Abstract
DNA enters the herpes simplex virus capsid by way of a ring-shaped structure called the portal. Each capsid contains a single portal, located at a unique capsid vertex, that is composed of 12 UL6 protein molecules. The position of the portal requires that capsid formation take place in such a way that a portal is incorporated into one of the 12 capsid vertices and excluded from all other locations, including the remaining 11 vertices. Since initiation or nucleation of capsid formation is a unique step in the overall assembly process, involvement of the portal in initiation has the potential to cause its incorporation into a unique vertex. In such a mode of assembly, the portal would need to be involved in initiation but not able to be inserted in subsequent assembly steps. We have used an in vitro capsid assembly system to test whether the portal is involved selectively in initiation. Portal incorporation was compared in capsids assembled from reactions in which (i) portals were present at the beginning of the assembly process and (ii) portals were added after assembly was under way. The results showed that portal-containing capsids were formed only if portals were present at the outset of assembly. A delay caused formation of capsids lacking portals. The findings indicate that if portals are present in reaction mixtures, a portal is incorporated during initiation or another early step in assembly. If no portals are present, assembly is initiated in another, possibly related, way that does not involve a portal.
Assembly of herpes simplex virus type 1 (HSV-1) can be considered to begin with formation of the capsid. Capsids are produced in the infected cell nucleus, where they are also packaged with the double-stranded DNA (dsDNA) genome. Promptly after DNA is introduced, capsids transit to the cytoplasm, where they are enveloped to form mature virions (5, 21, 23).
The HSV-1 capsid is closely similar in structure to the capsids of other herpesviruses. It is icosahedral in shape, and its major structural features are 162 capsomers. The capsomers are of three types: (i) hexons that form the capsid faces and edges, (ii) pentons that are located at 11 of the 12 capsid vertices, and (iii) the portal found at one of the 12 vertices. The 150 hexons are hexamers of the major capsid protein (UL19), while the 11 pentons are UL19 pentamers. The portal is a 12-mer of UL6, the portal protein. The portal is about the size of a hexon or penton and cylindrical, with an axial channel through which HSV-1 DNA passes as it is introduced into the capsid (32). The capsomers are connected in groups of three by the triplexes, trigonal structures that lie on the outer surface of the capsid floor (15, 27, 32, 36).
The mechanism of HSV-1 capsid formation has been examined in infected cells, in insect cells infected with recombinant baculoviruses (rBV) encoding HSV-1 capsid proteins, and in an in vitro assembly system (14, 28, 31, 34). Such studies have demonstrated that assembly of closed icosahedral capsids requires the major capsid protein, the triplex proteins, and a scaffolding protein. The major scaffolding protein, the product of the UL26.5 gene, is required for capsid formation, but thereafter it is lost and is not present in the mature capsid or virion.
Capsid assembly was found to proceed by way of a spherical, fragile intermediate called the procapsid. After it is formed, the procapsid undergoes a large-scale energy-independent conformational change to create the mature icosahedral capsid. Procapsids have been identified during capsid formation in infected cells and as capsids are assembled from purified proteins in vitro (4, 12, 13, 18, 22). In vitro studies have demonstrated that procapsids are assembled by way of angular segments of the closed structure. Called partial procapsids, the angular segments contain the major capsid, scaffolding, and triplex proteins. They are extended in small increments to create the closed procapsid. Extension of the partial procapsid occurs by addition of complexes containing the major capsid and scaffolding proteins. Like the mature capsid, the procapsid was found to contain a single portal, indicating that the portal is incorporated as the capsid is assembled (24).
The location of the portal at a unique site in the mature capsid suggests it is introduced by an unusual step in the assembly process. Each procapsid must incorporate one portal, but no more. Such assembly would be expected if the portal were involved in the initiation of procapsid formation. Since initiation is a singular step in assembly, involvement of the portal could result in its incorporation at a unique site. On the other hand, it is clear that capsid assembly can occur in the absence of the portal. Formation of capsids lacking portals is observed in cells infected with an HSV-1 mutant lacking the UL6 gene (35) and in similar UL6-negative assembly reactions carried out in vitro or in rBV-infected insect cells (14, 28, 31). Such observations demonstrate that the portal is not required for initiation of procapsid assembly and raise the possibility that it may be incorporated at a later step.
Here, we describe the results of experiments performed to test the idea that the portal is involved in initiation of capsid formation. Using an in vitro capsid assembly system, we compared the levels of portal incorporation in reactions where the portal was added at the outset of the assembly process and after a delay. It was reasoned that if the portal is involved specifically in initiation, then delay in its addition to reaction mixtures would permit formation of some capsids lacking portals and therefore decrease the overall level of portal incorporation.
MATERIALS AND METHODS
Protein purification.
Proteins used for in vitro capsid assembly were isolated from insect cells infected with appropriate rBV. The major capsid protein (UL19; VP5), scaffolding protein (UL26.5), portal protein (UL6), and an enzymatically inactive form of the protease (UL26/61 [30]) were prepared from singly infected cells, while cells coinfected with rBV encoding UL18 and UL38 were employed for triplex purification. Earlier publications have described the procedures used for creation of the rBV, growth and infection of Spodoptera frugiperda (Sf9) cells, and harvesting of the infected cells (13, 16, 31).
