Abstract
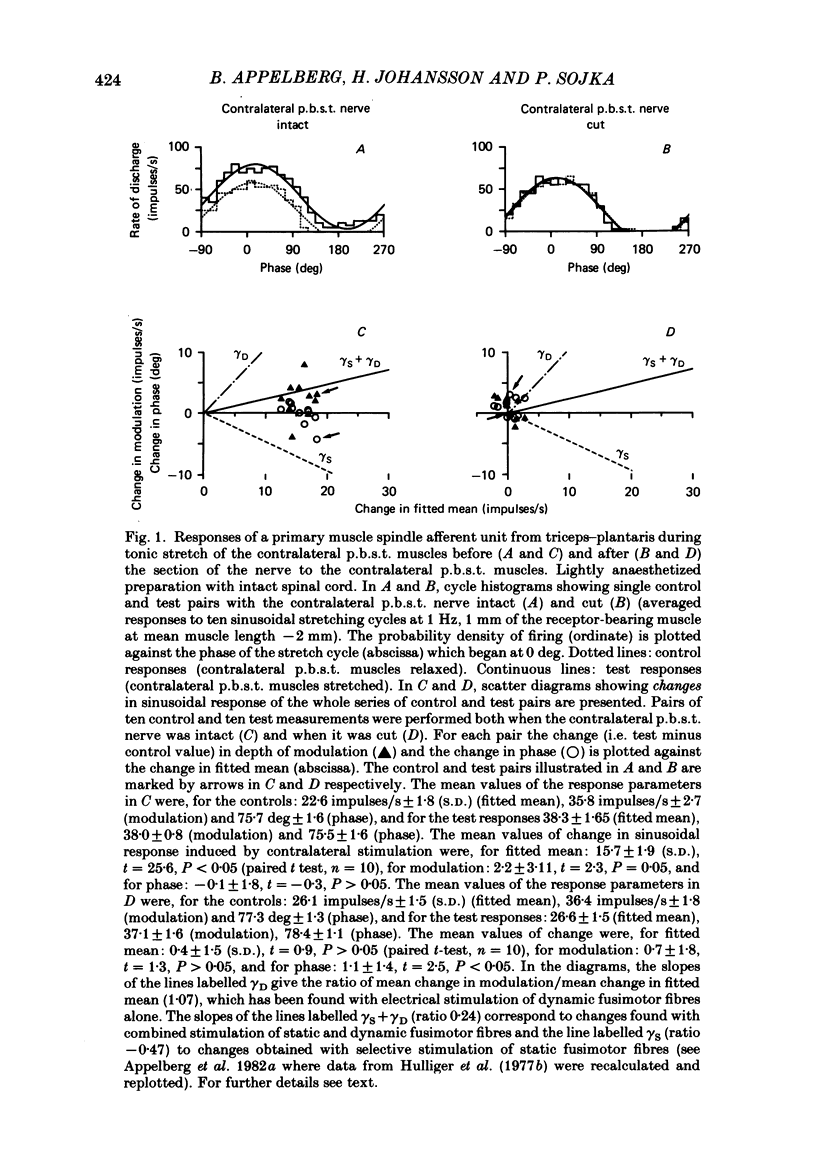
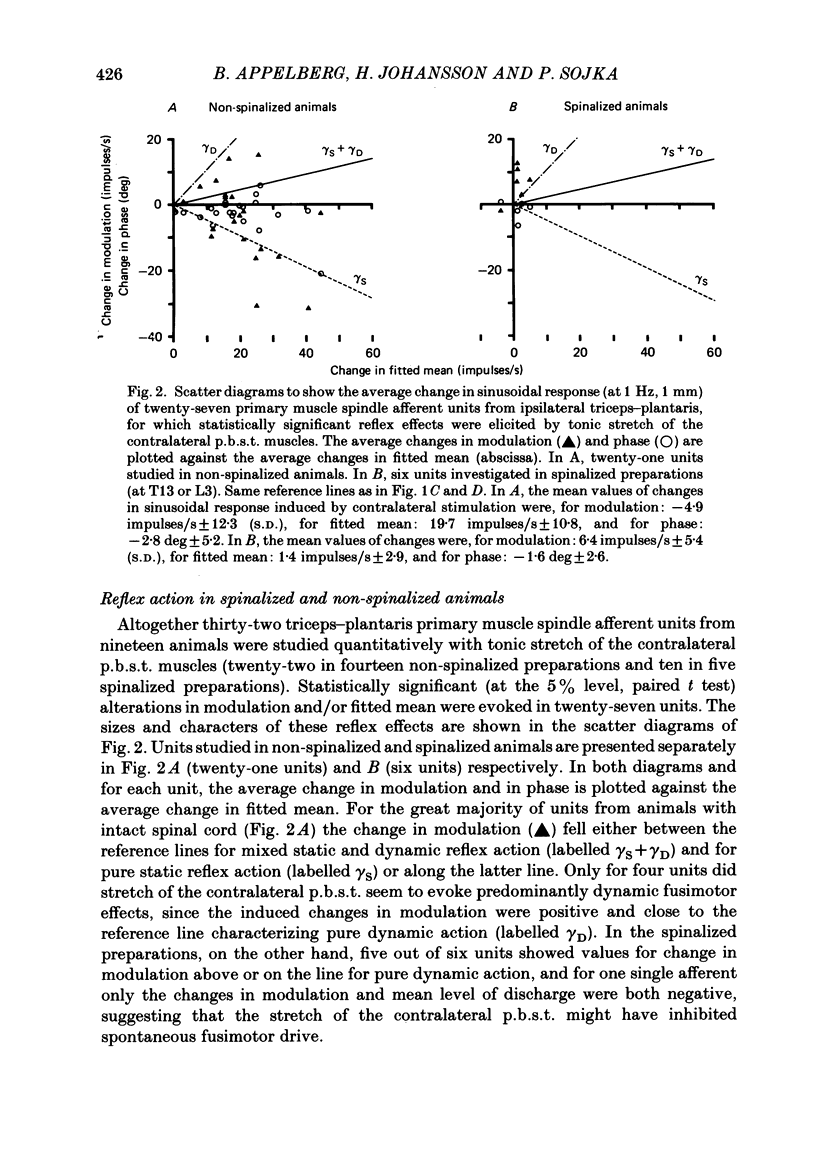
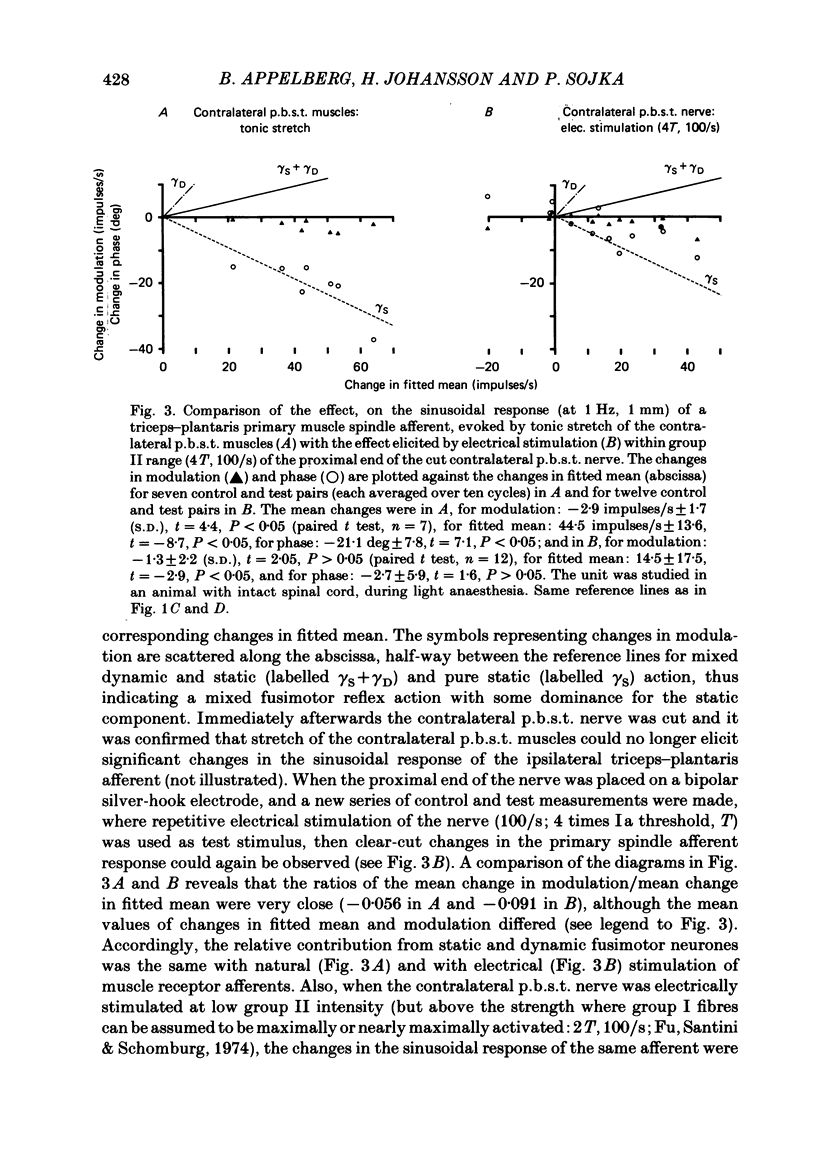
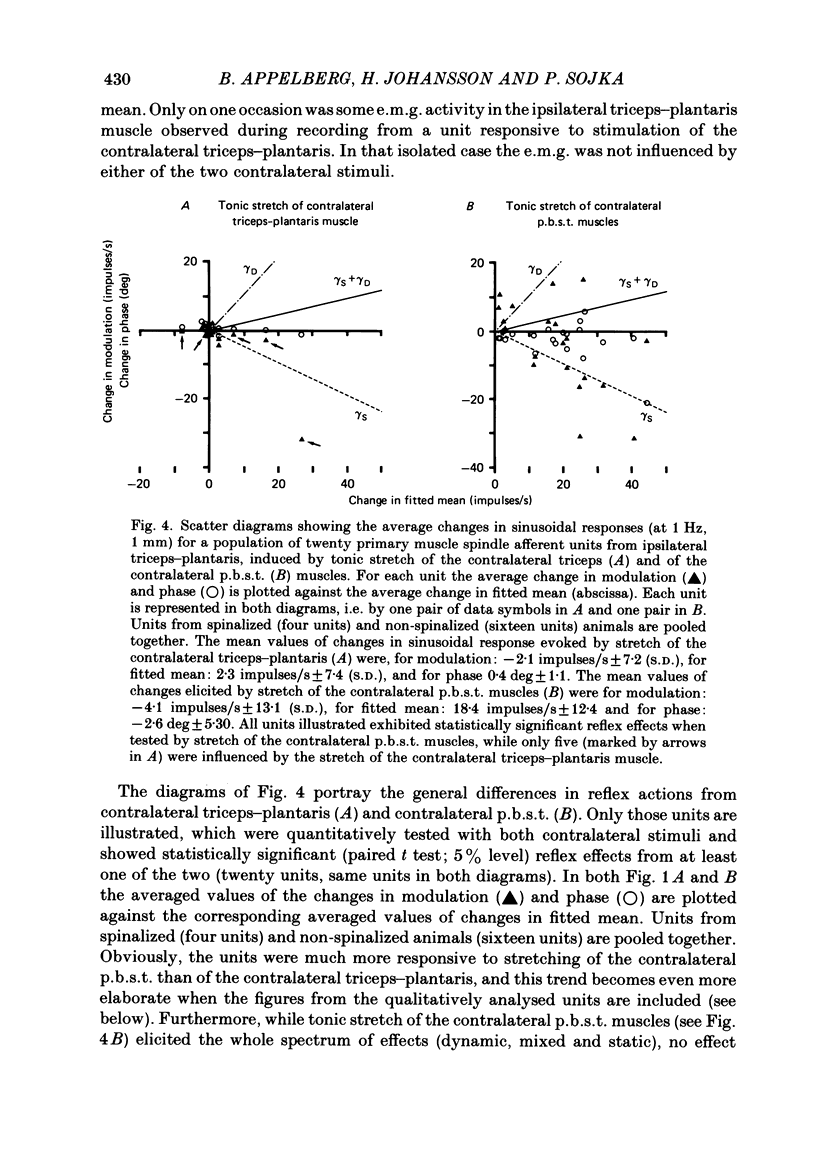
Experiments were performed on twenty-one cats anaesthetized with alpha-chloralose. The aim of this study was to investigate the reflex effects on triceps surae and plantaris fusimotor neurones elicited by tonic stretch of the contralateral posterior biceps and semitendinosus (p.b.s.t.) and the contralateral triceps surae and plantaris muscles, to compare these effects with the effects evoked by flexion or extension of the intact contralateral hind limb (Appelberg, Hulliger, Johansson & Sojka, 1984) and to clarify the interactions between the reflexes from contralateral and ipsilateral muscles. Activity in fusimotor neurones was studied indirectly by recording from primary muscle spindle afferents of the triceps surae and plantaris muscle. The mean rate of firing and the modulation of the afferent response to sinusoidal extension of the triceps surae and plantaris muscles was determined. Control measurements were made with the ipsilateral p.b.s.t., the contralateral p.b.s.t. and the contralateral triceps and plantaris muscles relaxed. Tests were