Abstract
Virus-specific CD8+ T cells are critical for the control of acute Friend virus (FV) infections, but are rendered impotent by CD4+ regulatory T cells during the chronic phase of infection. The current study examines this CD8+ T-cell dysfunction by analyzing the production and release of cytolytic molecules by CD8+ T cells. CD8+ T cells with an activated phenotype (CD43+) from acutely infected mice produced all three key components of lytic granules: perforin, granzyme A, and granzyme B. Furthermore, they displayed evidence of recent degranulation and in vivo cytotoxicity. In contrast, activated CD8+ T cells from chronically infected mice were deficient in cytolytic molecules and showed little evidence of recent degranulation and poor in vivo cytotoxicity. Evidence from tetramer-positive CD8+ T cells with known virus specificity confirmed the findings from the activated subset of CD8+ T cells. Interestingly, perforin and granzyme A mRNA levels were not significantly reduced during chronic infection, indicating control at a posttranscriptional level. Granzyme B deficiency was associated with a significant decrease in mRNA levels, but posttranscriptional control also appeared to contribute to deficiency. These results demonstrate a broad impairment of cytotoxic CD8+ T-cell effector function during chronic retroviral infection and explain the inability of virus-specific CD8+ T cells to eliminate persistent virus.
Many viruses such as herpesviruses, hepatitis viruses, and retroviruses evade immunological destruction during acute infection and establish chronic (persistent) infections that may culminate in life-threatening diseases. We have used infection of mice with Friend virus (FV) as a model to study basic mechanisms of immunological control and escape during a chronic retroviral infection (14, 21, 24, 25, 46). FV is a retroviral complex that induces lethal leukemia in most strains of mice. However, resistant strains of mice exist that develop potent immune responses which allow recovery from acute infection (13, 22). Mice that recover from acute infection never completely clear virus and remain persistently infected for life (10, 19). Virus levels are low during the persistent phase of infection, and B cells serve as the predominant reservoir for persistent virus (21).
The resolution of acute FV infection requires complex immune responses, including antibodies, CD4+ T cells, and CD8+ T cells (20, 41). CD8+ T cells are critical for recovery, as CD8-deficient or -depleted mice develop high viral loads and severe disease (19, 20, 41). Critical factors in the CD8+ T-cell-mediated control of acute FV infection are secretion of gamma interferon (15, 38, 39), and production of the cytotoxic molecules perforin, granzyme A, and granzyme B (55).
In contrast to acute infection, depletion of CD8+ T cells during chronic infection does not affect virus levels, suggesting that the chronic virus had somehow escaped the CD8+ T-cell response (21). It has previously been shown that FV was not latent during chronic infection, nor had the virus directly escaped recognition by virus-specific CD8+ T cells. Virus-specific CD8+ T cells adoptively transferred into persistently infected mice were rapidly activated and proliferated as well as the same cells transferred into acutely infected mice. However, the CD8+ T cells transferred into persistently infected mice failed to develop effector function as they did when transferred into acutely infected mice. Cotransfer experiments indicated that CD4+ regulatory T cells from persistently infected mice suppressed the ability of virus-specific CD8+ T cells to produce gamma interferon and eliminate infected cells (14).
The current study extends the results from these adoptive transfer experiments to the endogenous population of FV-specific CD8+ T cells and investigates the defects at the molecular level. All three of the major molecules involved in cytotoxicity were analyzed: perforin, granzyme A, and granzyme B. The results showed deficiencies in all three proteins compared to CD8+ T cells from acutely infected mice. Interestingly, the regulation of protein expression varied from protein to protein, with only granzyme B being regulated at the transcriptional level. These results suggest the possibility that multiple upstream signals control cytotoxic granule content.
MATERIALS AND METHODS
Mice.