VP5 was purified to homogeneity using a three-step procedure described previously (13). Preparations used for capsid assembly had concentrations in the range of 1.0 to 1.5 mg/ml in phosphate-buffered saline (PBS)-10 mM EDTA plus 0.1 volume of a protease inhibitor cocktail (prepared by dissolving 1 tablet of Roche Complete per 5 ml PBS). The scaffolding protein and protease were partially purified by ammonium sulfate precipitation of cell lysates as described previously (16). Both were dissolved in PBS-2 mM EDTA-5 mM dithiothreitol containing protease inhibitors and used for capsid assembly on the same day they were prepared. The scaffolding protein and protease solutions had concentrations of 1.5 to 2.0 mg/ml and ∼1 mg/ml, respectively. The scaffolding protein was present in solution in the form of 55-nm-diameter scaffold particles (11, 16). UL6 was purified to homogeneity by the previously described method, which yields intact portals in 20 mM Tris-HCl, pH 7.5, containing 1 M arginine and ∼20% sucrose (15). UL6 protein concentrations were in the range of 0.5 to 1.0 mg/ml. Triplexes were purified to homogeneity as described previously (16). Triplex solutions in PBS-10 mM EDTA plus protease inhibitors had concentrations in the range of 0.5 to 1.0 mg/ml.
In vitro capsid assembly.
The compositions of reaction mixtures yielding the highest level of capsid formation were found to vary with individual preparations of the reaction components. For each set of experiments, therefore, preliminary tests were carried out to determine the optimal proportions. Representative reaction mixtures contained 30 μl VP5, 10 μl UL26.5, 30 μl triplexes, 2 μl UL26.5/61, 2 μl UL6, 60 μl PBS, 16 μl 0.5 M EDTA, 12 μl 20 mM dithiothreitol, and 6 μl protease inhibitor cocktail (see above). Optimal capsid formation occurred when UL26.5/61 was present and VP5 was the limiting component of the reaction mixtures. Assembly was allowed to proceed for 20 h at 34°C with a 10-μl aliquot withdrawn as necessary for electron microscopy. The reaction mixtures were then added to 400 μl TNE (20 mM Tris-HCl, pH 7.5, 0.5 M NaCl, 1 mM EDTA), and the capsids were purified by centrifugation at 4°C in 600-μl-capacity tubes (6 by 42 mm) using a Beckman SW50.1 rotor. Insoluble material was removed by centrifugation at 8,000 rpm for 30 min, after which the supernatant was transferred to a fresh tube and underlaid with 50 μl 35% sucrose in TNE, and the capsids were pelleted by centrifugation for 1 h at 23,000 rpm. The capsids were then suspended in 50 μl TNE, layered on a gradient of 20% to 50% sucrose in TNE, and centrifuged for 1 h at 23,000 rpm. The gradient was fractionated (13 fractions; ∼45 μl each), and the capsid-containing fractions were identified by applying small aliquots (2 μl) to an Immobilon-P polyvinylidine difluoride (PVDF; Millipore, Billerica, MA) sheet and staining them with Ponceau S (16). Capsid fractions identified in this way corresponded to a light scattering band visible in the gradient. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and agarose gel analysis followed by Western immunoblotting were performed with the three peak capsid-containing fractions.
The amount of UL6 present in capsids assembled in vitro was determined beginning with fractions from the sucrose gradients used for capsid isolation, as described above. Small aliquots (2 μl) were applied to a PVDF sheet and stained with Ponceau S to measure the total amount of protein present. Ponceau staining was followed by immunostaining with UL6-specific antibody (described below) to measure the amount of UL6. At both stages, the spot stain was determined quantitatively by scanning on a flatbed scanner, followed by densitometry with Un-Scan-It (Silk Scientific, Orem, UT). The UL6 signal was scaled by the use of HSV-1 B capsids, which were assumed to contain one portal per capsid. The same PVDF sheet was used for analysis of B capsids and all experimental capsid preparations. Control experiments showed that capsids formed normally in the presence of the drug WAY-150138, but portal incorporation was inhibited as described previously (11, 16).
Localization of UL6 by immunoelectron microscopy.
UL6 was labeled specifically with primary UL6-specific antibody, followed by gold-labeled secondary antibody. The labeling procedure for capsids formed in vitro was the same as that used previously for capsids isolated from infected cells (15). Primary antibody staining was with anti-MBP-UL6 (29), which was absorbed with (i) capsids assembled in vitro in the absence of UL6 and (ii) capsids prepared from cells infected with the UL6 deletion strain hr74 (6).
Other methods.
