Abstract
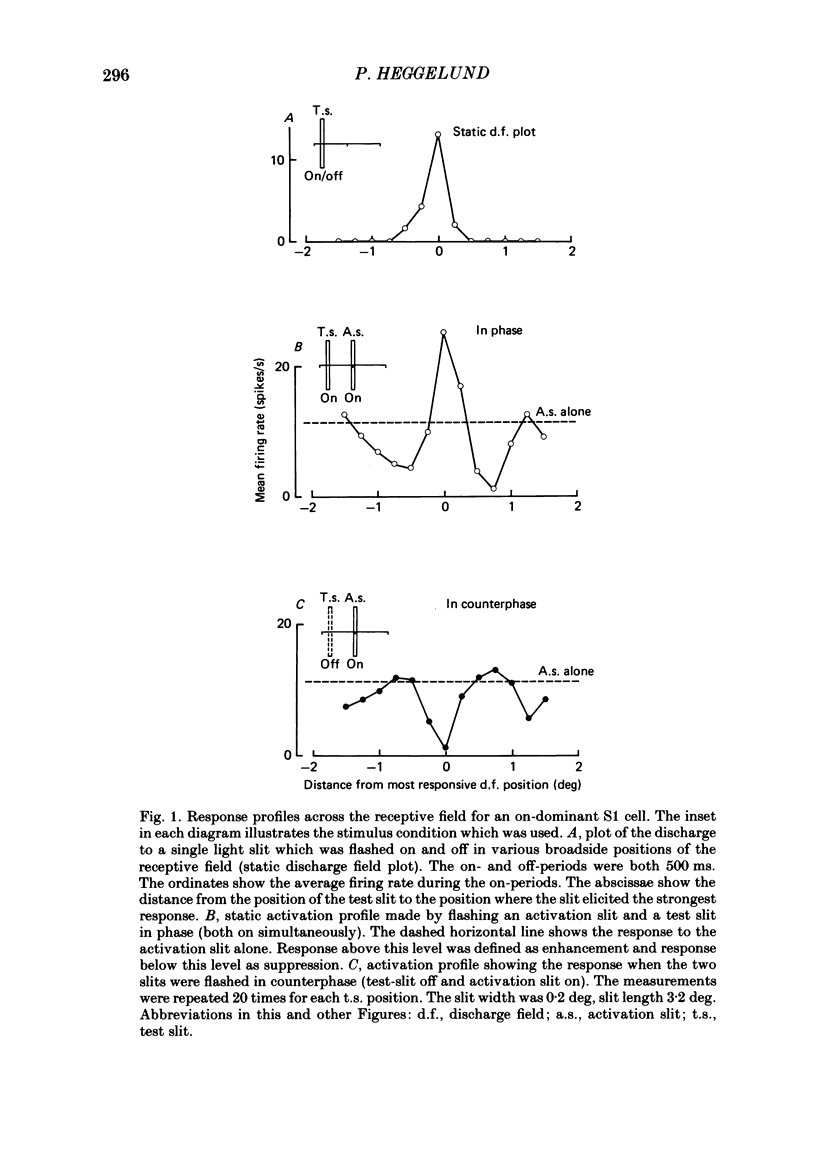
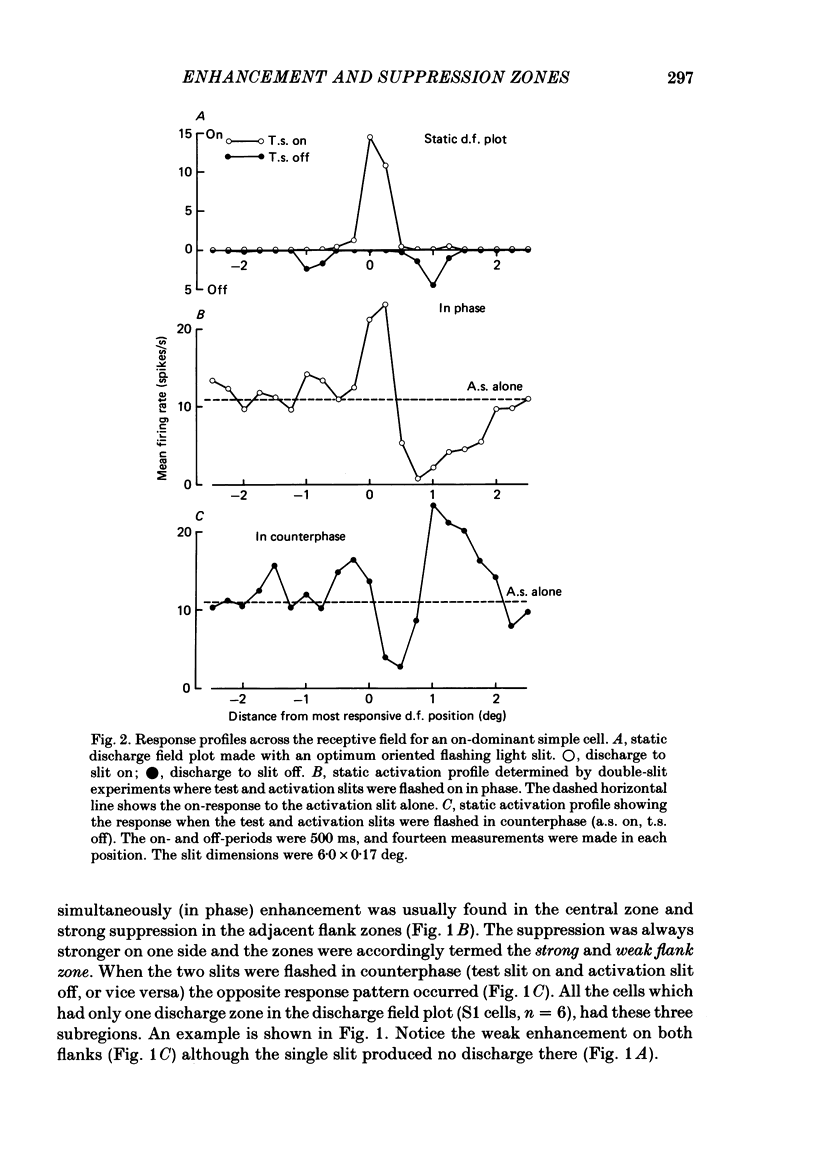
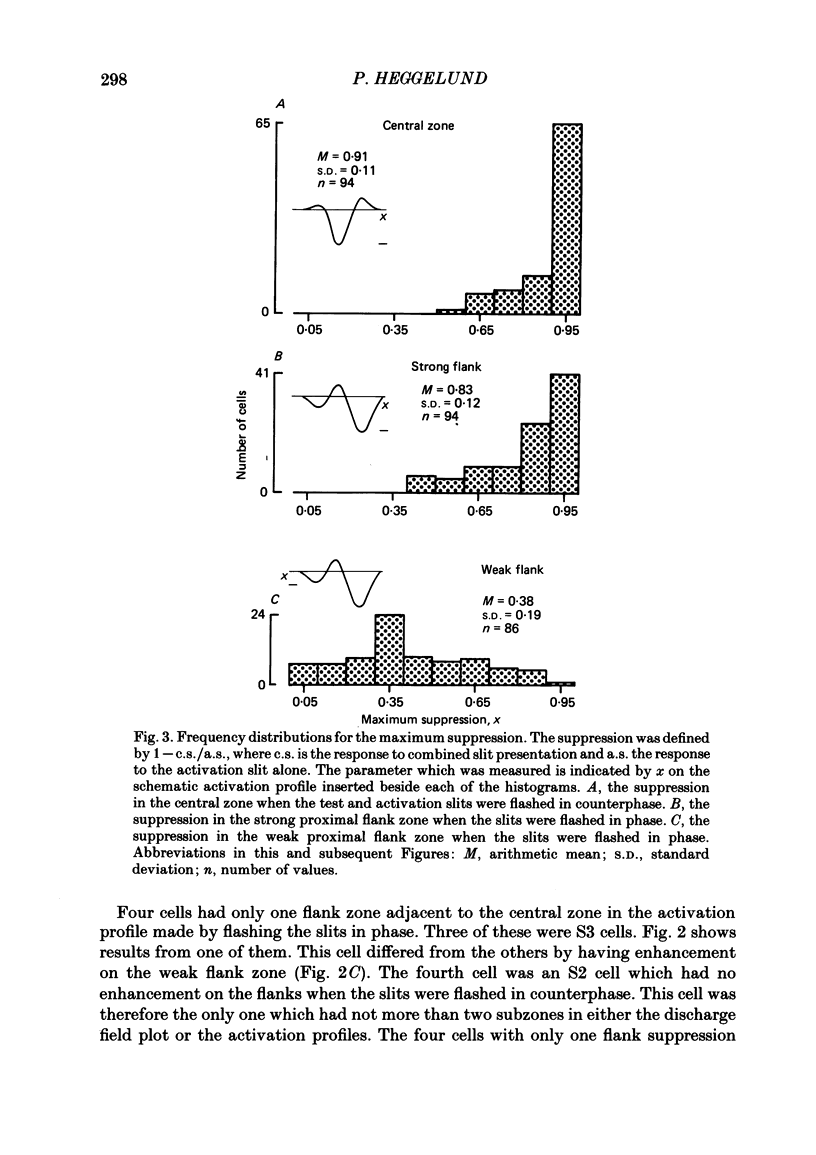
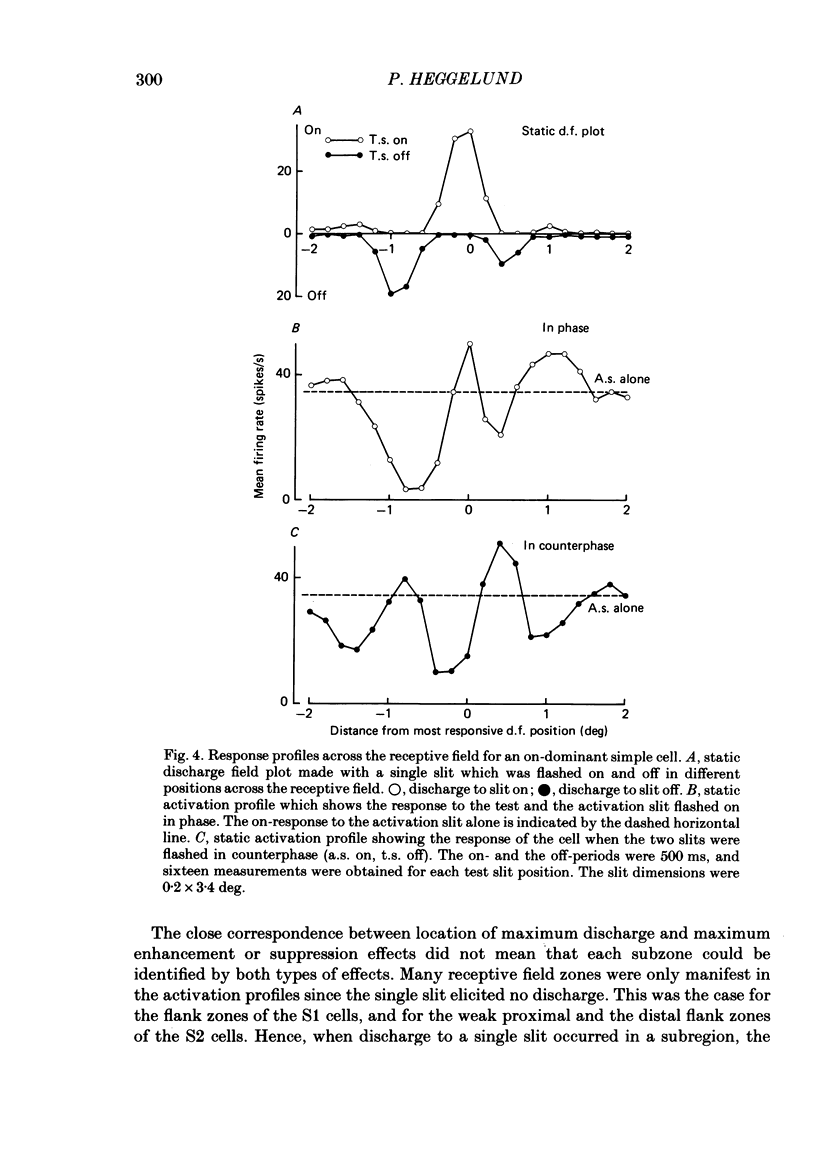
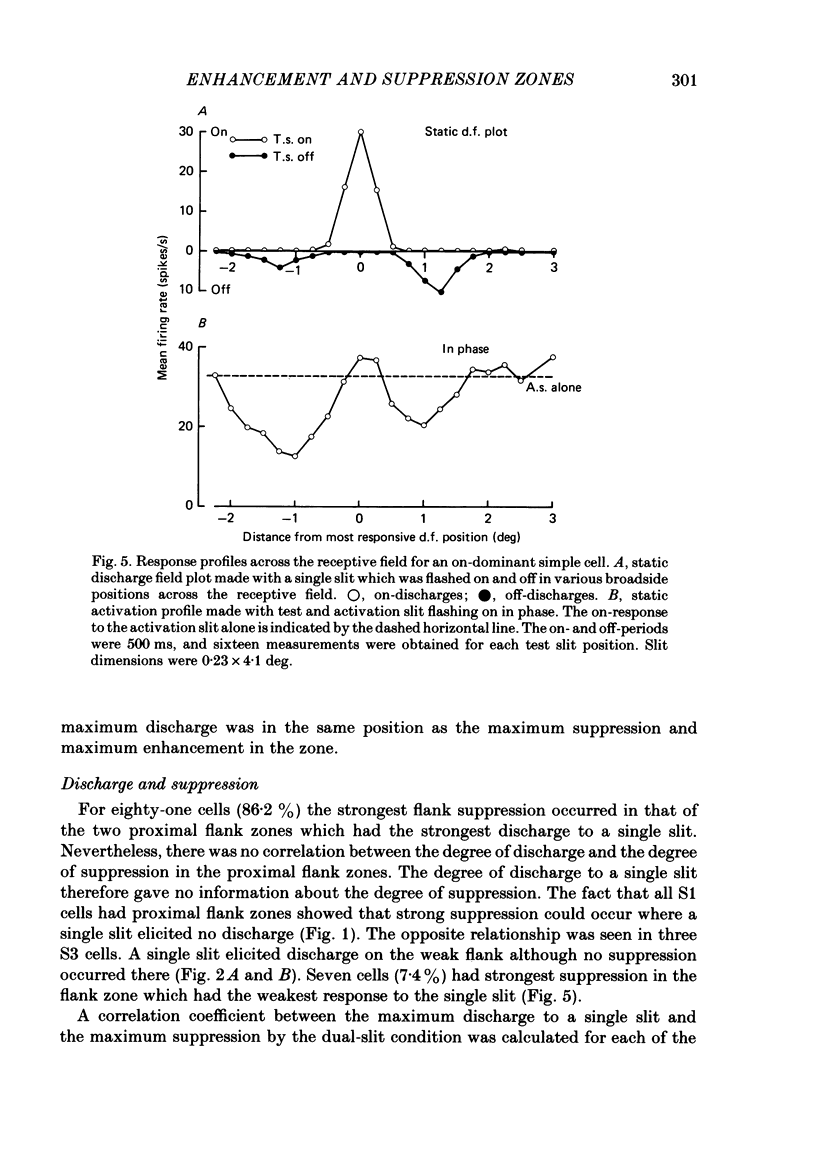
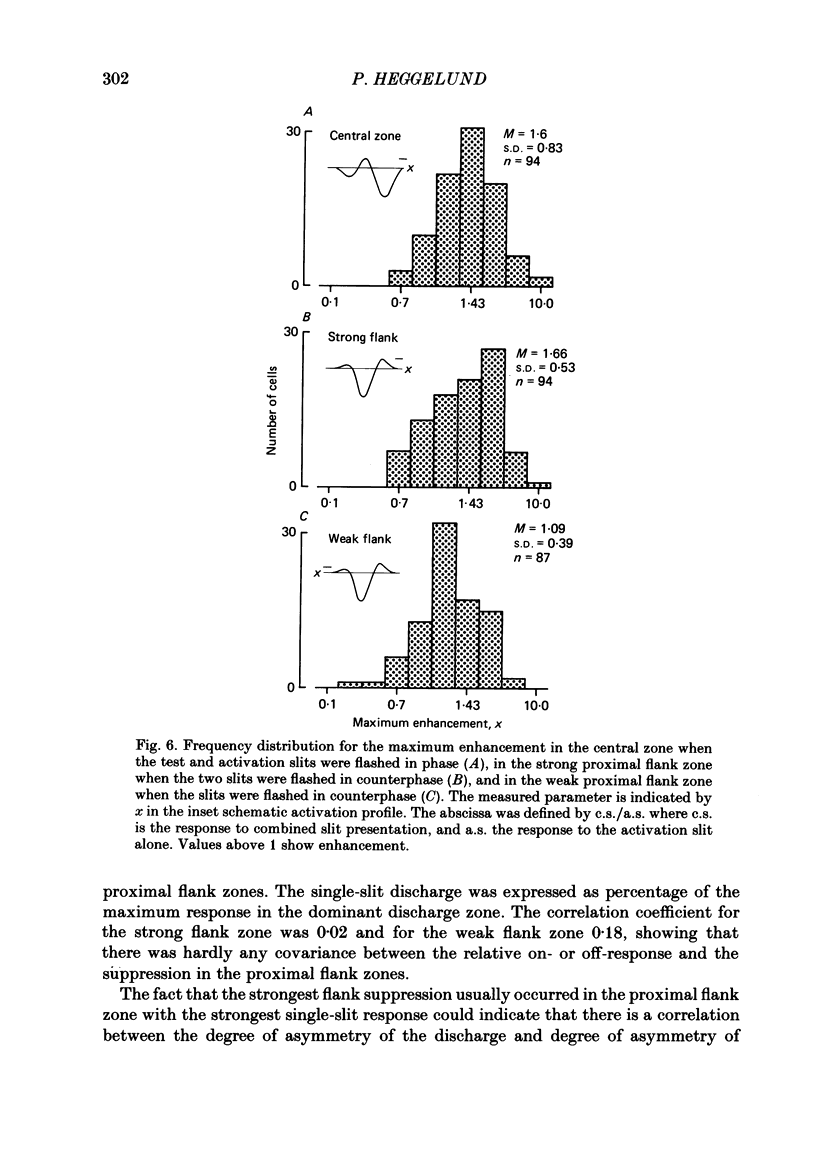
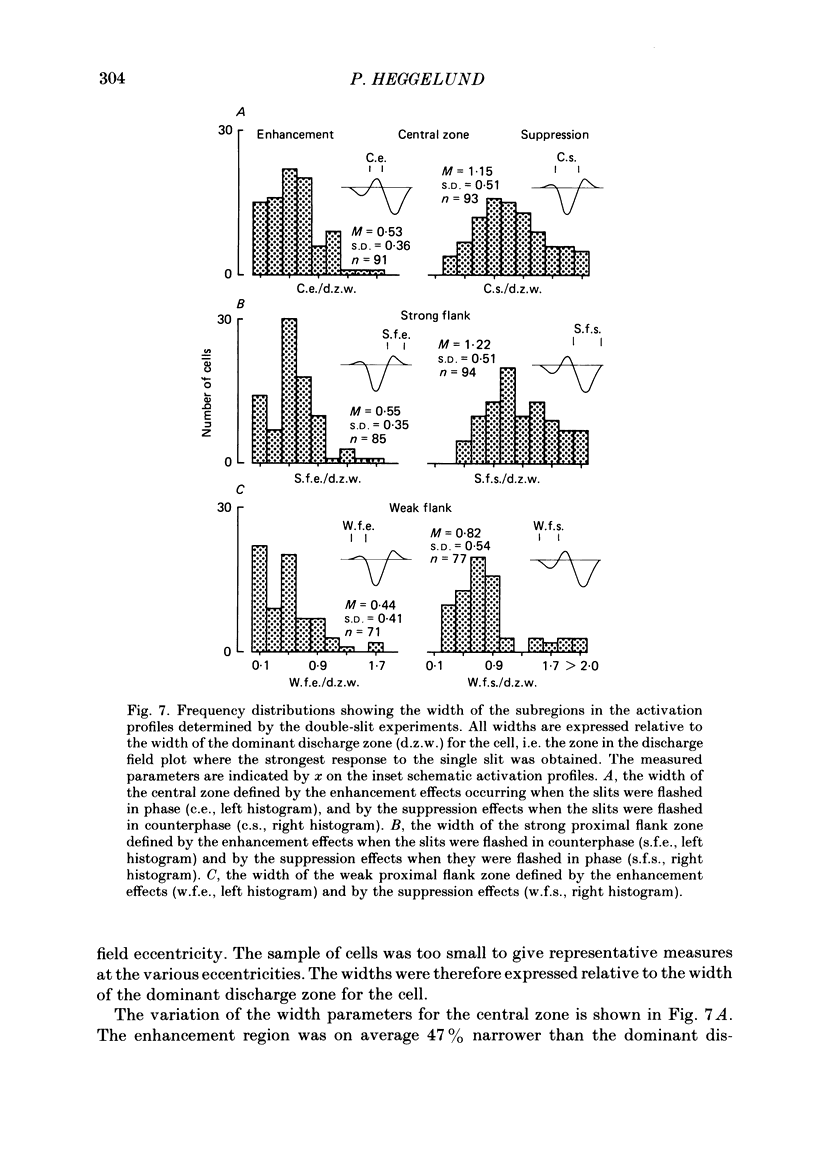
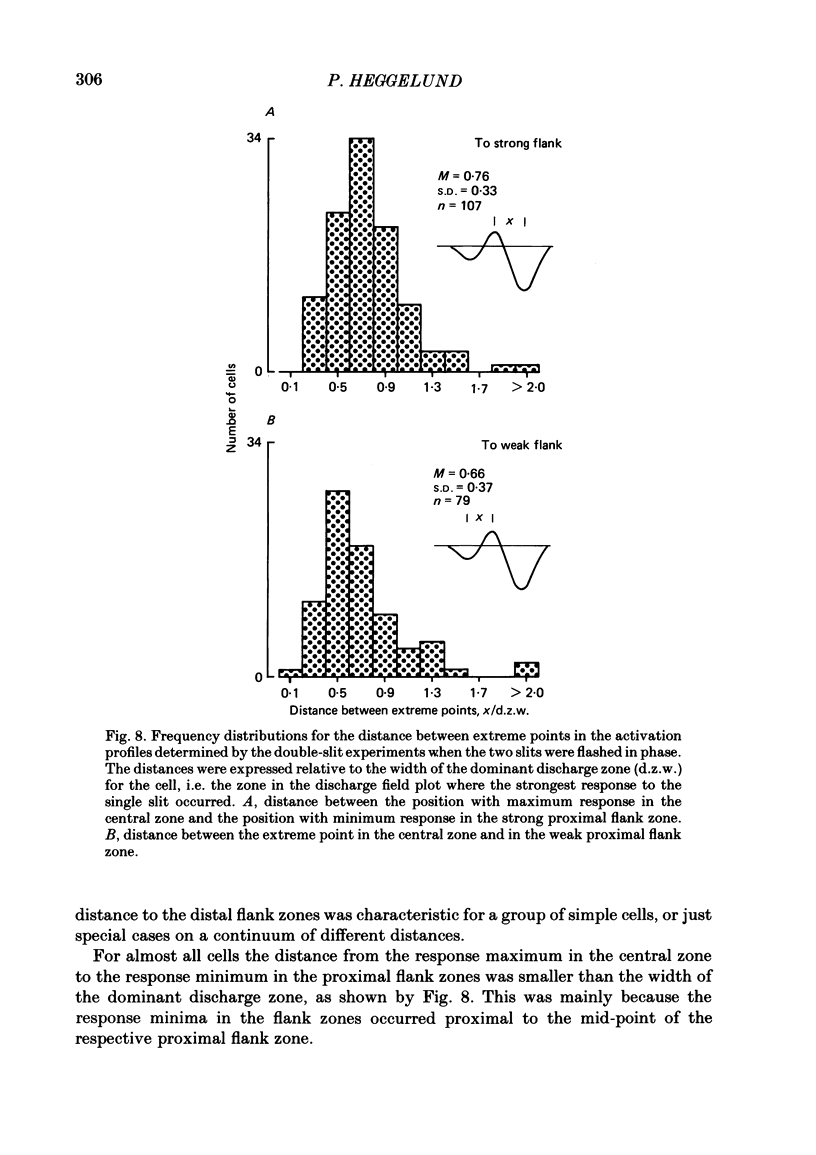
The configuration and extension of enhancement and suppression zones were compared with the configuration and extension of on- and off-response zones across the receptive field of simple cells in cat striate cortex. The enhancement and suppression zones were determined by a dual-stimulus technique where a stationary flashing light slit produced activity against which activation profiles across the receptive field were plotted with a parallel stationary test