Abstract
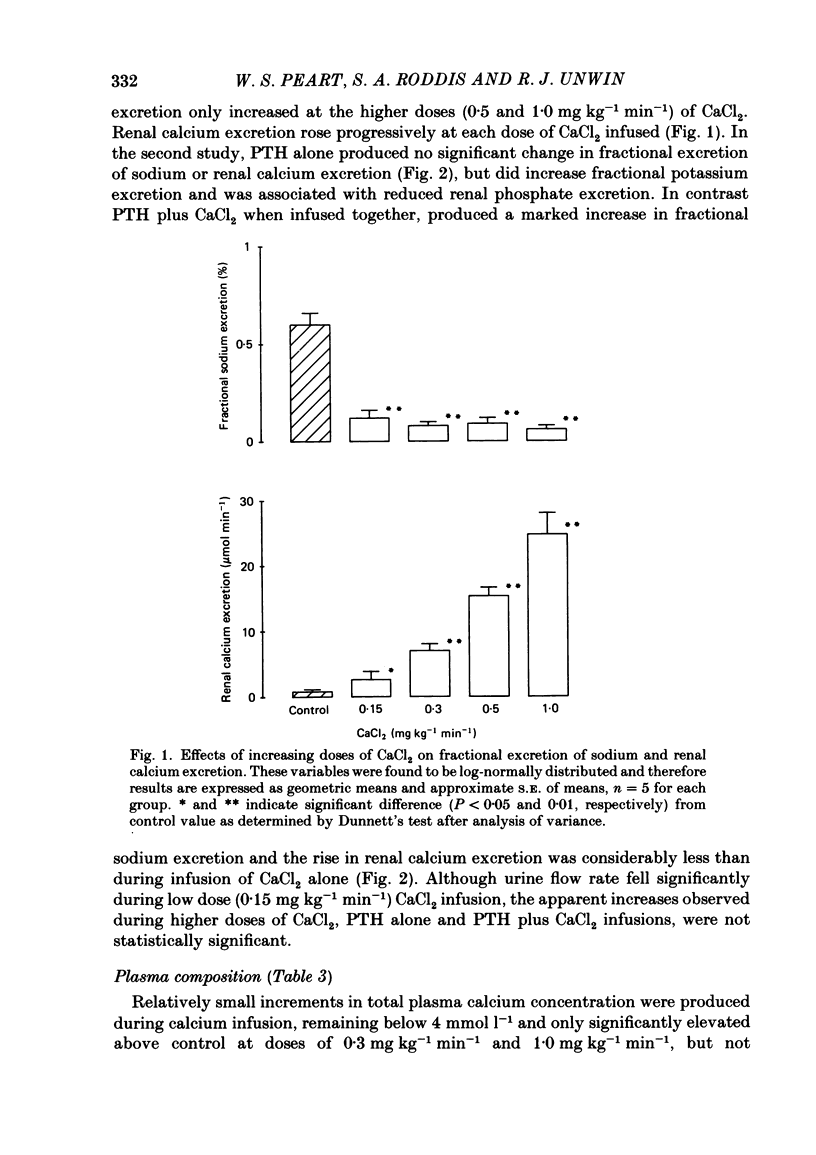
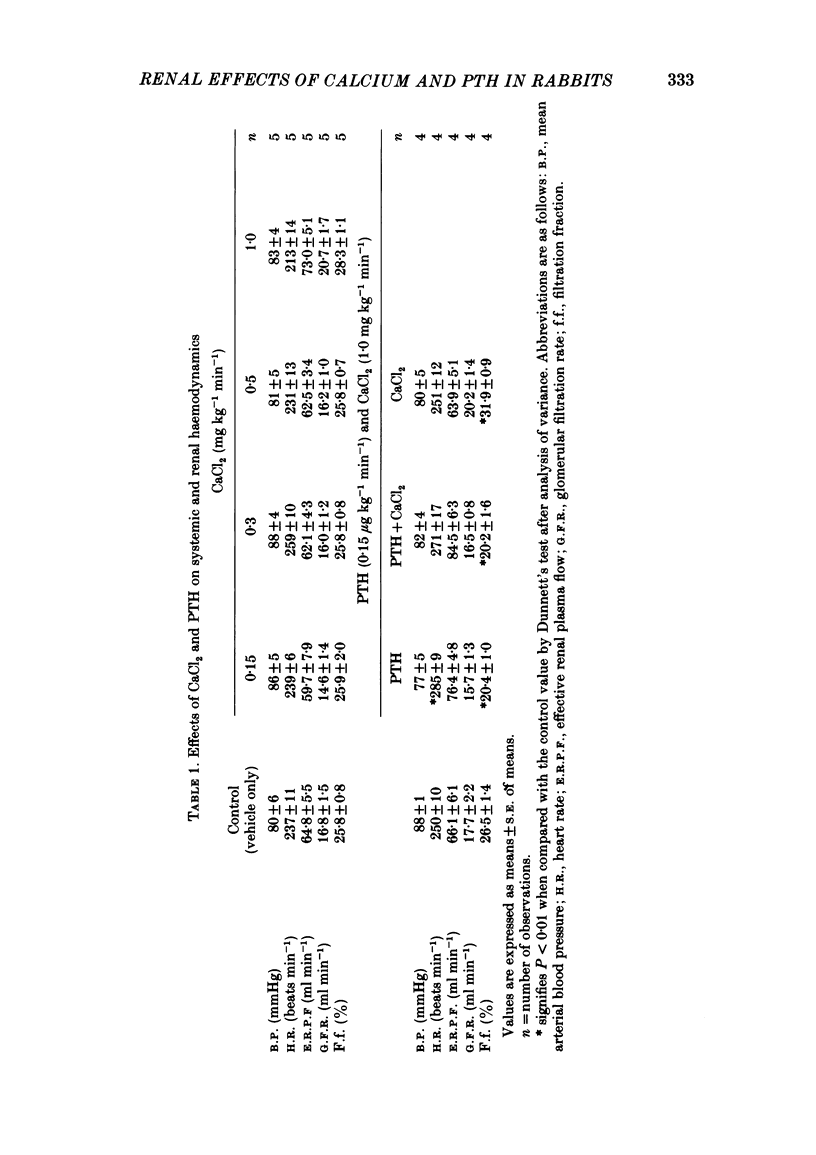
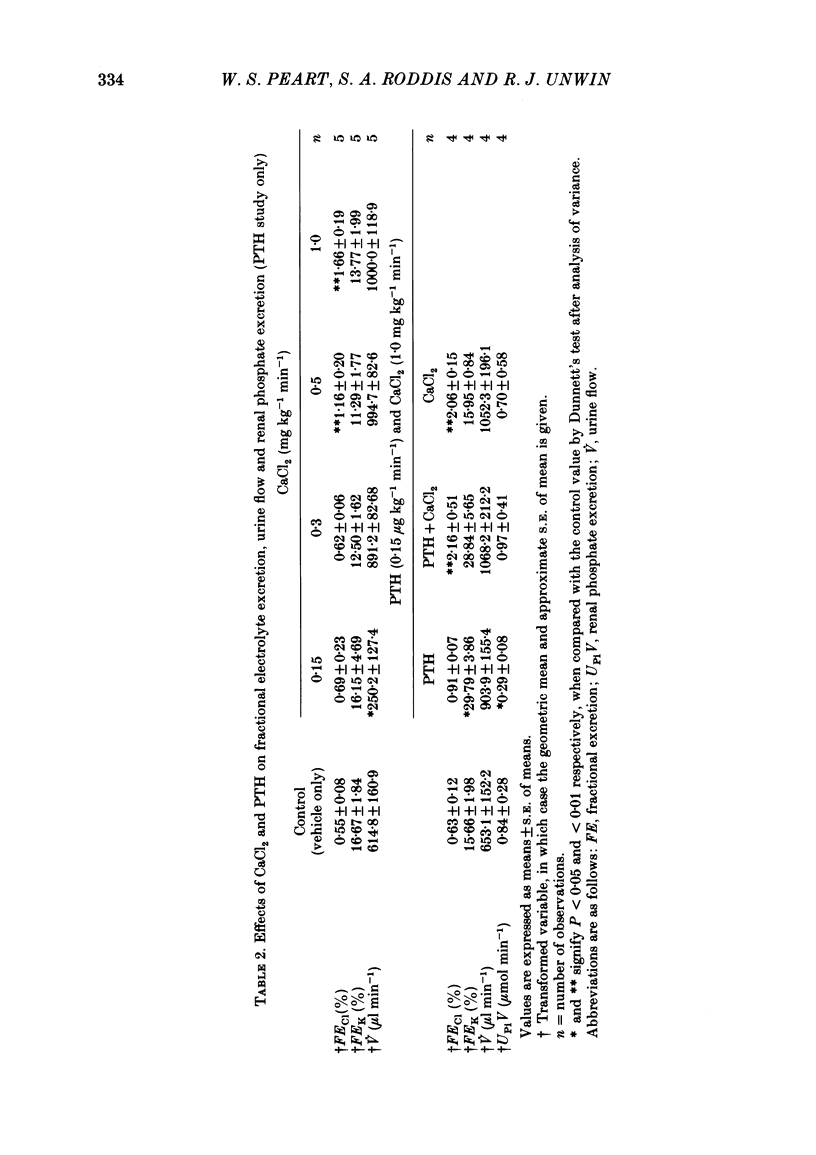
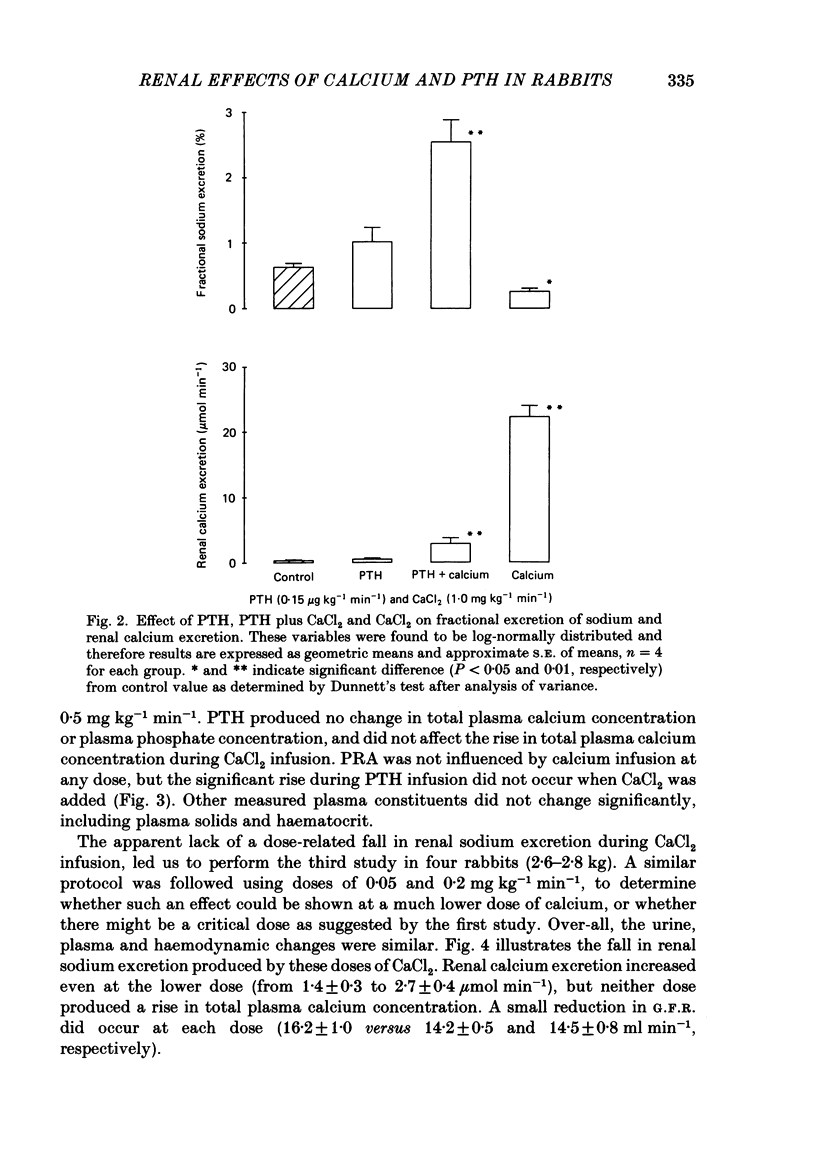
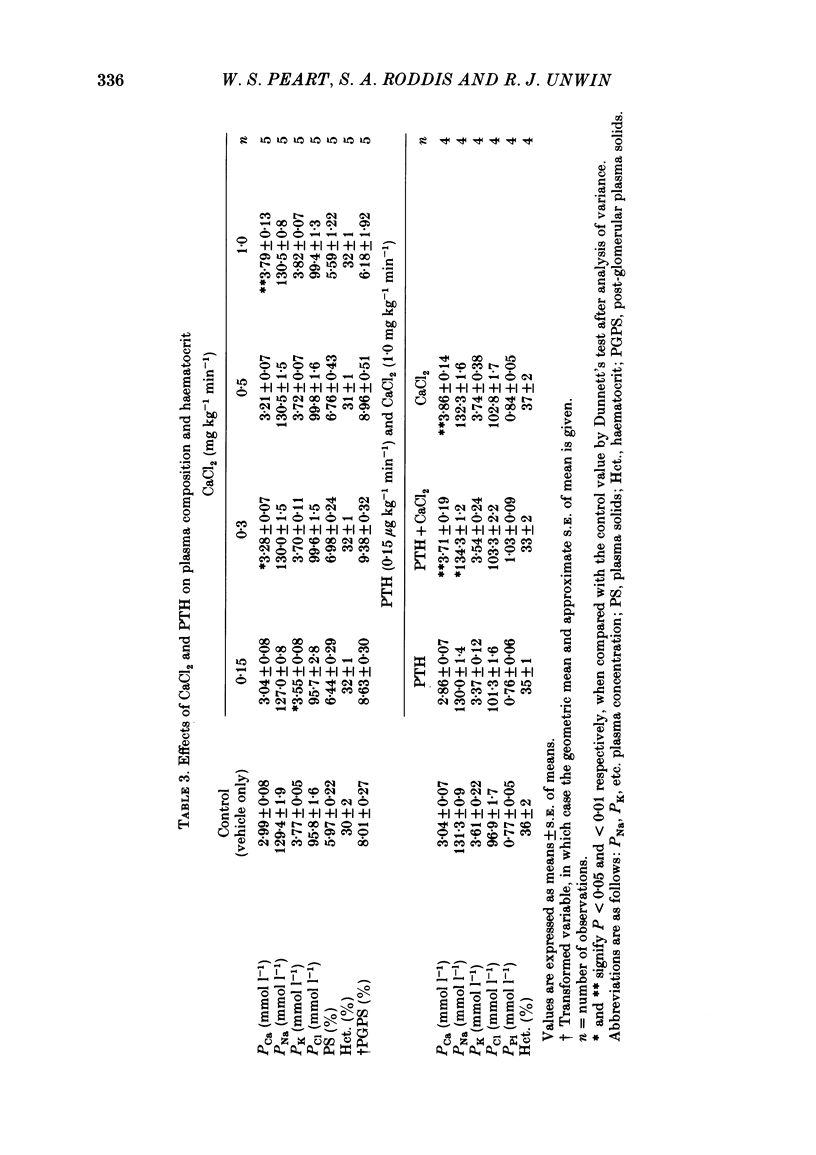
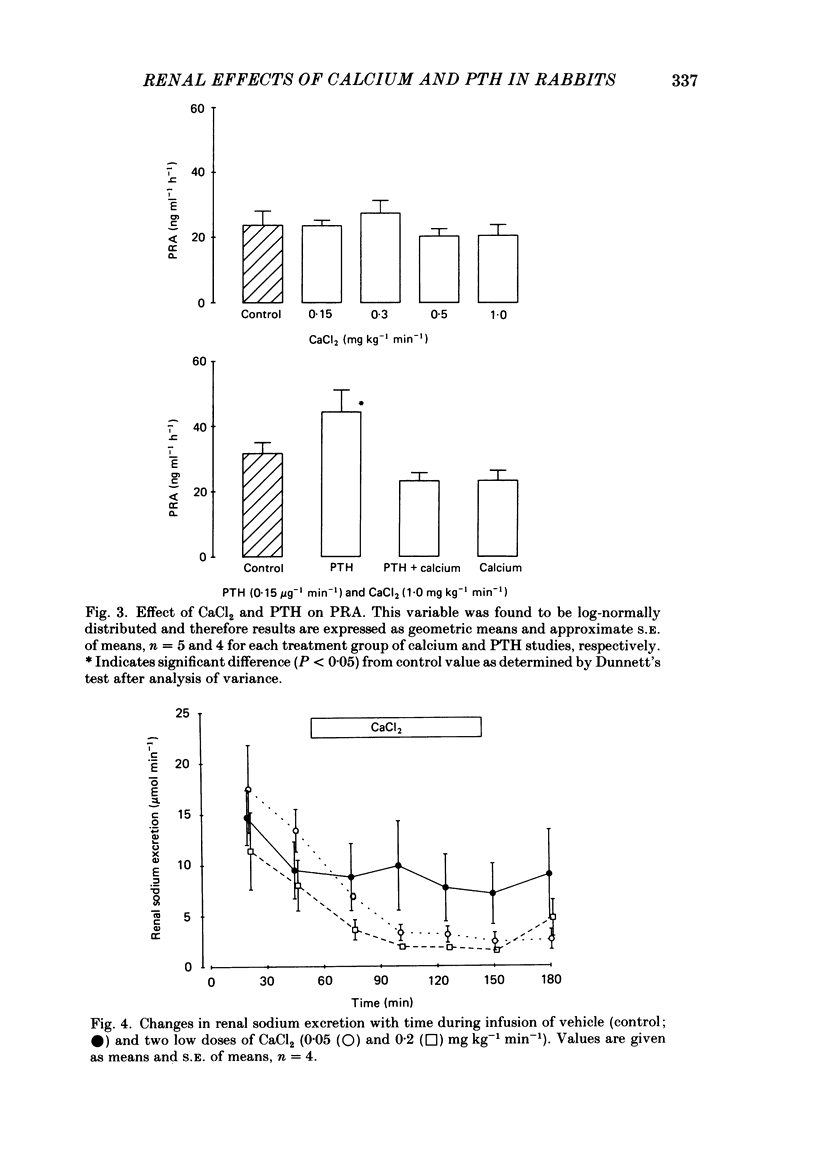
Following a random block experimental design in each case, three repeated measurement studies were carried out in three different groups of conscious rabbits, to investigate the renal effects of increasing doses of intravenous calcium chloride (CaCl2) and bovine parathyroid hormone (PTH). In the first study, each rabbit received either CaCl2 (0.15, 0.3, 0.5 or 1.0 mg kg-1 min-1) or vehicle alone (control) for 160 min. In the second study, rabbits were given either PTH (0.15 microgram kg-1 min-1), CaCl2 (1.0 mg kg-1 min-1), PTH plus CaCl2 (0.15 microgram kg-1 min-1 and 1.0 mg kg-1 min-1, respectively) or vehicle alone; PTH was infused for just over 60 min. In the third study, a much smaller dose (0.05 mg kg-1 min-1) of CaCl2 was infused for 100 min. CaCl2 infusion produced a striking fall in fractional excretion of sodium of at least 50% (P less than 0.01), but this was not dose related, being almost maximal at the smaller doses infused. Although this effect was evident in the absence of any changes in total plasma calcium concentration at the lower doses of CaCl2, renal calcium excretion was