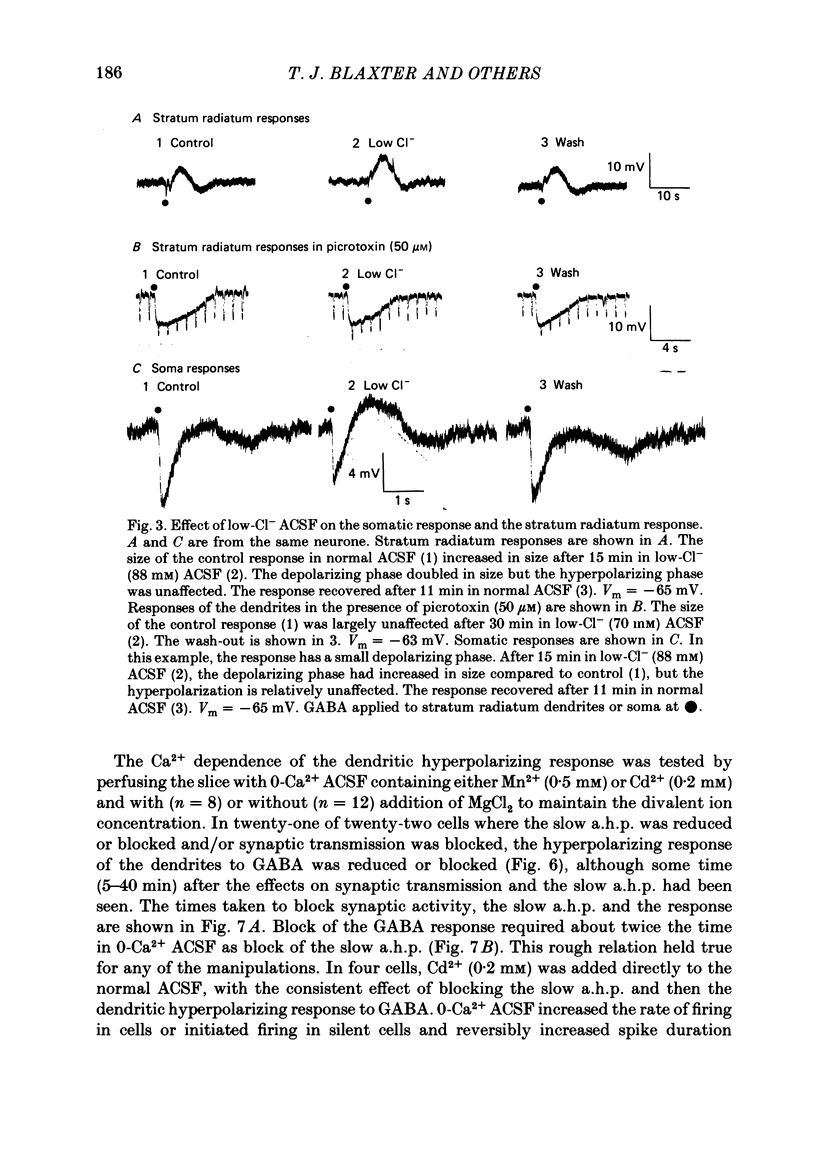
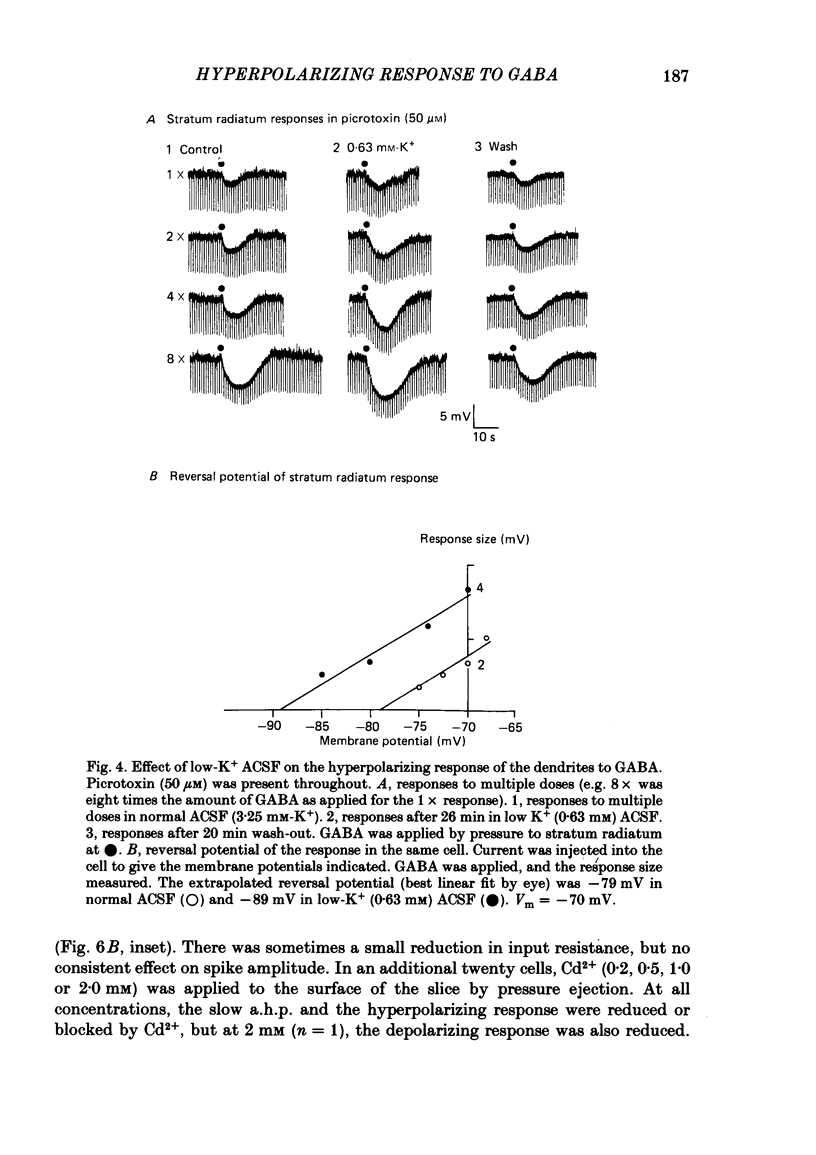
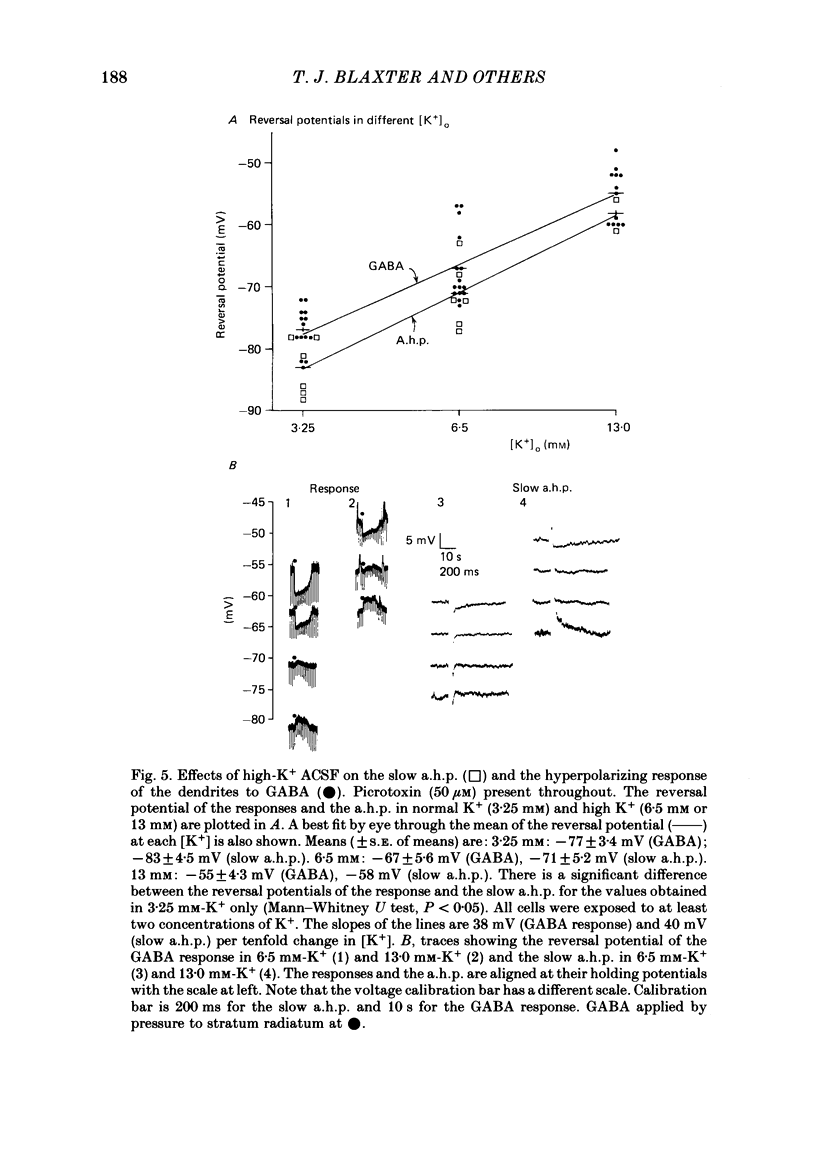
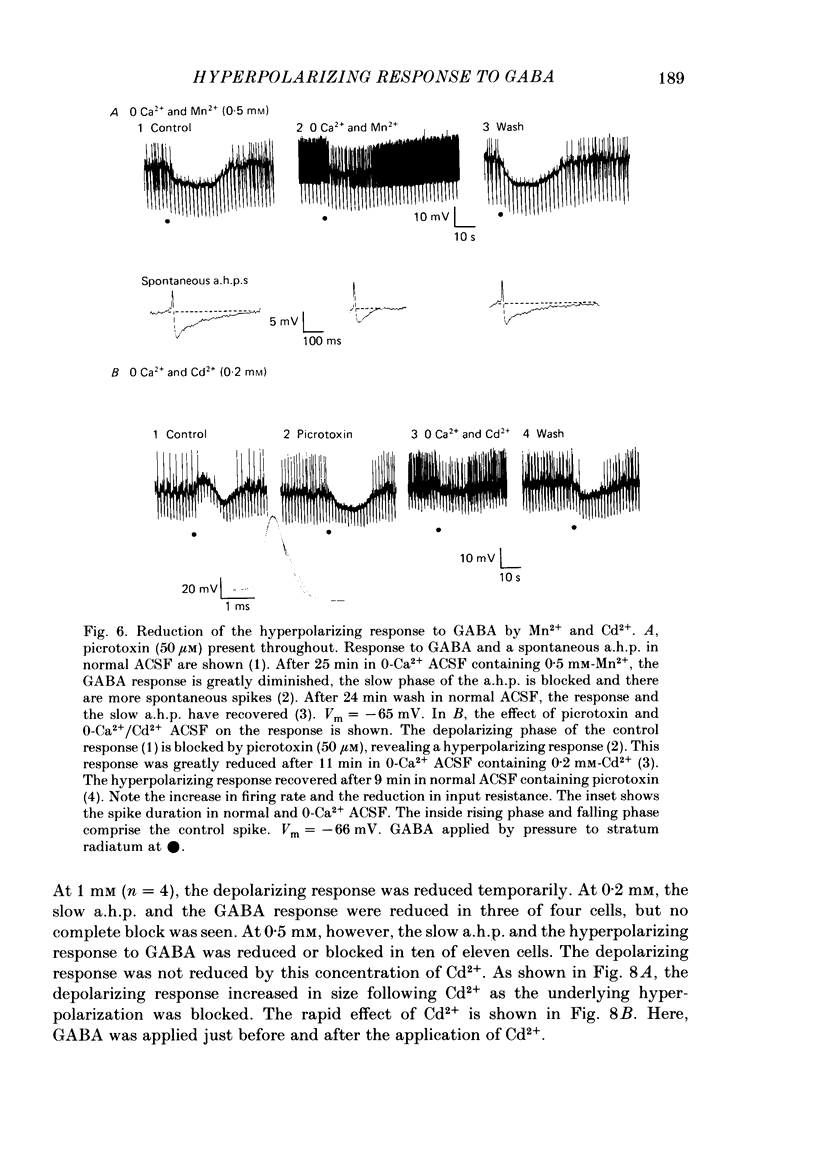
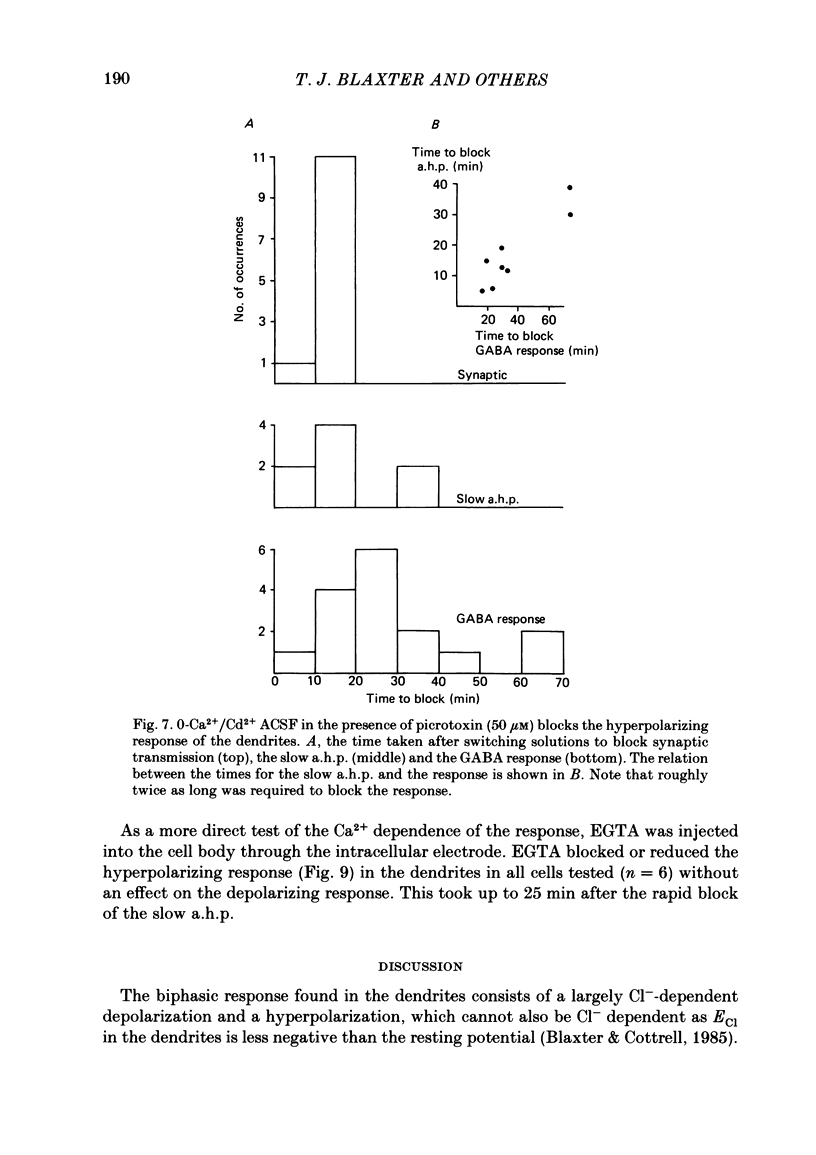
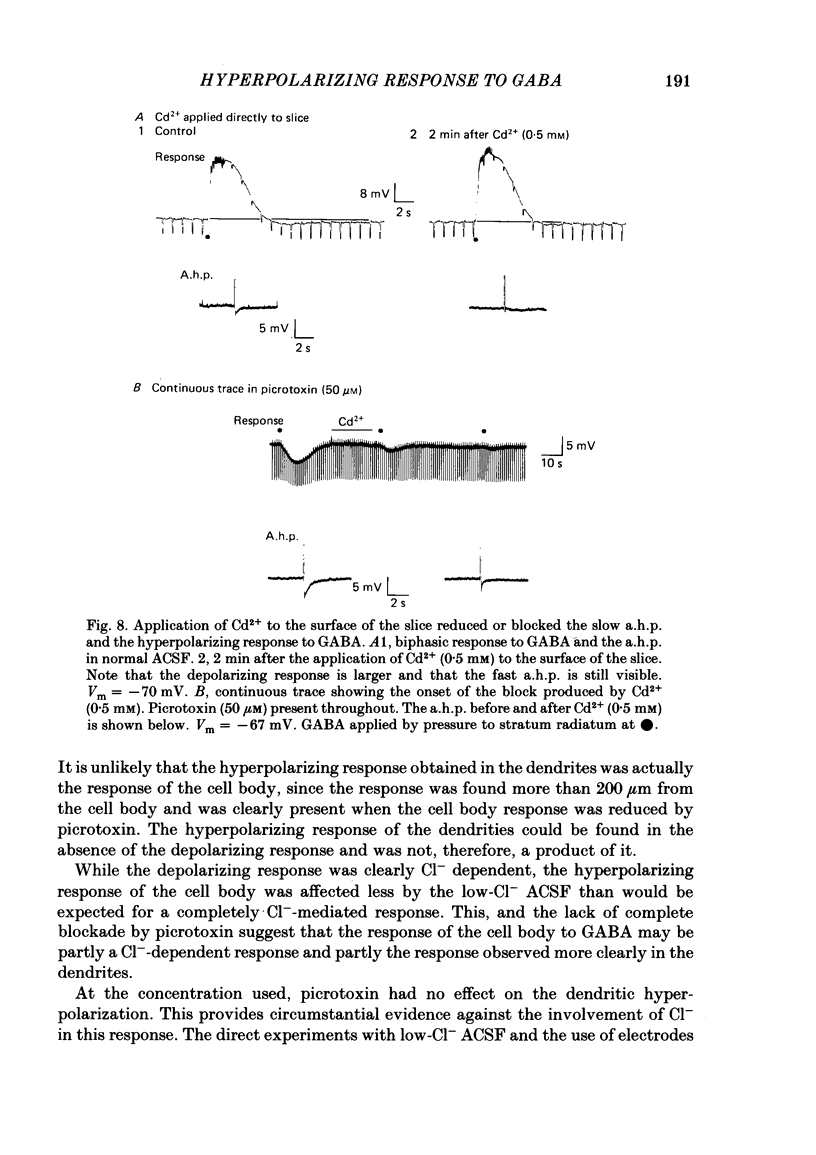
Application of gamma-aminobutyric acid (GABA) to the dendrites of CA1 pyramidal cells in hippocampal slices produced depolarizing and hyperpolarizing responses. Picrotoxin (50 microM) blocked the depolarizing response of the dendrites to GABA but not the hyperpolarizing responses of the dendrites. The hyperpolarizing response of the cell body to GABA was reduced but not blocked by picrotoxin, suggesting the presence of a complex response at the cell body. The depolarizing response of the dendrites and the hyperpolarizing response of the cell body appeared to be at least partly Cl- dependent as they were respectively increased and decreased in size in low-Cl- artificial