Abstract
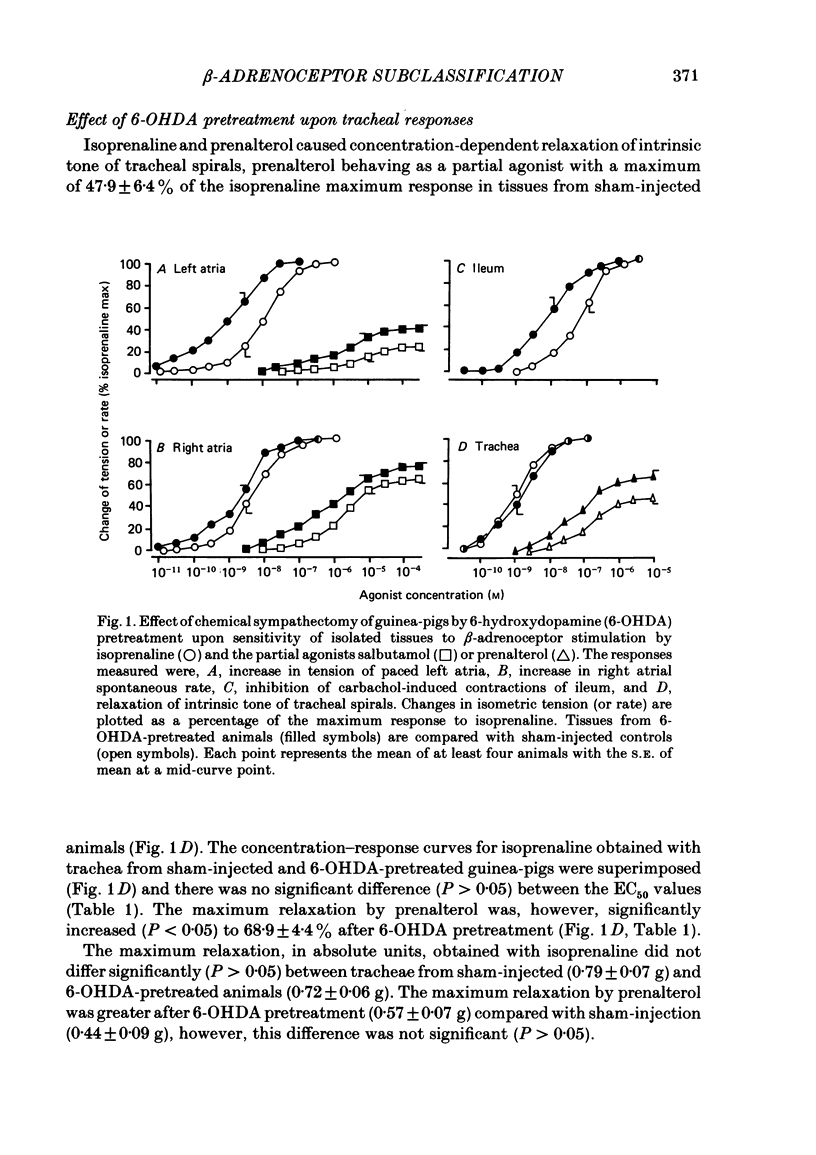
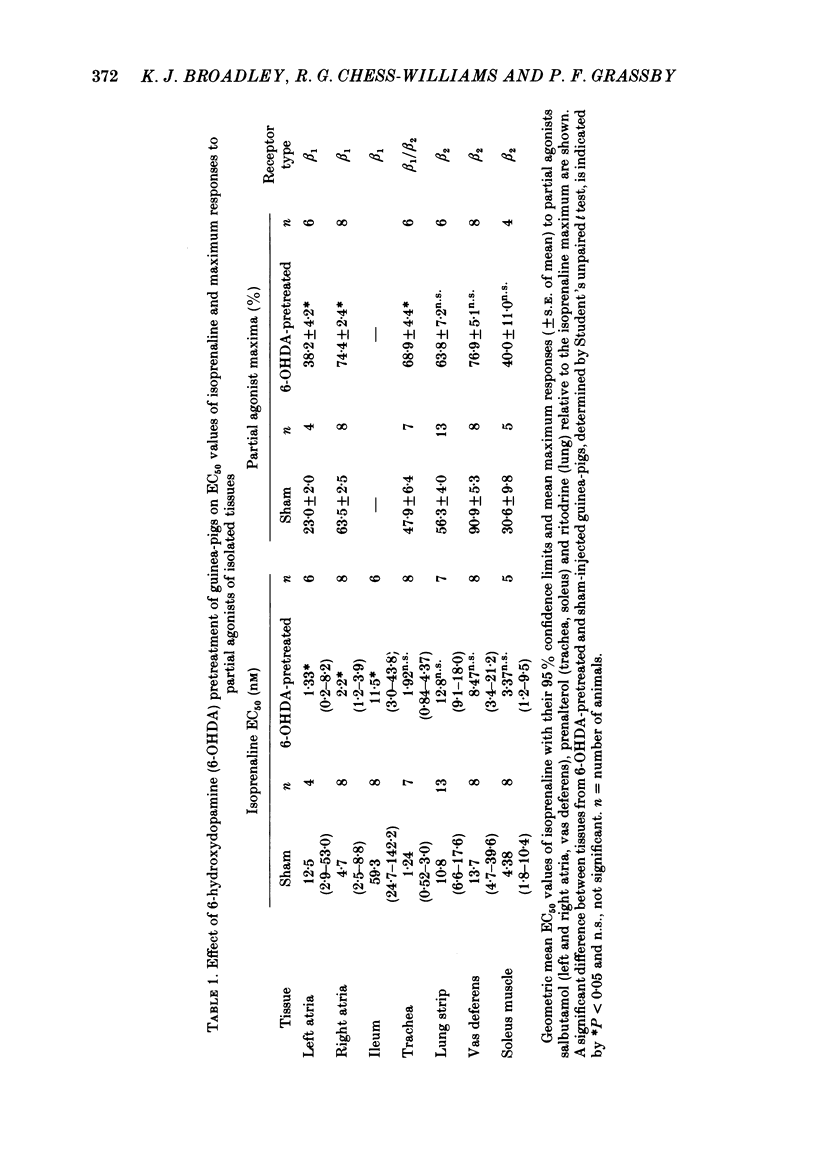
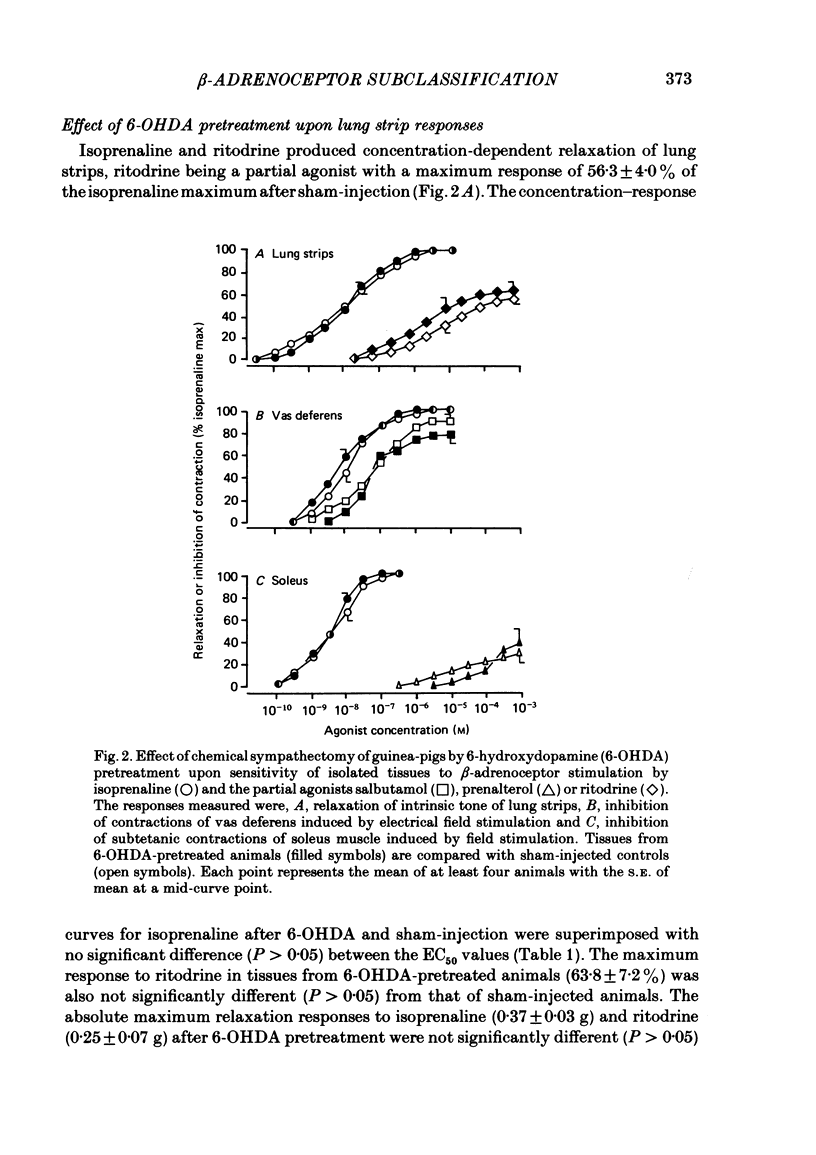
Chemical sympathectomy of guinea-pigs was induced by chronic pretreatment with 6-hydroxydopamine over a 20 day period. Control animals were sham injected with vehicle at the same times. Isolated tissues were removed from the animals and beta-adrenoceptor sensitivity assessed from cumulative concentration-response curves for isoprenaline, followed after wash-out by a partial agonist (salbutamol, ritodrine or prenalterol). The following responses were measured: increases in force and rate of contraction of left and right atria respectively, inhibition of carbachol-induced ileal contractions, relaxation of intrinsic tone of lung strips and tracheal spirals, inhibition of contractions of vas deferens and soleus muscle induced by field stimulation. Left and right atria and ileum from 6-hydroxydopamine-pretreated guinea-pigs exhibited supersensitivity to beta-adrenoceptor stimulation. This was measured as a leftwards shift of the concentration-response curve for isoprenaline and as an elevation of the partial agonist maximum response (relative to isoprenaline), when compared with tissues from sham-injected controls. The supersensitivity was assumed to be due to the loss of endogenous neurotransmitter release by chemical sympathectomy and specific for the beta-adrenoceptor. In contrast, lung strips, vas deferens and soleus muscle were not supersensitive. The responses of these tissues are thought to be mediated via beta 2-adrenoceptors whereas cardiac and ileal responses are beta 1-adrenoceptor mediated. The latter receptor subtype would therefore appear to be under the influence of sympathetic innervation, but since no supersensitivity