Abstract
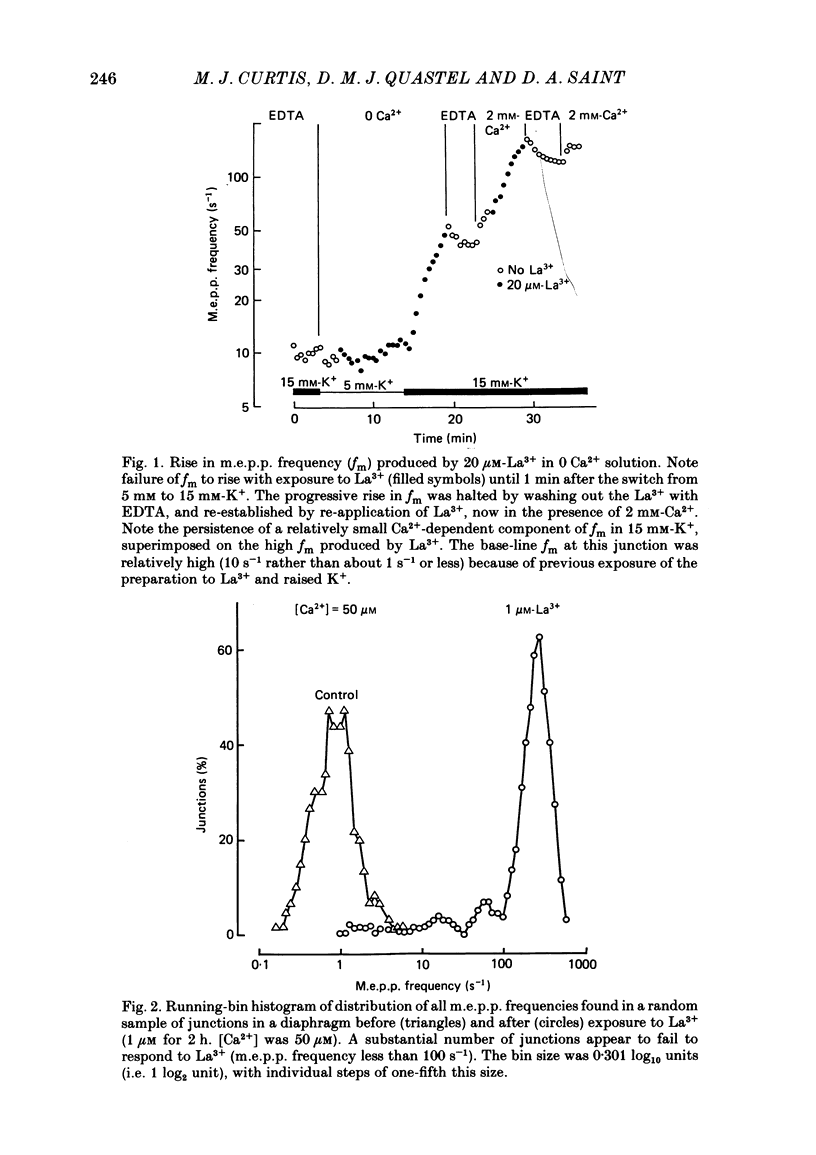
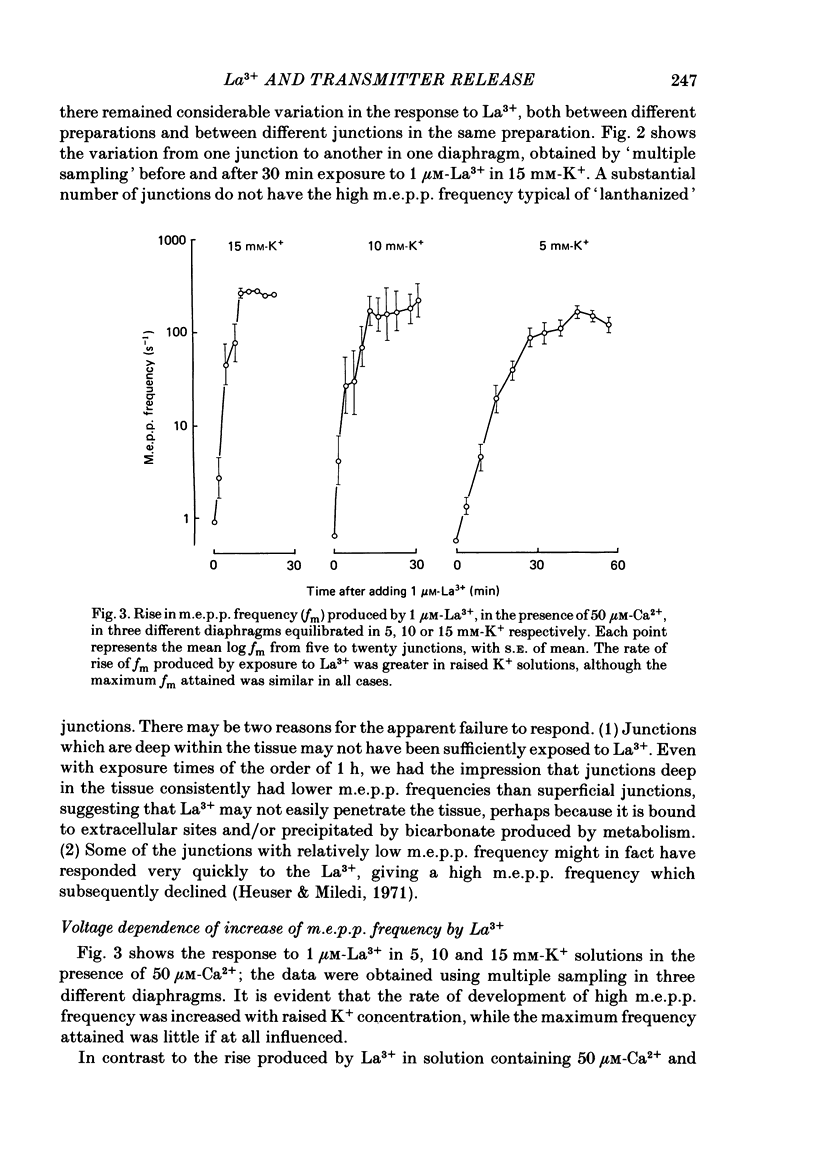
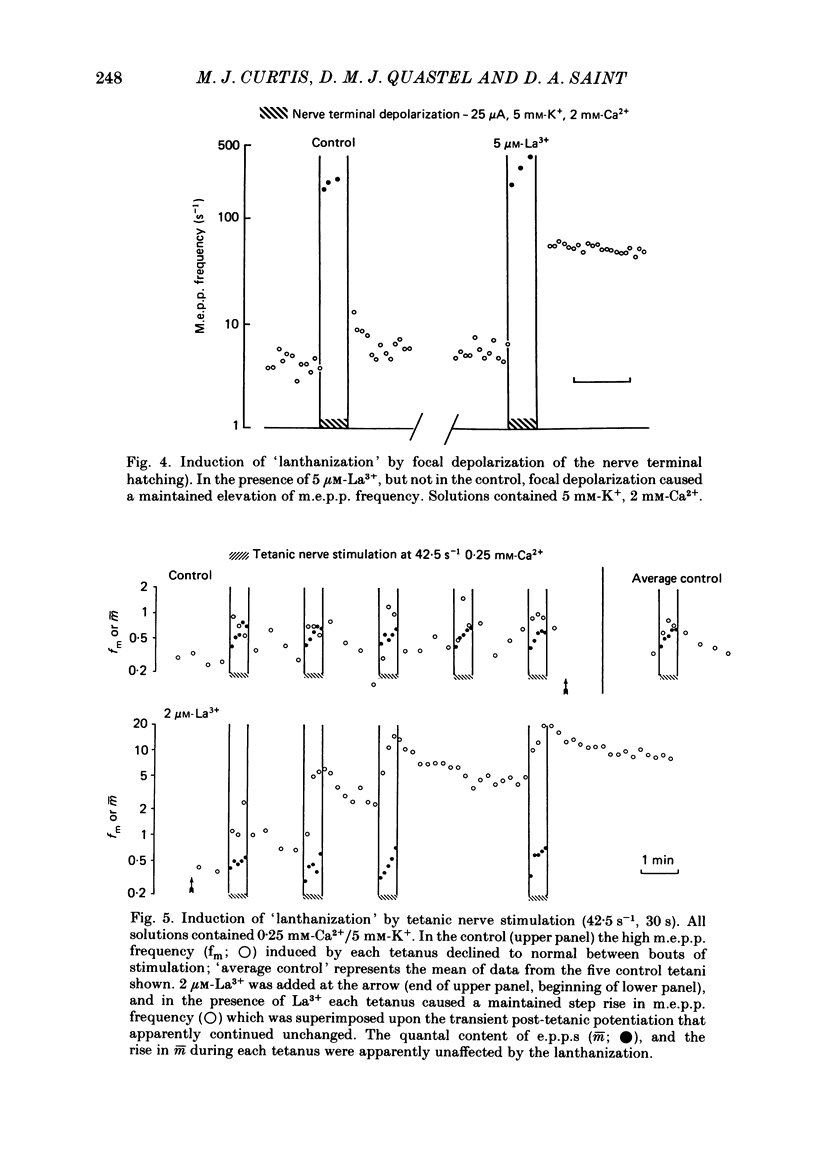
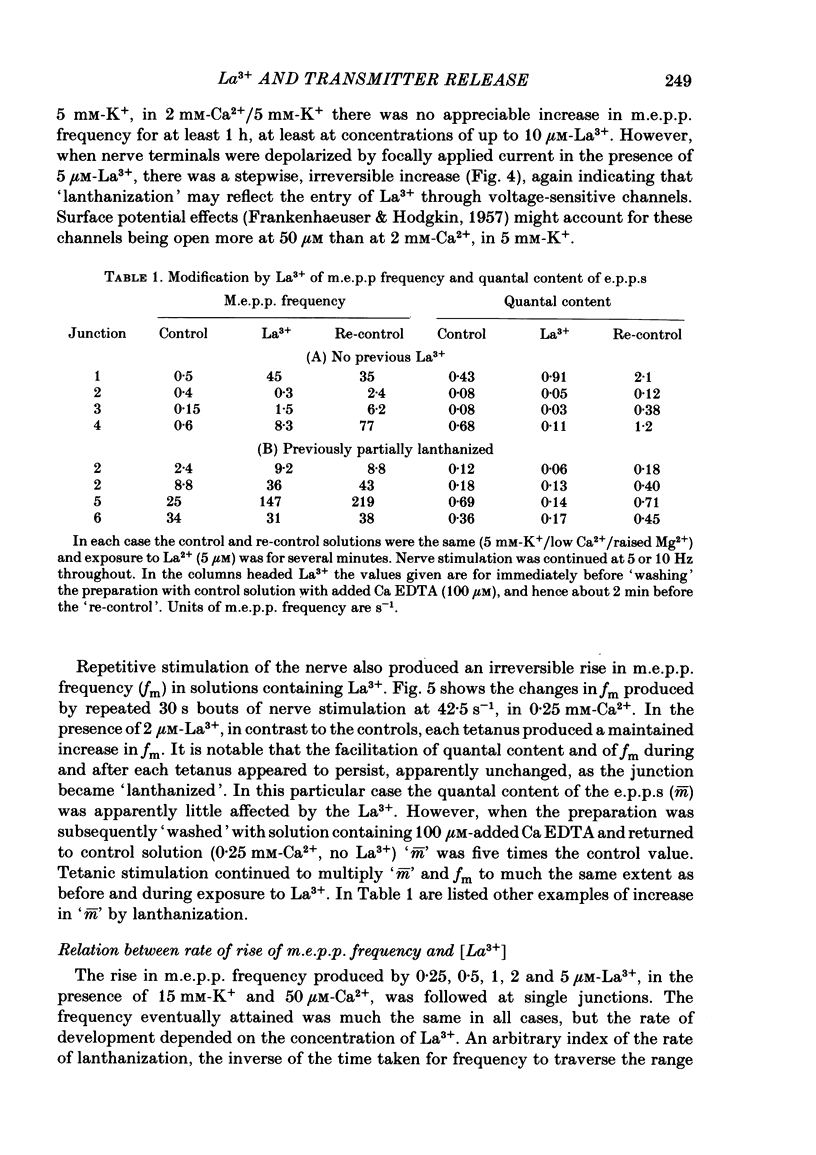
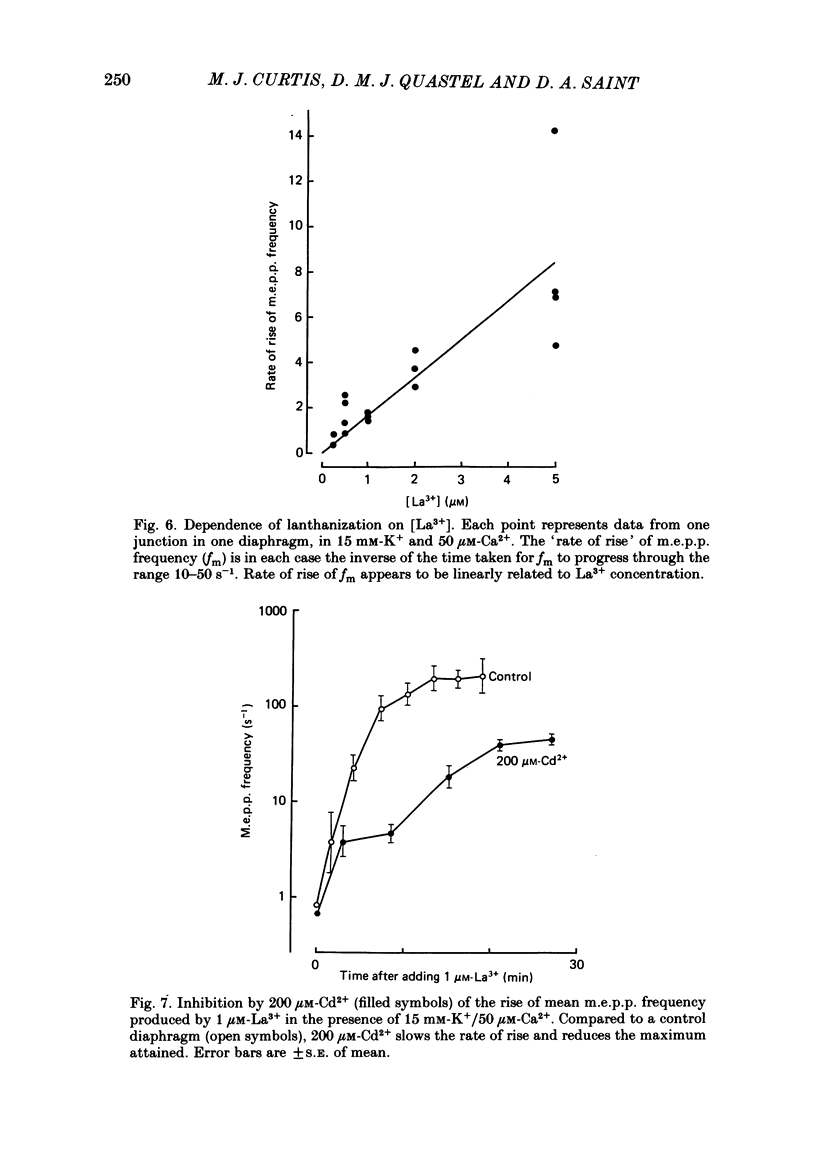
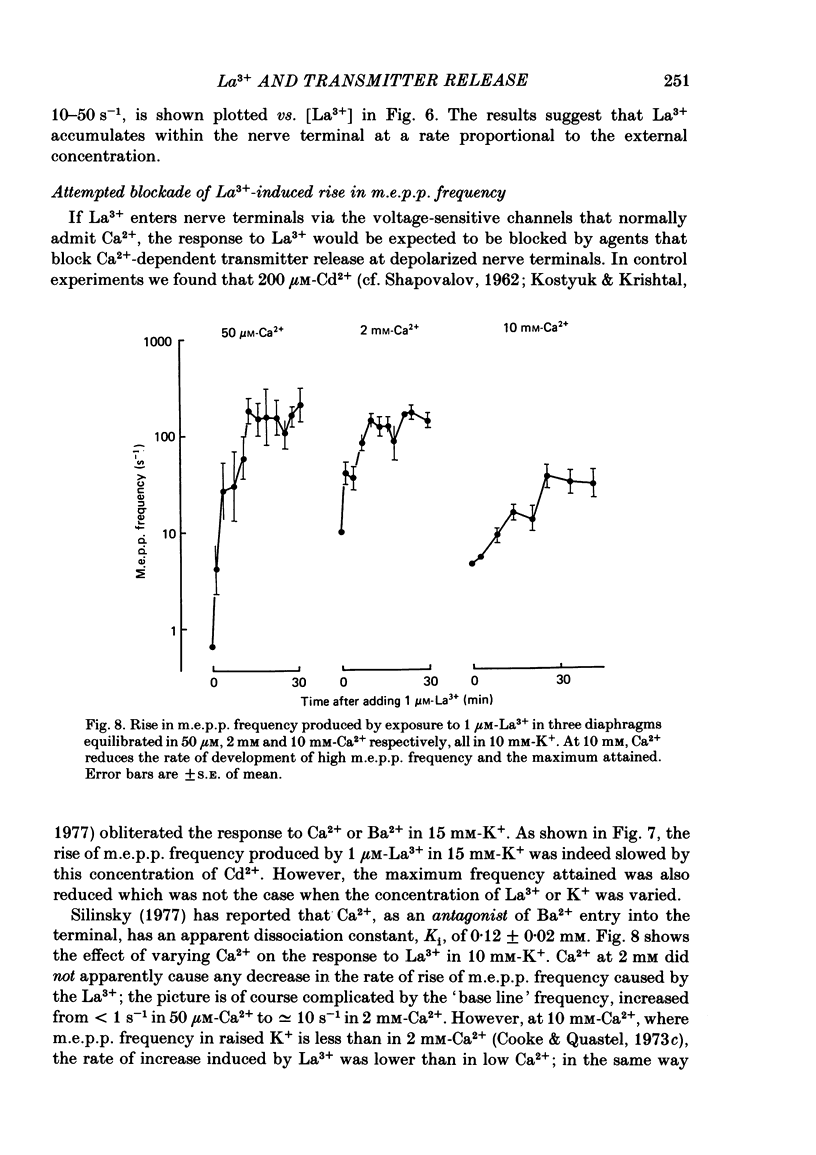
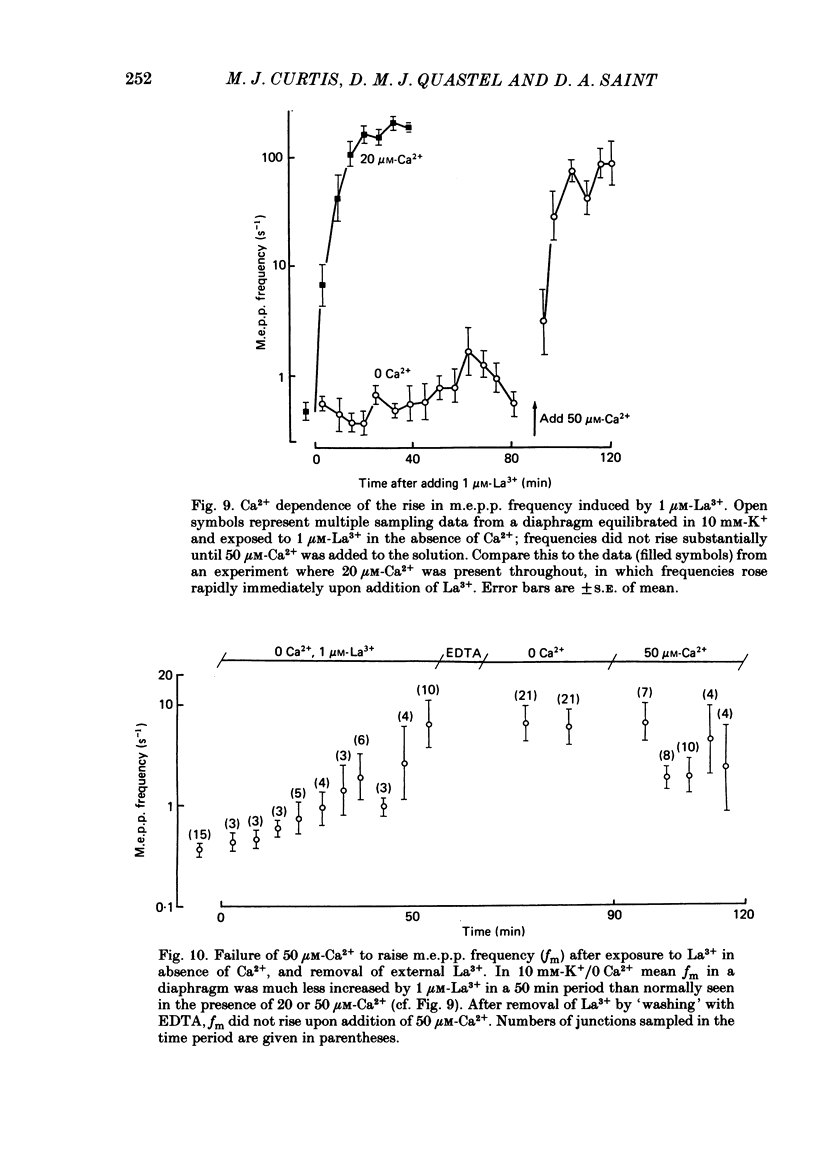
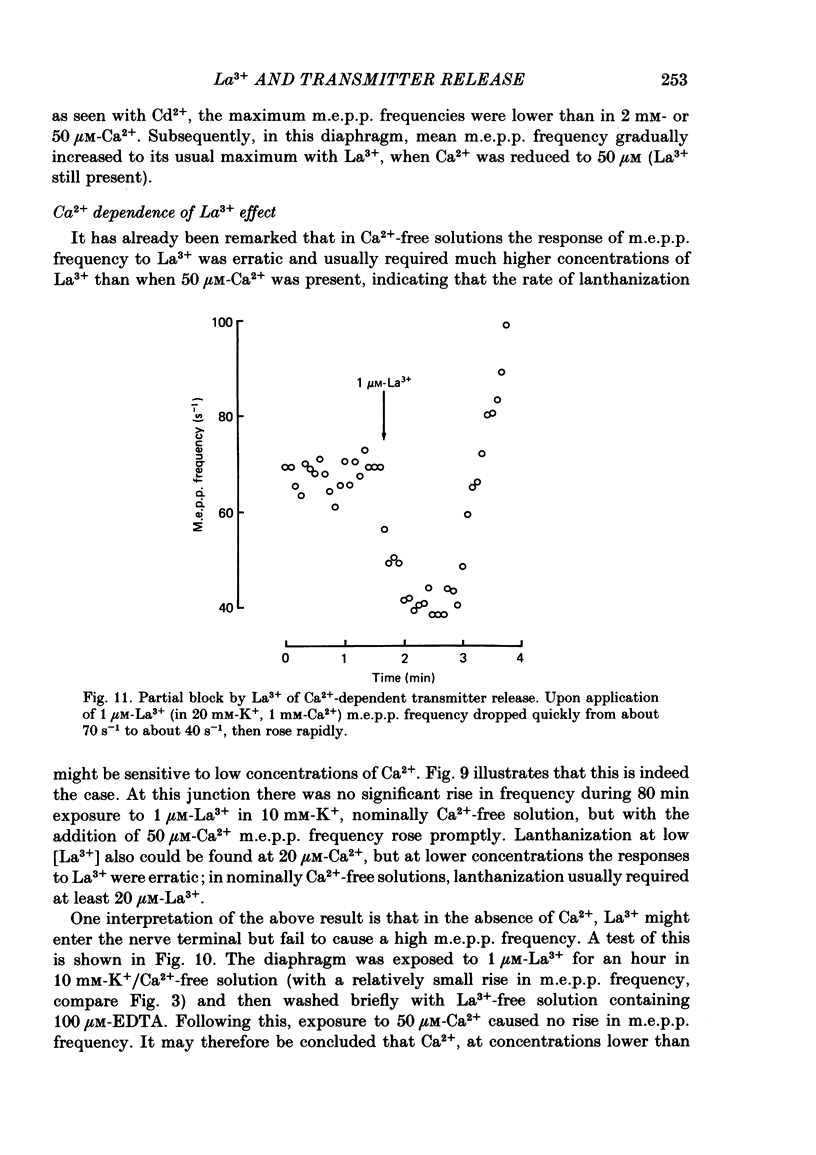
The mechanism by which lanthanum (La3+) causes an increased frequency of miniature end-plate potentials (m.e.p.p.s) was studied at the mouse neuromuscular junction. At concentrations as low as 0.25 microM, La3+ caused a progressive rise in m.e.p.p. frequency, to a maximum of several hundred per second. 'Washing' with solution containing EDTA arrested the rise, but did not substantially reduce the raised m.e.p.p. frequency. At partially 'lanthanized' junctions high frequencies of m.e.p.p.s were maintained indefinitely, even in 0 Ca2+/EDTA solutions. The rate of development of high m.e.p.p. frequency was increased by repetitive nerve stimulation or by depolarization of the nerve terminal (high K+ or focally applied current), and appeared to be proportional to the concentration of La3+ over the range of 0.25-5 microM. At low concentrations of La3+ the rise of m.e.p.p. frequency depended upon the co-presence of a small amount of Ca2+ (greater than 10 microM) and was slowed and partially blocked by Cd2+, or by Ca2+ at about 10 microM. The quantal content of end-plate potentials was usually reduced in the presence of La3+, but was increased over control values after removal of La3+ by 'washing' with solution containing EDTA, once a raised m.e.p.p. frequency had developed. At partially lanthanized junctions the absolute increases in m.e.p.p. frequency produced by Ca2+ (in raised K+), ethanol, or by nerve stimulation in the presence of Ba2+, were greater than at control junctions, but in each case the increases in the logarithm of m.e.p.p. frequency were less than at control junctions. It is concluded that La3+ causes transmitter release only after entry into the nerve terminal via voltage-sensitive channels, probably those that normally admit Ca2+, that La3+ and Ca2+ may co-operate at internal sites to induce transmitter release, and that these ions both co-operate and compete at external sites that regulate their entry into the nerve terminal.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alnaes E., Rahamimoff R. Dual action of praseodymium (Pr3+) on transmitter release at the frog neuromuscular synapse. Nature. 1974 Feb 15;247(5441):478–479. doi: 10.1038/247478a0. [DOI] [PubMed] [Google Scholar]
- Balnave R. J., Gage P. W. The inhibitory effect of manganese on transmitter release at the neuromuscular junction of the toad. Br J Pharmacol. 1973 Feb;47(2):339–352. doi: 10.1111/j.1476-5381.1973.tb08332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binah O., Meiri U., Rahamimoff H. The effects of HGCl2 and mersalyl on mechanisms regulating intracellular calcium and transmitter release. Eur J Pharmacol. 1978 Oct 15;51(4):453–457. doi: 10.1016/0014-2999(78)90438-7. [DOI] [PubMed] [Google Scholar]
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen J. M. Effects of rare earths and yttrium on striated muscle and the neuromuscular junction. Can J Physiol Pharmacol. 1972 Jun;50(6):603–611. doi: 10.1139/y72-089. [DOI] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Okamoto K., Quastel D. M. The role of calcium in depolarization-secretion coupling at the motor nerve terminal. J Physiol. 