increased between 2- and 20-fold (P less than 0.01) at all doses infused. Fractional excretion of chloride doubled at the two higher doses of CaCl2 (P less than 0.01), but potassium excretion was unchanged. There were no consistent alterations in mean arterial blood pressure, effective renal plasma flow, glomerular filtration rate or plasma renin activity (PRA); total plasma calcium concentration was consistently elevated only during infusion of the high dose by just under 1 mmol l-1. PTH infusion had no measured effect on fractional excretion of sodium or renal calcium excretion, but doubled fractional potassium excretion (P less than 0.05). Heart rate and PRA increased (P less than 0.01 and less than 0.05, respectively), the latter by 50%, but systemic pressure and renal haemodynamics were not significantly affected. By contrast, PTH infused with CaCl2 produced a 4-fold rise in fractional sodium excretion and although renal calcium excretion remained increased, it was reduced by ca. 80% when compared with renal calcium excretion during infusion of CaCl2 alone. Infusion of PTH alone increased PRA, but when PTH and CaCl2 were infused together, PRA did not change.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt T. J., Knox F. G. Effects of parathyroid hormone and calcitonin on electrolyte excretion in the rabbit. Kidney Int. 1980 Apr;17(4):473–478. doi: 10.1038/ki.1980.55. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Hodgkin A. L. The effect of cyanide on the efflux of calcium from squid axons. J Physiol. 1969 Feb;200(2):497–527. doi: 10.1113/jphysiol.1969.sp008704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsztyk K., George J. P., Wright F. S. Effects of luminal fluid anions on calcium transport by proximal tubule. Am J Physiol. 1984 May;246(5 Pt 2):F600–F608. doi: 10.1152/ajprenal.1984.246.5.F600. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- Borle A. B., Uchikawa T. Effects of parathyroid hormone on the distribution and transport of calcium in cultured kidney cells. Endocrinology. 1978 Jun;102(6):1725–1732. doi: 10.1210/endo-102-6-1725. [DOI] [PubMed] [Google Scholar]
- Chomdej B., Bell P. D., Navar L. G. Renal hemodynamic and autoregulatory responses to acute hypercalcemia. Am J Physiol. 1977 Jun;232(6):F490–F496. doi: 10.1152/ajprenal.1977.232.6.F490. [DOI] [PubMed] [Google Scholar]
- Costanzo L. S. Comparison of calcium and sodium transport in early and late rat distal tubules: effect of amiloride. Am J Physiol. 1984 Jun;246(6 Pt 2):F937–F945. doi: 10.1152/ajprenal.1984.246.6.F937. [DOI] [PubMed] [Google Scholar]
- Dennis V. W., Stead W. W., Myers J. L. Renal handling of phosphate and calcium. Annu Rev Physiol. 1979;41:257–271. doi: 10.1146/annurev.ph.41.030179.001353. [DOI] [PubMed] [Google Scholar]