occurred at beta 2-adrenoceptors these were presumed to be non-innervated but stimulated by circulating adrenaline. These results obtained by use of chemical sympathectomy with 6-hydroxydopamine support the contention that the physiological basis of beta-adrenoceptor subclassification is that the beta 1-subtype are innervated whereas the beta 2-subtype are non-innervated.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelakos E. T., King M. P., Millard R. W. Regional distribution of catecholamines in the hearts of various species. Ann N Y Acad Sci. 1969 Jan 31;156(1):219–240. doi: 10.1111/j.1749-6632.1969.tb16730.x. [DOI] [PubMed] [Google Scholar]
- Ariëns E. J., Simonis A. M. Physiological and pharmacological aspects of adrenergic receptor classification. Biochem Pharmacol. 1983 May 15;32(10):1539–1545. doi: 10.1016/0006-2952(83)90324-6. [DOI] [PubMed] [Google Scholar]
- Arnold A. Differentiation of receptors activated by catecholamines. 3. Farmaco Sci. 1972 Jan;27(1):79–100. [PubMed] [Google Scholar]
- Bevan J. A. Some functional consequences of variation in adrenergic synaptic cleft width and in nerve density and distribution. Fed Proc. 1977 Sep;36(10):2439–2443. [PubMed] [Google Scholar]
- Bowman W. C., Nott M. W. Actions of some sympathomimetic bronchodilator and beta-adrenoceptor blocking drugs on contractions of the cat soleus muscle. Br J Pharmacol. 1970 Jan;38(1):37–49. doi: 10.1111/j.1476-5381.1970.tb10334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman W. C., Nott M. W. Actions of sympathomimetic amines and their antagonists on skeletal muscle. Pharmacol Rev. 1969 Mar;21(1):27–72. [PubMed] [Google Scholar]
- CONSTANTINE J. W. THE SPIRALLY CUT TRACHEAL STRIP PREPARATION. J Pharm Pharmacol. 1965 Jun;17:384–385. doi: 10.1111/j.2042-7158.1965.tb07688.x. [DOI] [PubMed] [Google Scholar]