1973 Jan;228(2):459–497. doi: 10.1113/jphysiol.1973.sp010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Cumulative and persistent effects of nerve terminal depolarization on transmitter release. J Physiol. 1973 Jan;228(2):407–434. doi: 10.1113/jphysiol.1973.sp010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. The specific effect of potassium on transmitter release by motor nerve terminals and its inhibition by calcium. J Physiol. 1973 Jan;228(2):435–458. doi: 10.1113/jphysiol.1973.sp010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBassio W. A., Schnitzler R. M., Parsons R. L. Influence of lanthanum on transmitter release at the neuromuscular junction. J Neurobiol. 1971;2(3):263–278. doi: 10.1002/neu.480020307. [DOI] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Juang M. S. An electrophysiological study of the action of methylmercuric chloride and mercuric chloride on the sciatic nerve-sartorius muscle preparation of the frog. Toxicol Appl Pharmacol. 1976 Aug;37(2):339–348. doi: 10.1016/0041-008x(76)90097-1. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LETTVIN J. Y., PICKARD W. F., MCCULLOCH W. S., PITTS W. A THEORY OF PASSIVE ION FLUX THROUGH AXON MEMBRANES. Nature. 1964 Jun 27;202:1338–1339. doi: 10.1038/2021338a0. [DOI] [PubMed] [Google Scholar]
- Lev-Tov A., Rahamimoff R. A study of tetanic and post-tetanic potentiation of miniature end-plate potentials at the frog neuromuscular junction. J Physiol. 1980 Dec;309:247–273. doi: 10.1113/jphysiol.1980.sp013507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalis R. S., Cooper G. P. Evoked transmitter release increased by inorganic mercury at frog neuromuscular junction. Nature. 1975 Oct 23;257(5528):690–691. doi: 10.1038/257690a0. [DOI] [PubMed] [Google Scholar]
- Manalis R. S., Cooper G. P., Pomeroy S. L. Effects of lead on neuromuscular transmission in the frog. Brain Res. 1984 Feb 27;294(1):95–109. doi: 10.1016/0006-8993(84)91313-1. [DOI] [PubMed] [Google Scholar]
- Mela L. Inhibition and activation of calcium transport in mitochondria. Effect of lanthanides and local anesthetic drugs. Biochemistry. 1969 Jun;8(6):2481–2486. doi: 10.1021/bi00834a034. [DOI] [PubMed] [Google Scholar]