Experiments were done using (C57BL/10 × A.BY)F1 mice (H-2b/b, Fv1b/b, Fv2r/s, Rfv3r/s). All mice were females 8 to 16 weeks of age at the beginning of the experiments. Animals were treated in accordance with the regulations and guidelines of Germany or the Animal Care and Use Committee of the Rocky Mountain Laboratories of the National Institutes of Health.
Virus and virus infection.
The FV stock used in these experiments was FV complex containing B-tropic Friend murine leukemia helper virus (F-MuLV) and polycythemia-inducing spleen focus-forming virus (29). The stock was prepared as a 10% spleen cell homogenate from BALB/c mice infected 14 days previously with 3,000 spleen focus-forming units of uncloned virus stock. Persistently infected mice were mice that had been infected as above at least 8 weeks prior to experimentation and which had recovered from acute splenomegaly. Spleen virus levels in persistently infected mice are generally stable at approximately 104 infectious centers per spleen by 6 to 8 weeks postinfection (46).
In vivo cytotoxicity assay.
The in vivo cytotoxic T-lymphocyte assay described by Barber et al. (5) was modified to measure cytotoxicity in FV-infected mice. Briefly, FV-expressing FBL-3 and control EL-4 tumor cells were stained with 20 nM and 200 nM 5-carboxyfluoresceine diacetate succinimidyl ester (CFSE), respectively. The labeled cells (107 cells of each population) were then injected subcutaneously into the indicated groups of mice; 24 h later the injection site was surgically removed. The tumor cells were then separated from the skin using a metal filter and analyzed for CFSE fluorescence by flow cytometry. Target cells were distinguished from one another based on CFSE staining brightness. The percent killing was calculated by the formula 100 − [(% FBL-3 in infected/% EL-4 in infected)/(% FBL-3 in uninfected/% EL-4 in uninfected)] × 100.
Intracellular granzyme staining and flow cytometry.
Cell surface staining was performed using Becton Dickinson (Heidelberg) reagents. T-cell antibodies were anti-CD43, directed against the activation-associated glycosylated isoform of CD43 (1B11), anti-CD4 (RM4-5), anti-CD8 (53-6.7), and anti-CD107a (1D4B). Dead cells (TOPRO or propidium iodide positive) were excluded from all cell surface analyses. Intracellular granzyme A (polyclonal rabbit-anti-mouse granzyme A immunoglobulin G (IgG), protein A purified) and granzyme B (monoclonal anti-human granzyme B antibody allophycocyanin-conjugated, clone GB12, Caltag Laboratories, Hamburg) staining was performed using the Cytofix/Cytoperm intracellular staining kit (Becton Dickinson, Heidelberg). After labeling for cell surface markers the spleen cells were washed and intracellular staining for granzyme B was performed according to the manufacturer's protocol. Granzyme A was reacted with a polyclonal rabbit-anti-mouse granzyme A IgG antiserum (kindly provided by M. Simon) and stained with a fluorescein isothiocyanate-conjugated goat anti-rabbit IgG secondary antibody (Dianova, Hamburg, Germany). Data were acquired on a FACSCalibur flow cytometer (Becton Dickinson, Heidelberg, Germany) from 30,000 to 80,000 lymphocyte-gated events per sample. Analyses were done using B.D. Cellquest Pro software (Version 4.0.1, Becton Dickinson, Heidelberg, Germany).
Quantitative PCR.
Total RNA was extracted from 105 CD43+CD8+ T cells by using the RNeasy Micro kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. mRNA was transcribed by incubating total RNA with an oligo(dT)12-18 primer (500 ng; Pharmacia, Freiburg, Germany) and RevertAid H Minus Moloney murine leukemia virus reverse transcriptase (Fermentas, St Leon-Rot, Germany) as advised by the manufacturer. The resulting cDNA was used as a template for 18S rRNA (housekeeping gene), granzyme A, granzyme B, and perforin amplification in the LightCycler system (Roche Diagnostics, Mannheim, Germany) by using FastStart DNA Master SYBR Green I (Roche, Basel, Germany). Plasmid vectors expressing one of the cytotoxic molecules or 18S were used for quantification of mRNA molecules.