slit. The activation profiles showed less variation in receptive field configuration than plots of on- and off-discharge zones. Whereas the number of on- and off-zones varied between one and five, the activation profiles showed at least three distinct subregions in the receptive field, i.e. a central zone with an adjacent oppositely responding zone on each side. The responsivity was clearly stronger in one of these proximal flank zones. An additional zone occurred distal to the strong proximal flank zone in 53% of the cells, and in 10% such a distal zone occurred distal to both proximal flank zones. There was good correspondence between the location of on- and off-discharge zones and the location of the enhancement and suppression regions, although some subregions seen in the activation profiles did not appear in plots made with a single slit. The maximum discharge and the maximum enhancement and suppression effects in a subregion were found at the same receptive field location. The width of a subregion was measured as the width of the eventual on- or off-discharge zone determined with a single slit, as the width of the enhancement zone, and as the width of the suppression zone determined with the dual-slit technique. The enhancement zone was narrower, and the suppression zone wider than the discharge zone. The strong proximal flank zone had the same width as the central zone, but was wider than the weak proximal flank zone. For most cells the distances between successive extreme points across the activation profiles were constant, and this may explain the selectivity of the cells for spatial frequency of periodic stimuli. The strongest flank suppression occurred for most cells in that of the two proximal flank zones which had the strongest discharge to the single slit. Nevertheless, there was no correlation between the degree of discharge and the degree of suppression produced by opposite light cycles.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht D. G., De Valois R. L. Striate cortex responses to periodic patterns with and without the fundamental harmonics. J Physiol. 1981;319:497–514. doi: 10.1113/jphysiol.1981.sp013922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews B. W., Pollen D. A. Relationship between spatial frequency selectivity and receptive field profile of simple cells. J Physiol. 