- Carlsson E., Dahlöf C. G., Hedberg A., Persson H., Tångstrand B. Differentiation of cardiac chronotropic and inotropic effects of beta-adrenoceptor agonists. Naunyn Schmiedebergs Arch Pharmacol. 1977 Nov;300(2):101–105. doi: 10.1007/BF00505039. [DOI] [PubMed] [Google Scholar]
- Chess-Williams R. G., Grassby P. F., Culling W., Penny W., Broadley K. J., Sheridan D. J. Cardiac postjunctional supersensitivity to beta-agonists after chronic chemical sympathectomy with 6-hydroxydopamine. Naunyn Schmiedebergs Arch Pharmacol. 1985 Apr;329(2):162–166. doi: 10.1007/BF00501207. [DOI] [PubMed] [Google Scholar]
- Culling W., Penny W. J., Lewis M. J., Middleton K., Sheridan D. J. Effects of myocardial catecholamine depletion on cellular electrophysiology and arrhythmias during ischaemia and reperfusion. Cardiovasc Res. 1984 Nov;18(11):675–682. doi: 10.1093/cvr/18.11.675. [DOI] [PubMed] [Google Scholar]
- Fleming W. W., McPhillips J. J., Westfall D. P. Postjunctional supersensitivity and subsensitivity of excitable tissues to drugs. Ergeb Physiol. 1973;68:55–119. doi: 10.1007/3-540-06238-6_5. [DOI] [PubMed] [Google Scholar]
- Furness J. B. Transmission to the longitudinal muscle of the guinea-pig vas deferens: The effect of pretreatment with guanethidine. Br J Pharmacol. 1974 Jan;50(1):63–68. doi: 10.1111/j.1476-5381.1974.tb09593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorn M. H., Broadley K. J. Reserpine-induced supersensitivity occurs for beta-adrenoceptor-mediated responses of heart and trachea but not of the uterus and lung. Eur J Pharmacol. 1984 Oct 15;105(3-4):245–255. doi: 10.1016/0014-2999(84)90616-2. [DOI] [PubMed] [Google Scholar]