made with tonic stretch of one of these muscles alone or with two of them simultaneously. With stretch of the contralateral p.b.s.t. ten out of eighty-four primary afferents (11.9%) showed predominantly dynamic reflexes (six out of forty-one in spinalized preparations: 14.6%), twenty-two (26.2%) showed mixed or predominantly static effects (one spinalized: 2.4%) and fifty-two units (61.9%) showed no effect (thirty-four spinalized: 83.0%). The reflex effects could be reproduced by electrical stimulation of the cut contralateral p.b.s.t. nerve either at group II or at group III strength. With stretch of the contralateral triceps and plantaris muscles seventy out of seventy-six (92.1%) primary muscle spindle afferents showed no effect and six (7.9%) mixed or predominantly static reflex effects. In general, the reflex effects were not accompanied by detectable electromyographic (e.m.g.) activity in the ipsilateral triceps and plantaris (recorded with surface or needle electrodes), indicating that the reflexes mainly involved gamma-motoneurones. The difference in efficacy between contralateral flexor (p.b.s.t.) and extensor (triceps and plantaris) muscles seems to be in accordance with the response pattern found with extension or flexion of the intact contralateral hind limb (Appelberg et al. 1984).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








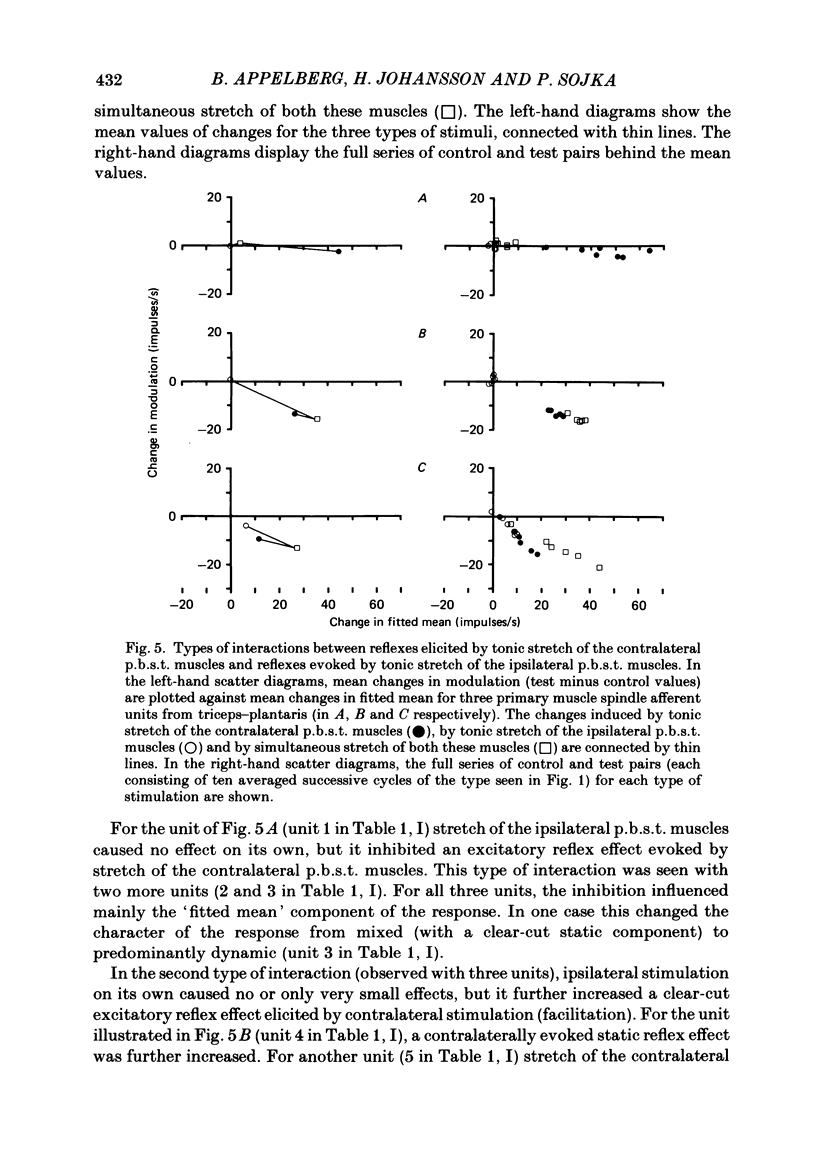
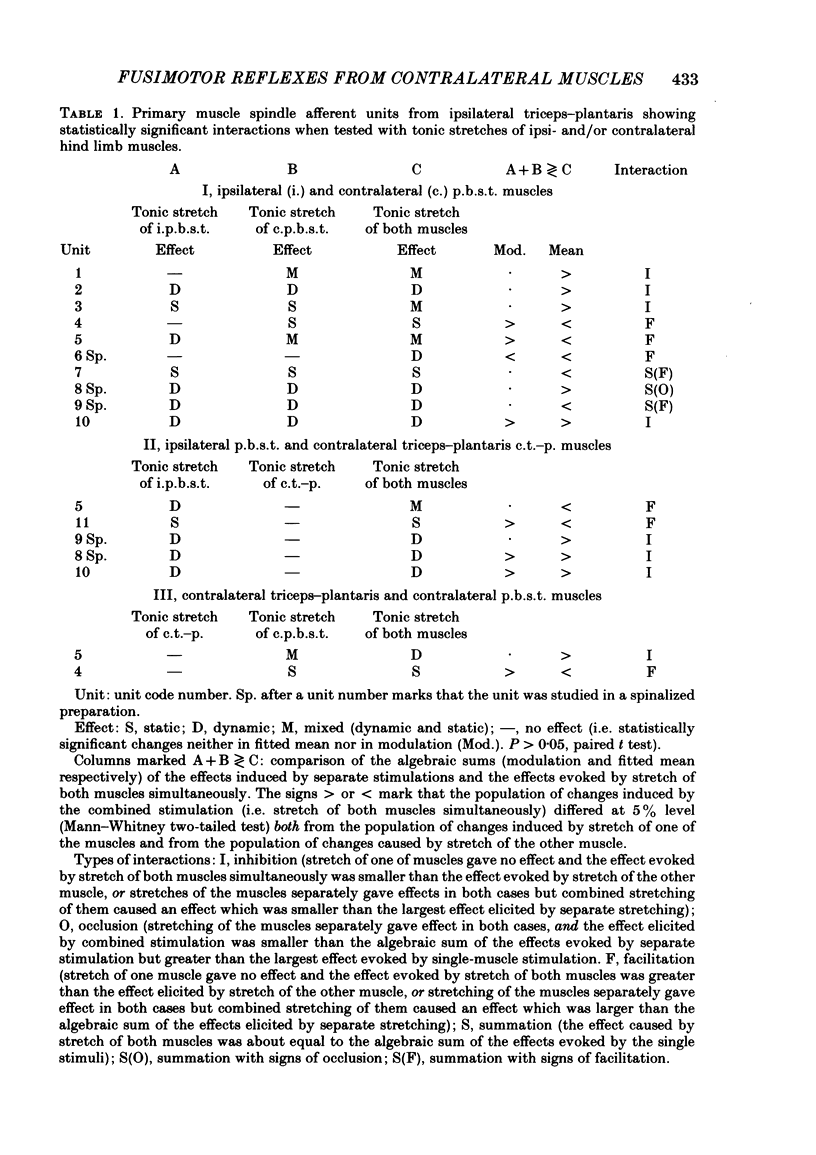

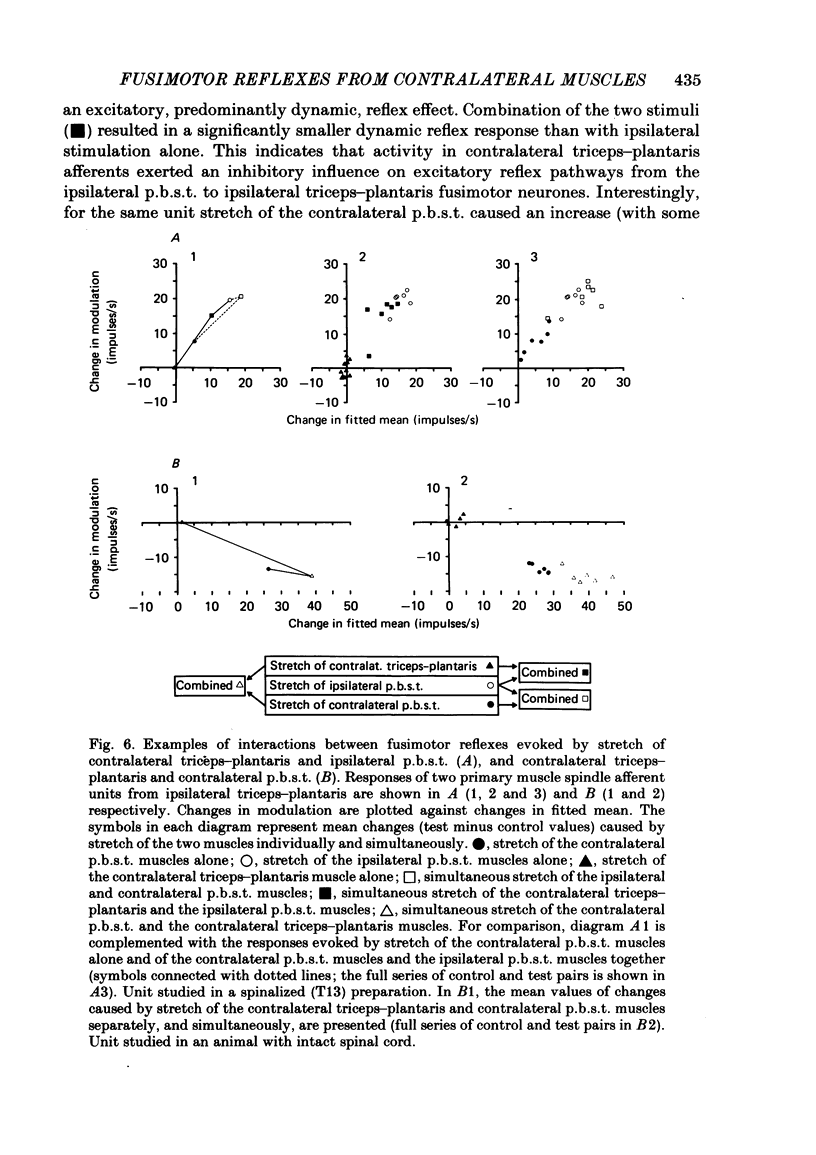






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALNAES E., JANSEN J. K., RUDJORD T. FUSIMOTOR ACTIVITY IN THE SPINAL CAT. Acta Physiol Scand. 1965 Mar;63:197–212. doi: 10.1111/j.1748-1716.1965.tb04060.x. [DOI] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group I muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:237–253. doi: 10.1113/jphysiol.1983.sp014531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group II muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:255–273. doi: 10.1113/jphysiol.1983.sp014532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group III muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:275–292. doi: 10.1113/jphysiol.1983.sp014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. An intracellular study of rubrospinal and rubro-bulbospinal control of lumbar gamma-motoneurones. Acta Physiol Scand. 