- Dimaline R., Peart W. S., Unwin R. J. Effects of vasoactive intestinal polypeptide (VIP) on renal function and plasma renin activity in the conscious rabbit. J Physiol. 1983 Nov;344:379–388. doi: 10.1113/jphysiol.1983.sp014946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Flatman P. W., Stewart G. W. Inhibition of human red cell sodium and potassium transport by divalent cations. J Physiol. 1983 Jul;340:1–17. doi: 10.1113/jphysiol.1983.sp014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein F. H. Calcium and the kidney. Am J Med. 1968 Nov;45(5):700–714. doi: 10.1016/0002-9343(68)90206-4. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Figueiredo J. F., Maack T., Windhager E. E. Sodium-calcium interactions in the renal proximal convoluted tubule of the rabbit. Am J Physiol. 1981 Jun;240(6):F558–F568. doi: 10.1152/ajprenal.1981.240.6.F558. [DOI] [PubMed] [Google Scholar]
- Frindt G., Windhager E. E., Taylor A. Hydroosmotic response of collecting tubules to ADH or cAMP at reduced peritubular sodium. Am J Physiol. 1982 Nov;243(5):F503–F513. doi: 10.1152/ajprenal.1982.243.5.F503. [DOI] [PubMed] [Google Scholar]
- Gordon D., Nashat F. S., Wilcox C. S. An analysis of the regulation of sodium excretion during induced changes in plasma sodium concentration in anaesthetized dogs. J Physiol. 1981 May;314:531–545. doi: 10.1113/jphysiol.1981.sp013723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habener J. F., Rosenblatt M., Potts J. T., Jr Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev. 1984 Jul;64(3):985–1053. doi: 10.1152/physrev.1984.64.3.985. [DOI] [PubMed] [Google Scholar]
- Jayakumar A., Cheng L., Liang C. T., Sacktor B. Sodium gradient-dependent calcium uptake in renal basolateral membrane vesicles. Effect of parathyroid hormone. J Biol Chem. 1984 Sep 10;259(17):10827–10833. [PubMed] [Google Scholar]
- Kotchen T. A., Galla J. H., Luke R. G. Effects of calcium on renin and aldosterone in the rat. Am J Physiol. 1977 Apr;232(4):E388–E393. doi: 10.1152/ajpendo.1977.232.4.E388. [DOI] [PubMed] [Google Scholar]
- Kotchen T. A., Mauli K. I., Luke R., Rees D., Flamenbaum W. Effect of acute and chronic calcium administration on plasma renin. J Clin Invest. 1974 Dec;54(6):1279–1286. doi: 10.1172/JCI107873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVITT M. F., HALPERN M. H., POLIMEROS D. P., SWEET A. Y., GRIBETZ D. The effect of abrupt changes in plasma calcium concentrations on renal function and electrolyte excretion in man and monkey. J Clin Invest. 