- Hedberg A., Mattsson H., Nerme V., Carlsson E. Effects of in vivo treatment with isoprenaline or prenalterol on beta-adrenoceptor mechanisms in the heart and soleus muscle of the cat. Naunyn Schmiedebergs Arch Pharmacol. 1984 Mar;325(3):251–258. doi: 10.1007/BF00495952. [DOI] [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Lulich K. M., Mitchell H. W., Sparrow M. P. The cat lung strip as an in vitro preparation of peripheral airways: a comparison of beta-adrenoceptor agonists, autacoids and anaphylactic challenge on the lung strip and trachea. Br J Pharmacol. 1976 Sep;58(1):71–79. doi: 10.1111/j.1476-5381.1976.tb07694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisheri K. D., Tenner T. E., Jr, McNeill J. H. Reserpine-induced supersensitivity to the cardiac effects of agonists. Life Sci. 1979 Feb 5;24(6):473–480. doi: 10.1016/0024-3205(79)90167-x. [DOI] [PubMed] [Google Scholar]
- Mian M. A., Malta E., Raper C. An homogeneous population of beta 1-adrenoceptors subserves inhibitory responses in guinea-pig ileal preparations. J Pharm Pharmacol. 1984 Oct;36(10):698–699. doi: 10.1111/j.2042-7158.1984.tb04849.x. [DOI] [PubMed] [Google Scholar]
- NORBERG K. A. ADRENERGIC INNERVATION OF THE INTESTINAL WALL STUDIED BY FLUORESCENCE MICROSCOPY. Int J Neuropharmacol. 1964 Sep;3:379–382. doi: 10.1016/0028-3908(64)90067-x. [DOI] [PubMed] [Google Scholar]
- Nadeau R. A., de Champlain J., Tremblay G. M. Supersensitivity of the isolated rat heart after chemical sympathectomy with 6-hydroxydopamine. Can J Physiol Pharmacol. 1971 Jan;49(1):36–44. doi: 10.1139/y71-005. [DOI] [PubMed] [Google Scholar]
- O'Donnell S. R., Saar N. Histochemical localization of adrenergic nerves in the guinea-pig trachea. Br J Pharmacol. 1973 Apr;47(4):707–710. doi: 10.1111/j.1476-5381.1973.tb08197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell S. R., Saar N., Wood L. J. Tne density of adrenergic nerves at various levels in the guinea-pig lung. Clin Exp Pharmacol Physiol. 1978 Jul-Aug;5(4):325–332. doi: 10.1111/j.1440-1681.1978.tb00681.x. [DOI] [PubMed] [Google Scholar]