1982 Dec;116(4):377–386. doi: 10.1111/j.1748-1716.1982.tb07155.x. [DOI] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Fusimotor reflexes in triceps surae elicited by natural stimulation of muscle afferents from the cat ipsilateral hind limb. J Physiol. 1982 Aug;329:211–229. doi: 10.1113/jphysiol.1982.sp014299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Fusimotor reflexes in triceps surae muscle elicited by extension of the contralateral hind limb in the cat. J Physiol. 1984 Oct;355:99–117. doi: 10.1113/jphysiol.1984.sp015409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B. Muscle spindles in a flexion reflex elicited by natural stimulation. Acta Physiol Scand. 1973 Oct;89(2):145–153. doi: 10.1111/j.1748-1716.1973.tb05506.x. [DOI] [PubMed] [Google Scholar]
- Bergmans J., Grillner S. Reciprocal control of spontaneous activity and reflex effects in static and dynamic flexor gamma-motoneurones revealed by an injection of DOPA. Acta Physiol Scand. 1969 Sep-Oct;77(1):106–124. doi: 10.1111/j.1748-1716.1969.tb04557.x. [DOI] [PubMed] [Google Scholar]
- Bessou P., Joffroy M., Pagès B. Efferents and afferents in an intact muscle nerve: background activity and effects of sural nerve stimulation in the cat. J Physiol. 