1958 Feb;37(2):294–305. doi: 10.1172/JCI103608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennane R. J., Stockigt J., Peart W. S. The effect of active immunization against aldosterone on the colonic potential response and sodium excretion in rabbits. Clin Sci Mol Med. 1976 Jun;50(6):545–549. doi: 10.1042/cs0500545. [DOI] [PubMed] [Google Scholar]
- Lins L. E. Renal function in hypercalcemic dogs during hydropenia and during saline infusion. Acta Physiol Scand. 1979 Jun;106(2):177–186. doi: 10.1111/j.1748-1716.1979.tb06387.x. [DOI] [PubMed] [Google Scholar]
- Lorenzen M., Lee C. O., Windhager E. E. Cytosolic Ca2+ and Na+ activities in perfused proximal tubules of Necturus kidney. Am J Physiol. 1984 Jul;247(1 Pt 2):F93–102. doi: 10.1152/ajprenal.1984.247.1.F93. [DOI] [PubMed] [Google Scholar]
- Manery J. F. Effects of Ca ions on membranes. Fed Proc. 1966 Nov-Dec;25(6):1804–1810. [PubMed] [Google Scholar]
- Ng R. C., Rouse D., Suki W. N. Calcium transport in the rabbit superficial proximal convoluted tubule. J Clin Invest. 1984 Sep;74(3):834–842. doi: 10.1172/JCI111500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. S., Malvin R. L. Calcium in the control of renin release. Am J Physiol. 1978 Jul;235(1):F22–F25. doi: 10.1152/ajprenal.1978.235.1.F22. [DOI] [PubMed] [Google Scholar]
- Peart W. S. Renin release. Gen Pharmacol. 1978;9(2):65–72. doi: 10.1016/0306-3623(78)90001-0. [DOI] [PubMed] [Google Scholar]