Previously described methods were employed for SDS-PAGE, agarose gel electrophoresis, electrophoretic blotting of proteins onto PVDF sheets, staining of blots with Ponceau S, and quantitative determination of protein bands in stained gels and radioautographs (15, 16, 18). Staining of blots with the UL6-specific monoclonal antibody 1C9 was performed as described by Newcomb et al. (16), with 1C9 used at a dilution of 1:10,000. HSV-1 B capsids were prepared as previously described from Vero cells infected with the KOS strain of HSV-1 (17). Electron microscopy was performed with 10-μl aliquots of assembly reaction mixtures. They were adsorbed to carbon-Formvar-coated copper electron microscope grids, stained for 1 min with 1% uranyl acetate, air dried, and photographed in a Philips 400T electron microscope operated at 80 keV. To count capsids and capsid-related structures, the electron microscope negatives were digitized and viewed with Photoshop 5.0.
RESULTS
In vitro assembly system.
In vitro capsid assembly was carried out in reaction mixtures containing the major capsid, scaffolding, triplex, and portal proteins as described in Materials and Methods. The proportion of reaction components was adjusted by trial and error to optimize the number of capsids formed. If portals were present, they were the last component added to the reaction mixtures.
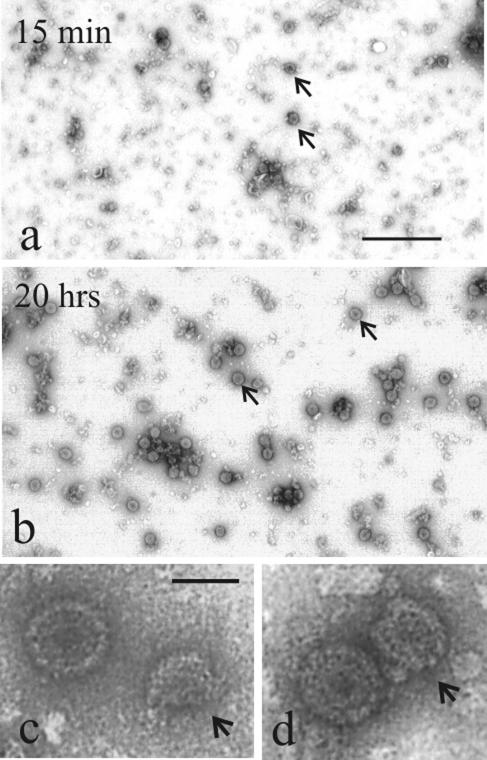
The progress of capsid formation was monitored by electron microscopy. Small samples were removed from reaction mixtures and examined after negative staining. Micrographs demonstrated the presence of assembly intermediates (i.e., partial procapsids) in reaction mixtures after 10 to 15 min of incubation (Fig. 1a, c, and d). Prior to this time, structures observed in reaction mixtures were too small to be identifiable as capsid precursors. Closed capsids were first observed after 30 to 60 min of incubation, and they increased in number during 20 h or more of incubation (Fig. 1b). By 20 h, closed capsids predominated over assembly intermediates in reaction mixtures.
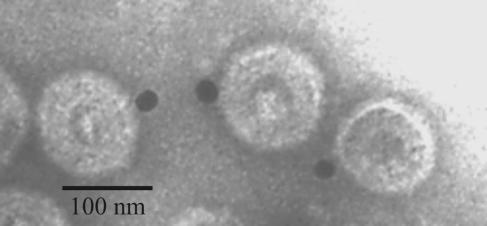
FIG. 1.
Electron micrographs showing the products of in vitro capsid assembly reactions. Samples of reaction mixtures were examined after 15 min (a, c, and d) and 20 h (b) of incubation. Note that partial procapsids predominate in 15-min reactions (arrows in panel a), while completed capsids predominate after 20 h (arrows in panel b). Partial procapsids (arrows) are shown at higher magnification in panels c and d, with more mature capsids close by. Bars = 500 nm (a and b) and 100 nm (c and d).
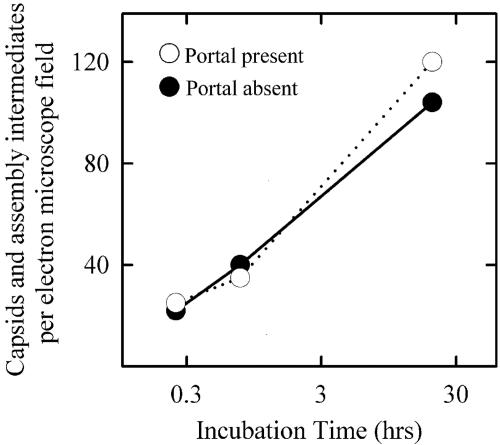
Control experiments were carried out to test the effect of the portal on capsid assembly. Analysis of micrographs such as those shown in Fig. 1 demonstrated little effect of the portal on either the rate or extent of capsid formation (Fig. 2). The morphologies of assembly intermediates were the same in the two reactions (data not shown). This result is consistent with previous in vivo and in vitro studies, indicating that capsid formation does not require the presence of the portal (14, 19, 28, 31).
FIG. 2.
Time course for appearance of HSV-1 capsids and capsid assembly intermediates during in vitro capsid formation. The total number of completed capsids and capsid assembly intermediates were counted in electron micrographs, such as those shown in Fig. 1. Counts for each time point were made in one ×10,000 electron microscope negative. Note that the rate of appearance of assembly products was not affected by the presence of the portal in reaction mixtures.