- Mela L. Interactions of La3+ and local anesthetic drugs with mitochondrial Ca++ and Mn++ uptake. Arch Biochem Biophys. 1968 Feb;123(2):286–293. doi: 10.1016/0003-9861(68)90136-7. [DOI] [PubMed] [Google Scholar]
- Metral S., Bonneton C., Hort-Legrand C., Reynes J. Dual action of erbium on transmitter release at the frog neuromuscular synapse. Nature. 1978 Feb 23;271(5647):773–775. doi: 10.1038/271773a0. [DOI] [PubMed] [Google Scholar]
- Miledi R. Lanthanum ions abolish the "calcium response" of nerve terminals. Nature. 1971 Feb 5;229(5284):410–411. doi: 10.1038/229410a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Thies R. Tetanic and post-tetanic rise in frequency of miniature end-plate potentials in low-calcium solutions. J Physiol. 1971 Jan;212(1):245–257. doi: 10.1113/jphysiol.1971.sp009320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K. Effects of alcohols and acetone on the neuromuscular junction of frog. Jpn J Physiol. 1967 Jun;17(3):245–261. doi: 10.2170/jjphysiol.17.245. [DOI] [PubMed] [Google Scholar]
- Quastel D. M., Hackett J. T., Cooke J. D. Calcium: is it required for transmitter secretion? Science. 1971 Jun 4;172(3987):1034–1036. doi: 10.1126/science.172.3987.1034. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Erulkar S. D., Lev-Tov A., Meiri H. Intracellular and extracellular calcium ions in transmitter release at the neuromuscular synapse. Ann N Y Acad Sci. 1978 Apr 28;307:583–598. doi: 10.1111/j.1749-6632.1978.tb41983.x. [DOI] [PubMed] [Google Scholar]
- Rahamimoff R., Lev-Tov A., Meiri H. Primary and secondary regulation of quantal transmitter release: calcium and sodium. J Exp Biol. 1980 Dec;89:5–18. doi: 10.1242/jeb.89.1.5. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAPOVALOV A. I. A study of action of drugs on neuromuscular transmission with the aid of multibarrelled intracellular microelectrodes. Biochem Pharmacol. 1962 Aug;9:213–220. doi: 10.1016/0006-2952(62)90030-8. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. An estimate of the equilibrium dissociation constant for calcium as an antagonist of evoked acetylcholine release: implications for excitation-secretion coupling. Br J Pharmacol. 1977 Dec;61(4):691–693. doi: 10.1111/j.1476-5381.1977.tb07562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the role of barium in supporting the asynchronous release of acetylcholine quanta by motor nerve impulses. J Physiol. 1978 Jan;274:157–171. doi: 10.1113/jphysiol.1978.sp012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. The biophysical pharmacology of calcium-dependent acetylcholine secretion. Pharmacol Rev. 1985 Mar;37(1):81–132. [PubMed] [Google Scholar]
- Takata M., Pickard W. F., Lettvin J. Y., Moore J. W. Ionic conductance changes in lobster axon membrane when lanthanum is substituted for calcium. J Gen Physiol. 1966 Nov;50(2):461–471. doi: 10.1085/jgp.50.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Washio H. A dual effect of cobalt ions on the spontaneous release of transmitter at insect motor nerve terminals. J Exp Biol. 1982 Jun;98:353–361. doi: 10.1242/jeb.98.1.353. [DOI] [PubMed] [Google Scholar]
- Washio H., Miyamoto T. Effect of lanthanum ions on neuromuscular transmission in insects. J Exp Biol. 1983 Nov;107:405–414. doi: 10.1242/jeb.107.1.405. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]