- Sejersted O. M., Steen P. A., Kiil F. Inhibition of transcellular NaCl reabsorption in dog kidneys during hypercalcemia. Acta Physiol Scand. 1984 Apr;120(4):543–549. doi: 10.1111/j.1748-1716.1984.tb07419.x. [DOI] [PubMed] [Google Scholar]
- Shirley D. G., Poujeol P., Le Grimellec C. Phosphate, calcium and magnesium fluxes into the lumen of the rat proximal convoluted tubule. Pflugers Arch. 1976 Apr 6;362(3):247–254. doi: 10.1007/BF00581177. [DOI] [PubMed] [Google Scholar]
- Sutton R. A., Wong N. L., Dirks J. H. Effects of parathyroid hormone on sodium and calcium transport in the dog nephron. Clin Sci Mol Med. 1976 Oct;51(4):345–351. doi: 10.1042/cs0510345. [DOI] [PubMed] [Google Scholar]
- Taylor A., Windhager E. E. Possible role of cytosolic calcium and Na-Ca exchange in regulation of transepithelial sodium transport. Am J Physiol. 1979 Jun;236(6):F505–F512. doi: 10.1152/ajprenal.1979.236.6.F505. [DOI] [PubMed] [Google Scholar]
- Ullrich K. J., Rumrich G., Klöss S. Active Ca2+ reabsorption in the proximal tubule of the rat kidney. Dependence on sodium- and buffer transport. Pflugers Arch. 1976 Aug 24;364(3):223–228. doi: 10.1007/BF00581759. [DOI] [PubMed] [Google Scholar]
- Vanherweghem J. L., Ducobu J., d'Hollander A., Toussaint C. Effects of hypercalcemia on water and sodium excretion by the isolated dog kidney. Pflugers Arch. 1976 May 6;363(1):75–80. doi: 10.1007/BF00587405. [DOI] [PubMed] [Google Scholar]