UL6 incorporation.
UL6 incorporation into capsids was tested in standard reactions as described above and in those in which portal addition was delayed for various times. In all cases, the reaction mixtures were incubated for a total of 20 h. After incubation, the capsids were isolated by sucrose gradient centrifugation and analyzed by SDS-PAGE, followed by Western immunostaining for UL6. In parallel studies, capsids isolated by sucrose gradient centrifugation were also analyzed by agarose gel electrophoresis, followed by UL6 immunostaining.
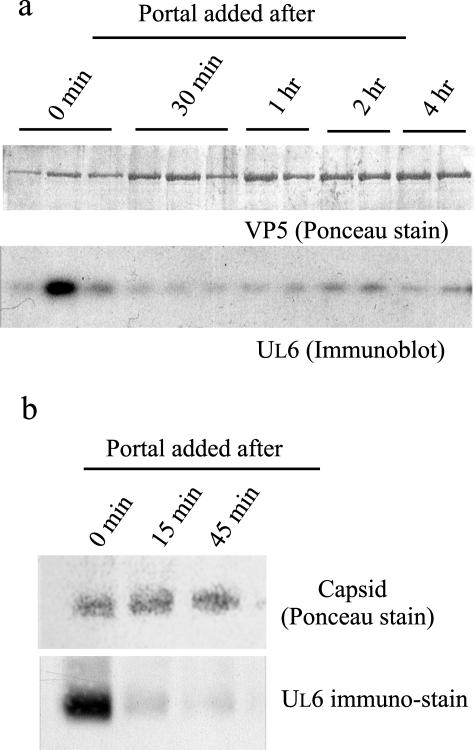
SDS-PAGE was carried out with the two or three peak capsid-containing fractions isolated from sucrose gradients. After electrophoresis, proteins on the gel were transferred to a PVDF sheet and stained with Ponceau S. VP5 staining, as shown in Fig. 3a (top), provides a measure of the total number of capsids analyzed. UL6 immunostaining demonstrated a high level of UL6 incorporation in complete reactions but little in reactions in which portal addition was delayed for 30 min, 1 h, 2 h, or 4 h (Fig. 3a, bottom). The decreases were 7.9-, 12.1-, 6.2-, and 10.6-fold, respectively, for the four time points.
FIG. 3.
Determination of the UL6 contents in capsids assembled in vitro. Analyses were carried out with capsids formed in incubations in which the time of portal addition varied, as described in the text. After incubation, the capsids were purified by sucrose gradient ultracentrifugation and analyzed in two ways: by SDS-PAGE, followed by Western immunoblotting (a), and by agarose gel electrophoresis, followed by SDS-PAGE and immunoblotting (b). Note that the amount of UL6 incorporated into capsids was greater when UL6 was present at the outset of incubation than when its addition was delayed.
Figure 3b shows the results obtained when capsid-containing sucrose gradient fractions were analyzed by agarose gel electrophoresis followed by blotting onto PVDF. Examination of the blot after Ponceau staining demonstrated that capsids migrated as a single band during electrophoresis (Fig. 3b, top). UL6 staining showed a substantial level of UL6 in capsids isolated from control incubations but a decreased level when the addition of UL6 was delayed (Fig. 3b, bottom). The reductions were 10.0-fold and 16.4-fold in incubations where UL6 addition was delayed for 15 min and 45 min, respectively.
Amount of UL6 incorporated.
If the portal is involved at an early step of procapsid assembly, as suggested by the results described above, then it is further expected that (i) no more than one portal will be able to be incorporated into the capsid and (ii) the portal will be located at a vertex in capsids formed in vitro as it is in capsids isolated from infected cells. Tests of both expectations were performed using capsids assembled in the in vitro system.
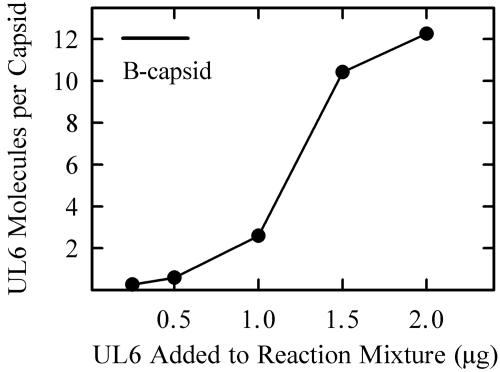
The amount of UL6 present was measured beginning with reaction mixtures in which the number of input portals was varied. After incubation to allow them to form, capsids were isolated by sucrose gradient ultracentrifugation, and the amount of UL6 was determined by dot blot analysis. The blots were stained with Ponceau S, followed by immunostaining for UL6 and densitometry as described in Materials and Methods.
The results (Fig. 4) indicated that the amount of UL6 incorporated into capsids increased with increasing input UL6 over the concentration range tested (i.e., 0.25 to 2.0 μg/170-μl reaction mixture). A sigmoid relationship was observed with little UL6 incorporation observed below ∼1.0 μg and evidence of saturation above ∼1.5 μg. The maximum UL6 incorporation corresponded to 10 to 12 UL6 molecules per capsid or approximately 25% of the UL6 added to standard incubations. Efforts to add greater amounts of UL6 to reaction mixtures were affected by an inhibition of capsid formation by the 1 M arginine required to maintain UL6 in solution (15).