cerebrospinal fluid (ACSF), while the hyperpolarizing response of the dendrites was unaffected. The hyperpolarizing response of the dendrites was increased in amplitude in low-K+ ACSF and the extrapolated reversal potential of the response became more negative, suggesting that the response was K+ dependent. The hyperpolarizing response of the dendrites was decreased in size in high-K+ ACSF and could be readily inverted by current injection. The reversal potential became less negative in high-K+ ACSF in a similar manner to that of the slow after-hyperpolarization following a train of spikes, indicating that the response was a K+ conductance. Perfusion of the slice with normal or 0-Ca2+ ACSF containing Cd2+ or Mn2+ blocked synaptic transmission, increased spike duration and blocked the slow phase of the spike after-hyperpolarization (a.h.p.). This latter potential is thought to be mediated by a Ca2+-dependent K+ conductance. Later, the hyperpolarizing response of the dendrites to GABA was blocked without an effect on the other GABA responses. Pressure application of Cd2+ (0.2-2 mM) onto the surface of the slice rapidly reduced or blocked the slow a.h.p. and the dendritic hyperpolarizing response to GABA. Intracellular injection of EGTA rapidly blocked the slow phase of the a.h.p. and then later blocked or reduced the dendritic hyperpolarizing response to GABA. We conclude that the hyperpolarizing response of the dendrites to GABA is mediated by a Ca2+-dependent K+ conductance.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Constanti A., Brown D. A., Clark R. B. Intracellular Ca2+ activates a fast voltage-sensitive K+ current in vertebrate sympathetic neurones. Nature. 1982 Apr 22;296(5859):746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. GABA-mediated biphasic inhibitory responses in hippocampus. Nature. 