1979 Feb;287:163–176. doi: 10.1113/jphysiol.1979.sp012652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Dreher B., Henry G. H. Simple striate cells: comparison of responses to stationary and moving stimuli. J Physiol. 1972 Dec;227(2):15P–17P. [PubMed] [Google Scholar]
- Campbell F. W., Cooper G. F., Enroth-Cugell C. The spatial selectivity of the visual cells of the cat. J Physiol. 1969 Jul;203(1):223–235. doi: 10.1113/jphysiol.1969.sp008861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt O., Ito M. Functional synaptic organization of primary visual cortex neurones in the cat. Exp Brain Res. 1968;6(4):324–352. doi: 10.1007/BF00233183. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund P., Krekling S., Skottun B. C. Spatial summation in the receptive field of simple cells in the cat striate cortex. Exp Brain Res. 1983;52(1):87–98. doi: 10.1007/BF00237153. [DOI] [PubMed] [Google Scholar]
- Heggelund P. Quantitative studies of the discharge fields of single cells in cat striate cortex. J Physiol. 1986 Apr;373:277–292. doi: 10.1113/jphysiol.1986.sp016047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund P. Receptive field organization of simple cells in cat striate cortex. Exp Brain Res. 1981;42(1):89–98. doi: 10.1007/BF00235733. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Coombs J. S. Inhibitory and sub-liminal excitatory receptive fields of simple units in cat striate cortex. Vision Res. 1969 Oct;9(10):1289–1296. doi: 10.1016/0042-6989(69)90116-3. [DOI] [PubMed] [Google Scholar]
- Kulikowski J. J., Bishop P. O. Linear analysis of the responses of simple cells in the cat visual cortex. Exp Brain Res. 1981;44(4):386–400. doi: 10.1007/BF00238831. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978 Oct;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. A., Davis T. L. Receptive-field structure in cat striate cortex. J Neurophysiol. 1981 Aug;46(2):260–276. doi: 10.1152/jn.1981.46.2.260. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory mechanisms influencing complex cell orientation selectivity and their modification at high resting discharge levels. J Physiol. 1979 Apr;289:33–53. doi: 10.1113/jphysiol.1979.sp012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson I. D., Tolhurst D. J. Variation in the spatial frequency selectivity of neurones in the cat visual cortex [proceedings]. J Physiol. 1979 Oct;295:33P–33P. [PubMed] [Google Scholar]