FIG. 4.
Effect of input UL6 on the amount of UL6 incorporated into HSV-1 capsids assembled in vitro. Capsids were formed in vitro and analyzed for the presence of UL6 as described in Materials and Methods. The bar indicates the amount of UL6 present in HSV-1 B capsids isolated from infected cells.
Portal location.
The location of the portal in the capsid was examined by immunoelectron microscopy in a procedure based on the use of a polyclonal antibody specific for UL6 (29). Capsids were prepared for immunoelectron microscopy by in vitro assembly in reaction mixtures containing portals. They were then purified by sucrose gradient centrifugation and adsorbed onto carbon-Formvar-coated electron microscope grids. All staining operations were performed with adsorbed capsids. They were stained with the primary antibody, followed by a secondary, gold-labeled antibody as described in Materials and Methods.
Electron microscopic examination showed that in capsids containing gold label, the label was confined to a single site that appeared to correspond to a vertex (Fig. 5). Capsids labeled at more than one site were not observed. The proportion of capsids containing label (32% [Table 1]) was in satisfactory agreement with the labeled fraction observed in capsids isolated from infected cells, as reported earlier (32% and 41%, respectively, in two experiments) (15). In contrast, labeling was 6% in control experiments performed with capsids lacking UL6 (Table 1).
FIG. 5.
Electron micrograph showing HSV-1 capsids that were assembled in vitro and stained to identify the location of UL6 protein. The capsids were stained with antibody specific for UL6, followed by a gold-labeled secondary antibody. The gold beads (black dots; ∼15 nm in diameter) identify the locations of UL6.
TABLE 1.
In vitro-assembled capsids: immunogold staining for UL6
| Capsid typea | No. of capsids with gold label | Total capsids counted | Percent |
|---|---|---|---|
| UL6 present | 27 | 85 | 32 |
| UL6 absent | 5 | 87 | 6 |
Capsids were assembled in vitro as described in Materials and Methods in the presence or absence of added portals.
DISCUSSION
Role of the scaffolding protein in portal incorporation.
The scaffolding protein has been the focus of previous efforts to understand the way in which the portal becomes assembled into the HSV-1 capsid. Intact portals were found to bind UL26.5 when the two purified proteins were mixed in vitro (16). Also, studies with deletion mutants identified a region of the scaffolding protein required for interaction with the portal (26). Experiments with one such deletion, UL26.5 Δ143-151, were particularly revealing. Δ143-151 protein failed to bind to the portal, although it supported the formation of portalless capsids (which were otherwise morphologically normal) in an in vitro assembly system. The results indicate that the scaffolding protein plays an important role in promoting incorporation of the portal into capsids as they are formed. In particular, the portal is suggested to be donated to the nascent capsid by way of a complex with the scaffolding protein in a process resembling the way the major capsid protein is donated (Fig. 6).
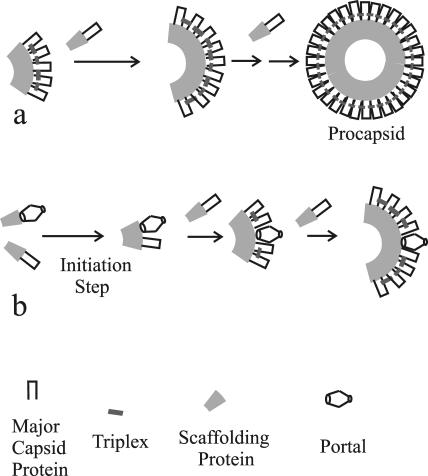
FIG. 6.
Schematic diagram illustrating HSV-1 procapsid formation in the absence (a) and presence (b) of the portal. The diagram illustrates the observation that procapsids are assembled normally in the absence of the portal (a). If the portal is present, however, it is suggested to be involved in the initiation of procapsid assembly and to be donated by way of a complex with the scaffolding protein, as shown in panel b.
A role for the scaffolding protein in portal incorporation is also suggested by results with WAY-150138, a small molecule inhibitor of HSV-1 replication (11, 16). WAY-150138 was found to antagonize the in vitro interaction between UL26.5 and the portal. When the drug was present in infected cells, or in in vitro assembly reaction mixtures, capsids formed normally but portal incorporation was suppressed (11). The results support the view that the portal cannot be incorporated into capsids if it is prevented from interacting with the scaffolding protein.
Delay in portal addition.