1979 Sep 27;281(5729):315–317. doi: 10.1038/281315a0. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:125–141. doi: 10.1113/jphysiol.1982.sp014256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P., Dingledine R., Gjerstad L., Langmoen I. A., Laursen A. M. Two different responses of hippocampal pyramidal cells to application of gamma-amino butyric acid. J Physiol. 1980 Aug;305:279–296. doi: 10.1113/jphysiol.1980.sp013363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter T. J., Carlen P. L. Pre- and postsynaptic effects of baclofen in the rat hippocampal slice. Brain Res. 1985 Aug 19;341(1):195–199. doi: 10.1016/0006-8993(85)91489-1. [DOI] [PubMed] [Google Scholar]
- Blaxter T. J., Cottrell G. A. Actions of GABA and ethylenediamine on CA1 pyramidal neurones of the rat hippocampus. Q J Exp Physiol. 1985 Jan;70(1):75–93. doi: 10.1113/expphysiol.1985.sp002898. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L. gamma-Aminobutyric acid reduces the evoked release of [3H]-noradrenaline from sympathetic nerve terminals [proceedings]. Br J Pharmacol. 1979 May;66(1):108P–108P. [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Griffith W. H. Calcium-activated outward current in voltage-clamped hippocampal neurones of the guinea-pig. J Physiol. 1983 Apr;337:287–301. doi: 10.1113/jphysiol.1983.sp014624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M., Okabayashi T. Effect of picrotoxin on benzodiazepine receptors and GABA receptors with reference to the effect of C1- ion. Life Sci. 1981 Feb 23;28(8):895–901. doi: 10.1016/0024-3205(81)90051-5. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Wigström H. Evidence for two types of afterhyperpolarization in CA1 pyramidal cells in the hippocampus. Brain Res. 1981 Feb 16;206(2):462–468. doi: 10.1016/0006-8993(81)90548-5. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell J. V., Adams P. R. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982 Oct 28;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Johnston D., Hablitz J. J., Wilson W. A. Voltage clamp discloses slow inward current in hippocampal burst-firing neurones. Nature. 1980 Jul 24;286(5771):391–393. doi: 10.1038/286391a0. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Weight F. F. Action potential repolarization may involve a transient, Ca2+-sensitive outward current in a vertebrate neurone. Nature. 1982 Nov 11;300(5888):185–188. doi: 10.1038/300185a0. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Stafstrom C. E. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980 Dec 5;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: potassium conductances. J Neurophysiol. 1984 Jun;51(6):1409–1433. doi: 10.1152/jn.1984.51.6.1409. [DOI] [PubMed] [Google Scholar]