1981 Nov;320:81–102. doi: 10.1113/jphysiol.1981.sp013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Stuart D. G. Responses of Ia and spindle group II afferents to single motor-unit contractions. J Neurophysiol. 1980 Mar;43(3):621–629. doi: 10.1152/jn.1980.43.3.621. [DOI] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. FURTHER STUDIES OF STATIC AND DYNAMIC FUSIMOTOR FIBRES. J Physiol. 1964 Oct;174:132–151. doi: 10.1113/jphysiol.1964.sp007477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles J. C. Crossed extensor reflexes and their interaction. J Physiol. 1929 Feb 28;67(1):97–118. doi: 10.1113/jphysiol.1929.sp002556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Grigg P. Mechanical factors influencing response of joint afferent neurons from cat knee. J Neurophysiol. 1975 Nov;38(6):1473–1484. doi: 10.1152/jn.1975.38.6.1473. [DOI] [PubMed] [Google Scholar]
- Grillner S., Hongo T., Lund S. Descending monosynaptic and reflex control of gamma-motoneurones. Acta Physiol Scand. 1969 Apr;75(4):592–613. doi: 10.1111/j.1748-1716.1969.tb04414.x. [DOI] [PubMed] [Google Scholar]
- Grillner S. The influence of DOPA on the static and the dynamic fusimotor activity to the triceps surae of the spinal cat. Acta Physiol Scand. 1969 Dec;77(4):490–509. doi: 10.1111/j.1748-1716.1969.tb04592.x. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., McINTYRE A. K. An analysis of fibre diameter and receptor characteristics of myelinated cutaneous afferent fibres in cat. J Physiol. 1960 Aug;153:99–112. doi: 10.1113/jphysiol.1960.sp006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. The reflex activity of mammalian small-nerve fibres. J Physiol. 1951 Dec 28;115(4):456–469. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Zytnicki D. Crossed actions of group I muscle afferents in the cat. J Physiol. 1984 Nov;356:263–273. doi: 10.1113/jphysiol.1984.sp015463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M., Matthews P. B., Noth J. Effects of combining static and dynamic fusimotor stimulation on the response of the muscle spindle primary ending to sinusoidal stretching. J Physiol. 1977 Jun;267(3):839–856. doi: 10.1113/jphysiol.1977.sp011840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M., Matthews P. B., Noth J. Static and dynamic fusimotor action on the response of Ia fibres to low frequency sinusoidal stretching of widely ranging amplitude. J Physiol. 1977 Jun;267(3):811–838. doi: 10.1113/jphysiol.1977.sp011839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulliger M. The mammalian muscle spindle and its central control. Rev Physiol Biochem Pharmacol. 1984;101:1–110. doi: 10.1007/BFb0027694. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The central control of the dynamic response of muscle spindle receptors. J Physiol. 1962 May;161:357–378. doi: 10.1113/jphysiol.1962.sp006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSEN J. K., MATTHEWS P. B. The effects of fusimotor activity on the static responsiveness of primary and secondary endings of muscle spindles in the decerebrate cat. Acta Physiol Scand. 1962 Aug;55:376–386. doi: 10.1111/j.1748-1716.1962.tb02451.x. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., RUDJORD T. FUSIMOTOR ACTIVITY IN A FLEXOR MUSCLE OF THE DECEREBRATE CAT. Acta Physiol Scand. 1965 Mar;63:236–246. doi: 10.1111/j.1748-1716.1965.tb04063.x. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., RUDJORD T. ON THE SILENT PERIOD AND GOLGI TENDON ORGANS OF THE SOLEUS MUSCLE OF THE CAT. Acta Physiol Scand. 1964 Dec;62:364–379. doi: 10.1111/j.1748-1716.1964.tb10435.x. [DOI] [PubMed] [Google Scholar]