The present study extends the previous results by demonstrating that a delay in addition of the portal to in vitro assembly reaction mixtures causes a decrease in incorporation of portals into product capsids. The decrease in portal incorporation was observed despite the fact that few assembly intermediates or completed capsids were present in reaction mixtures at the end of the time window (15 to 30 min of incubation) found to be permissive for portal incorporation. We interpret the findings to indicate that initiation, and perhaps other early steps in procapsid formation, takes place during the first 15 to 30 min of incubation in vitro. If the portal is present during this time, then it becomes incorporated into such early precursors and is found in product capsids. If not, then assembly takes place in the absence of the portal as described previously (14). This would be the case, for instance, if nucleation of capsid formation involved a complex of the major capsid, scaffolding, and triplex proteins to which the portal could bind if it were present. If not, then assembly would proceed without the portal. Alternatively, initiation of assembly could normally occur at a portal with VP5 or a combination of VP5 with other protein(s) substituting for the portal if it is absent. In either case, subsequent assembly events would alter the initiation complex and thereby prevent the portal from becoming incorporated at a later time.
It is interesting that the degree to which UL6 incorporation was suppressed by delay in its addition was not dependent on the length of the delay over the time range examined (i.e., 15 min to 4 h) (Fig. 3). Suppression was ∼6-fold to ∼16-fold in all cases with no systematic dependence on the extent of delay. The absence of dependence on the time of delay suggests that the opportunity for portal incorporation is lost after 15 min of incubation in vitro and is not regained.
If it is true that early assembly intermediates are present in 15- to 30-min reaction mixtures as postulated above, then it must be that they are not identifiable as assembly precursors in electron micrographs, such as Fig. 1 (top). Examination of 15- to 30-min reaction mixtures most often revealed a few partial procapsids and many other smaller structures that did not appear uniform in morphology and did not resemble capsids or partial procapsids (Fig. 1, top). The postulated early assembly intermediates may be among this uncharacterized group of structures.
The amount of UL6 assembled into product capsids was found to depend on the amount added to reaction mixtures. Little UL6 was incorporated in reactions containing less than ∼0.5 μg UL6, whereas above ∼1.5 μg, the amount incorporated corresponded to approximately one portal (i.e., 12 UL6 monomers) per capsid (Fig. 5). Higher levels of UL6 incorporation were not observed despite the fact that UL6 was present in excess in reaction mixtures. UL6 recovered in product capsids, for example, amounted to 25% or less of the input UL6 in standard reaction mixtures (see Materials and Methods). These results support the view that the assembly pathway limits portal incorporation to one per capsid.
In assembly reactions in which total UL6 incorporation corresponded to less than one portal per capsid (Fig. 4), we assume that product capsids were a mixture of capsids containing one portal and those containing none. The alternative possibility, that capsids contain portal fragments, seems less likely because the oligomeric state of UL6 as isolated from insect cells has been observed only in the range of 12. For example, values between ∼11 and 14 were reported in studies using two different experimental methods (15, 32). UL6 11- to 14-mers would need to disassemble for partial portals to be incorporated into capsids.
Examination of the product capsids by immunoelectron microscopy demonstrated that UL6 was present at a single vertex, as it is in capsids isolated from infected cells (Fig. 5 and Table 1). The presence of UL6 at a capsid vertex is consistent with the view that portal incorporation in vitro is a faithful recapitulation of capsid formation as it occurs in vivo.
Portal assembly in dsDNA bacteriophages.
The mechanism of portal assembly into a polyhedral capsid has been studied for many years in dsDNA bacteriophages, such as T4, φ29, P22, and SPP1 (2, 7, 8, 9, 25, 33). As in the case of HSV-1, bacteriophage portals are (i) cone-shaped structures formed from 12 or 13 copies of a single polypeptide, the portal protein; (ii) located at a unique vertex of the capsid polyhedron, where they serve as a channel for DNA to enter and exit; and (iii) present in the procapsid and not able to be added after the procapsid is formed (2, 7, 20).
Murialdo and Becker were the first to propose that the portal is involved in initiation of bacteriophage procapsid assembly (10). The proposal was made to account for the location of the portal at a unique location in the capsid structure. Studies with phage T4 supported a role for the portal in initiation of head formation. The T4 portal protein, gp20, was found associated with the scaffolding protein at membrane sites involved in nucleation of procapsid assembly (33). Results with capsid assembly in the Bacillus subtilis phages SPP1 and φ29 have also supported a role for the portal in initiation of procapsid formation. In these phages, uniform populations of morphologically normal procapsids are formed only in the presence of the portal. In its absence, populations include smaller and malformed structures (2, 3). The results suggest that the portal is involved at an early stage of procapsid assembly, when the radius of curvature and triangulation number become fixed.
In contrast, studies with P22 have emphasized the possibility that the portal may not be involved in initiation of procapsid assembly. Morphologically normal (T = 7; T, triangulation number) procapsids are formed both in vivo and in vitro in the absence of the portal protein, indicating that the portal is not obligatorily involved in the initiation process (8, 9). The portal was found to have little effect on the rate of procapsid formation, and results with mutants in which portal incorporation was affected suggested that the portal could be incorporated at any stage of assembly (1, 8). It is possible, therefore, that viruses differ in the manner in which the portal becomes incorporated into the nascent procapsid. In some, such as T4, SPP1, and HSV-1, the portal participates in nucleation or other early steps, while in others, such as P22, initiation can be accomplished without involvement of the portal.