- Richardson J. B. Nerve supply to the lungs. Am Rev Respir Dis. 1979 May;119(5):785–802. doi: 10.1164/arrd.1979.119.5.785. [DOI] [PubMed] [Google Scholar]
- Shibata S., Kuchii M., Kurahashi K. The supersensitivity of isolated rabbit atria and aortic strips produced by 6-hydroxydopamine. Eur J Pharmacol. 1972 May;18(2):271–280. doi: 10.1016/0014-2999(72)90253-1. [DOI] [PubMed] [Google Scholar]
- Tenner T. E., Jr Propranolol withdrawal supersensitivity in rat cardiovascular tissue, in vitro. Eur J Pharmacol. 1983 Aug 19;92(1-2):91–97. doi: 10.1016/0014-2999(83)90112-7. [DOI] [PubMed] [Google Scholar]
- Waldeck B. An in vitro method for the study of beta-receptor mediated effects on slow contracting skeletal muscle. J Pharm Pharmacol. 1976 May;28(5):434–436. doi: 10.1111/j.2042-7158.1976.tb04649.x. [DOI] [PubMed] [Google Scholar]
- Waldeck B. Analysis of the beta-receptor mediated effect on slow-contracting skeletal muscle in vitro. J Pharm Pharmacol. 1977 Sep;29(9):550–554. doi: 10.1111/j.2042-7158.1977.tb11394.x. [DOI] [PubMed] [Google Scholar]
- Yamada S., Yamamura H. I., Roeske W. R. Alterations in cardiac autonomic receptors following 6-hydroxydopamine treatment in rats. Mol Pharmacol. 1980 Sep;18(2):185–192. [PubMed] [Google Scholar]
- Zaagsma J., van der Heijden P. J., van der Schaar M. W., Bank C. M. Comparison of functional beta-adrenoceptor heterogeneity in central and peripheral airway smooth muscle of guinea pig and man. J Recept Res. 1983;3(1-2):89–106. doi: 10.3109/10799898309041925. [DOI] [PubMed] [Google Scholar]