- Mense S., Stahnke M. Responses in muscle afferent fibres of slow conduction velocity to contractions and ischaemia in the cat. J Physiol. 1983 Sep;342:383–397. doi: 10.1113/jphysiol.1983.sp014857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERL E. R. Crossed reflex effects evoked by activity in myelinated afferent fibers of muscle. J Neurophysiol. 1958 Mar;21(2):101–112. doi: 10.1152/jn.1958.21.2.101. [DOI] [PubMed] [Google Scholar]
- PERL E. R. Effects of muscle stretch on excitability of contralateral motoneurones. J Physiol. 1959 Jan 28;145(1):193–203. doi: 10.1113/jphysiol.1959.sp006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post E. M., Rymer W. Z., Hasan Z. Relation between intrafusal and extrafusal activity in triceps surae muscles of the decerebrate cat: evidence for beta action. J Neurophysiol. 1980 Aug;44(2):383–404. doi: 10.1152/jn.1980.44.2.383. [DOI] [PubMed] [Google Scholar]
- Windhorst U., Meyer-Lohmann J. The influence of extrafusal muscle activity on discharge patterns of primary muscle spindle endings. Pflugers Arch. 1977 Dec 12;372(2):131–138. doi: 10.1007/BF00585326. [DOI] [PubMed] [Google Scholar]
- Windhorst U., Schmidt J., Meyer-Lohmann J. Analysis of the dynamic responses of deefferented primary muscle spindle endings to ramp stretch. Pflugers Arch. 1976 Nov 5;366(2-3):233–240. doi: 10.1007/BF00585883. [DOI] [PubMed] [Google Scholar]