In future studies of HSV-1 capsid formation, it will be of interest to determine how portal incorporation is limited to one per capsid. For instance, if the portal is involved in initiation of procapsid formation or is donated at an early assembly step, as suggested here (Fig. 6), then how is it that another portal cannot be donated at a later assembly step? To account for how portal incorporation is limited, we suggest a model in which (i) the scaffolding protein forms a linear, head-to-tail aggregate containing many UL26.5 molecules and (ii) the portal binds selectively to only one end of the aggregate and not to internal UL26.5 molecules. Procapsid assembly would involve a nucleation step in which a portal binds to one end of the scaffold string, followed by extension steps involving major capsid protein molecules bound at internal positions, as illustrated schematically in Fig. 7. Procapsid assembly in the absence of a portal would involve binding of a major capsid protein molecule rather than a portal at the end of the scaffold polymer. It is consistent with the model proposed, which we call the string model, that UL26.5 is known to form large aggregates called scaffold particles in insect cells and in cells infected with HSV-1 (11, 16). It would be a critical test of the model to determine whether UL26.5 forms linear aggregates in scaffold particles or other aggregation states and how capsids form under conditions where the scaffold does not appear to form large aggregates (13).
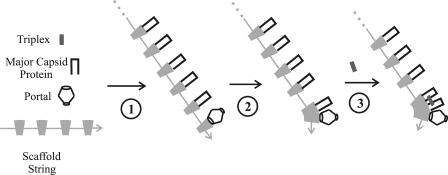
FIG. 7.
Schematic diagram illustrating the proposed mechanism by which portal incorporation is limited to the initiation step of capsid assembly. The model postulates that (i) the scaffolding protein, UL26.5, is found in the form of a head-to-tail polymer and (ii) the portal attaches only at one end of the polymer, as illustrated in step 1, with major capsid protein molecules bound to all other scaffold molecules. Procapsid formation is suggested to occur by successive steps in which one major capsid protein molecule is delivered to a growing edge of the nascent procapsid (steps 2 and 3). Note that the proposed mechanism restricts portal incorporation to the initiation step.
Acknowledgments
We thank Greg Singer and Anna Maria Copeland for helpful discussions during the course of this investigation and Joel Baines for the gift of anti-MBP-UL6 antibody.
The work was supported by NIH award AI41466.
REFERENCES
- 1.Bazinet, C., and J. King. 1988. Initiation of P22 procapsid assembly in vivo. J. Mol. Biol. 202:77-86. [DOI] [PubMed] [Google Scholar]
- 2.Droge, A., M. A. Santos, A. C. Stiege, J. C. Alonso, R. Lurz, T. A. Trautner, and P. Tavares. 2000. Shape and DNA packaging activity of bacteriophage SPP1 procapsid: protein components and interactions during assembly. J. Mol. Biol. 296:117-132. [DOI] [PubMed] [Google Scholar]
- 3.Guo, P., S. Erickson, W. Xu, N. Olson, T. S. Baker, and D. Anderson. 1991. Regulation of the phage phi 29 prohead shape and size by the portal vertex. Virology 183:366-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heymann, J. B., N. Cheng, W. W. Newcomb, B. L. Trus, J. C. Brown, and A. C. Steven. 2003. Dynamics of herpes simplex virus capsid maturation visualized by time-lapse cryo-electron microscopy. Nat. Struct. Biol. 10:334-341. [DOI] [PubMed] [Google Scholar]
- 5.Homa, F. L., and J. C. Brown. 1997. Capsid assembly and DNA packaging in herpes simplex virus. Rev. Med. Virol. 7:107-122. [DOI] [PubMed] [Google Scholar]
- 6.Lamberti, C., and S. K. Weller. 1996. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology 226:403-407. [DOI] [PubMed] [Google Scholar]
- 7.Lee, C. S., and P. Guo. 1995. Sequential interactions of structural proteins in phage phi 29 procapsid assembly. J. Virol. 69:5024-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore, S. D. and P. E. Prevelige, Jr. 2002. A P22 scaffold protein mutation increases the robustness of head assembly in the presence of excess portal protein. J. Virol. 76:10245-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore, S. D. and P. E. Prevelige, Jr. 2002. Bacteriophage p22 portal vertex formation in vivo. J. Mol. Biol. 315:975-994. [DOI] [PubMed] [Google Scholar]
- 10.Murialdo, H., and A. Becker. 1978. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol. Rev. 42:529-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newcomb, W. W., and J. C. Brown. 2002. Inhibition of herpes simplex virus replication by WAY-150138: assembly of capsids depleted of the portal and terminase proteins involved in DNA encapsidation. J. Virol. 76:10084-10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newcomb, W. W., F. L. Homa, D. R. Thomsen, F. P. Booy, B. L. Trus, A. C. Steven, J. V. Spencer, and J. C. Brown. 1996. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid assembly. J. Mol. Biol. 263:432-446. [DOI] [PubMed] [Google Scholar]
- 13.Newcomb, W. W., F. L. Homa, D. R. Thomsen, B. L. Trus, N. Cheng, A. C. Steven, F. P. Booy, and J. C. Brown. 1999. Assembly of the herpes simplex virus procapsid from purified components and identification of small complexes containing the major capsid and scaffolding proteins. J. Virol. 73:4239-4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newcomb, W. W., F. L. Homa, D. R. Thomsen, Z. Ye, and J. C. Brown. 1994. Cell-free assembly of the herpes simplex virus capsid. J. Virol. 68:6059-6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newcomb, W. W., R. M. Juhas, D. R. Thomsen, F. L. Homa, A. D. Burch, S. K. Weller, and J. C. Brown. 2001. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 75:10923-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newcomb, W. W., D. R. Thomsen, F. L. Homa, and J. C. Brown. 2003. Assembly of the herpes simplex virus capsid: identification of soluble scaffold-portal complexes and their role in formation of portal-containing capsids. J. Virol. 77:9862-9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newcomb, W. W., B. L. Trus, F. P. Booy, A. C. Steven, J. S. Wall, and J. C. Brown. 1993. Structure of the herpes simplex virus capsid: molecular composition of the pentons and the triplexes. J. Mol. Biol. 232:499-511. [DOI] [PubMed] [Google Scholar]
- 18.Newcomb, W. W., B. L. Trus, N. Cheng, A. C. Steven, A. K. Sheaffer, D. J. Tenney, S. K. Weller, and J. C. Brown. 2000. Isolation of herpes simplex virus procapsids from cells infected with a protease-deficient mutant virus. J. Virol. 74:1663-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel, A. H., F. J. Rixon, C. Cunningham, and A. J. Davison. 1996. Isolation and characterization of a herpes simplex virus type-1 mutant defective in the UL6 gene. Virology 217:111-123. [DOI] [PubMed] [Google Scholar]
- 20.Poteete, A. R., V. Jarvik, and D. Botstein. 1979. Encapsulation of phage P22 DNA in vitro. Virology 95:550-564. [DOI] [PubMed] [Google Scholar]
- 21.Rixon, F. J. 1993. Structure and assembly of herpesviruses. Semin. Virol. 4:135-144. [Google Scholar]
- 22.Rixon, F. J., and D. McNab. 1999. Packaging-competent capsids of a herpes simplex virus temperature-sensitive mutant have properties similar to those of in vitro-assembled procapsids. J. Virol. 73:5714-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 24.Sheaffer, A. K., W. W. Newcomb, M. Gao, D. Yu, S. K. Weller, J. C. Brown, and D. J. Tenney. 2001. Herpes simplex virus DNA cleavage and packaging proteins associate with the procapsid prior to its maturation. J. Virol. 75:687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Showe, M. K., and L. Onorato. 1978. Kinetic factors and form determination of the head of bacteriophage T4. Proc. Natl. Acad. Sci. USA 75:4165-4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singer, G. P., W. W. Newcomb, D. R. Thomsen, F. L. Homa, and J. C. Brown. 2005. Identification of a region in the herpes simplex virus scaffolding protein required for interaction with the portal. J. Virol. 79:132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steven, A. C., and P. G. Spear. 1997. Herpesvirus capsid assembly and envelopment, p. 312-351. In W. Chiu, R. Burnett, and R. L. Garcea (ed.), Structural biology of viruses. Oxford University Press, New York, N.Y.
- 28.Tatman, J. D., V. G. Preston, P. Nicholson, R. M. Elliott, and F. J. Rixon. 1994. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J. Gen. Virol. 75:1101-1113. [DOI] [PubMed] [Google Scholar]
- 29.Taus, N. S., B. Salmon, and J. D. Baines. 1998. The herpes simplex virus 1 UL17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged. Virology 252:115-125. [DOI] [PubMed] [Google Scholar]
- 30.Thomsen, D. R., W. W. Newcomb, J. C. Brown, and F. L. Homa. 1995. Assembly of the herpes simplex virus capsid: requirement for the carboxyl-terminal 25 amino acids of the proteins encoded by the UL26 and UL26.5 genes. J. Virol. 69:3690-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomsen, D. R., L. L. Roof, and F. L. Homa. 1994. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J. Virol. 68:2442-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trus, B. L., N. Cheng, W. W. Newcomb, F. L. Homa, J. C. Brown, and A. C. Steven. 2004. Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1. J. Virol. 78:12668-12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Driel, R., and E. Couture. 1978. Assembly of the scaffolding core of bacteriophage T4 proheads. J. Mol. Biol. 123:713-719. [DOI] [PubMed] [Google Scholar]
- 34.Ward, P. L., W. O. Ogle, and B. Roizman. 1996. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins on herpes simplex virus 1. J. Virol. 70:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weller, S. K., E. P. Carmichael, D. P. Aschman, D. J. Goldstein, and P. A. Schaffer. 1987. Genetic and phenotypic characterization of mutants in four essential genes that map to the left of HSV-1 UL DNA. Virology 161:198-210. [DOI] [PubMed] [Google Scholar]
- 36.Zhou, Z. H., M. Dougherty, J. Jakana, J. He, F. J. Rixon, and W. Chiu. 2000. Seeing the herpesvirus capsid at 8.5 Å. Science 288:877-880. [DOI] [PubMed] [Google Scholar]