The primers used were as follows: perforin (380 bp), 5′ GCT GGT GAA AAG GAC CTC C 3′ (sense) and 5′ CAC AGG ACT AGA ACA CCT GC 3′ (antisense); granzyme A (563 bp), 5′ GGG GAT CTA CAA CTT GTA CGG 3′ (sense) and 5′ ATT GCA GGA GTC CTT TCC ACC AC 3′(antisense); granzyme B (135 bp), 5′ TCA GGC TGC TGA TCC TTG ATC G 3′ (sense) and 5′ATG AAG ATC CTC CTG CTA CTG C 3′ (antisense); 18s rRNA (99 bp), 5′ GCC CGA GCC GCC TGG ATA C 3′ (sense) and 5′ CCG GCG GGT CAT GGG AAT AAC 3′ (antisense).
Infectious-center assays.
Serial dilutions of spleen cells from infected mice were plated onto susceptible Mus dunni cells, cocultivated for 3 days, fixed with ethanol, stained with F-MuLV envelope-specific monoclonal antibody 720, and developed with peroxidase-conjugated goat anti-mouse IgG and substrate to detect foci of infected cells (12).
Tetramers and tetramer staining.
The DbGagL tetramers were constructed by Koen Schepers and Ton Schumacher (The Netherlands Cancer Institute, Amsterdam, The Netherlands) using a peptide in which all three cysteine residues were replaced with aminobutyric acid to prevent interpeptide disulfide bonding as has been described (44). This variant peptide is recognized by polyclonal DbGagL-specific CD8+ T cells as determined by intracellular gamma interferon staining. For detection of DbGagL-specific CD8+ T cells, nucleated spleen cells were dually stained with fluorescein isothiocyanate-labeled anti-CD8 (Ly-2) (BD Pharmingen, Heidelberg, Germany) and phycoerythrin-labeled major histocompatibility complex class I H2-Db tetramers specific for the FV GagL peptide (9) for 15 min at room temperature. Cells were washed two times, resuspended in buffer containing TOPRO, and analyzed by flow cytometry.
Western blotting.
CD8+CD43+ T cells were isolated from spleens of FV-infected (C57BL/10 × A.BY)F1 mice at 2 weeks postinfection (acute) and 8 weeks postinfection (persistent). CD8+ T cells were first enriched by positive selection using anti-CD8 MicroBeads and magnetic-activated cell sorting (MACS, Miltenyi Biotech, Auburn, Calif.). CD8+-enriched cells were then stained with anti-CD43-fluorescein isothiocyanate (1B11) and anti-CD8-allophycocyanin (53-6.7) (BD Biosciences-Pharmingen, San Jose, Calif.). CD8+CD43+ T cells were sorted on a FACSAria flow cytometer (BD Biosciences-Immunocytometry Systems) from a population gated on side scatter, forward scatter, and exclusion of propidium iodide.
Protein samples were prepared from 8 × 105 highly purified CD8+CD43+ T cells (>96%) using the M-PER reagent containing the HALT protease inhibitor cocktail (Pierce Biotechnology, Rockford, Illinois). Protein from approximately 2.4 × 105 cell equivalents was separated on a 10% Tris-HCl Ready Gel (Bio-Rad, Hercules, Calif.), and transferred to Immobilon P membranes (Millipore, Billerica, Mass.). The membranes were probed with rabbit anti-mouse perforin antibody (Torrey Pines Biolabs, Houston, Texas), then with horseradish peroxidase-conjugated antibody to rabbit IgG (Pierce) and revealed by chemiluminescence (SuperSignalWest Pico, Pierce). For quantification, the blots were stripped and reprobed sequentially with rabbit antiactin antibody (Sigma-Aldrich, St. Louis, Missouri) and horseradish peroxidase-labeled anti-rabbit IgG and revealed by chemiluminescence.
RESULTS
Virus-specific CD8+ T cells with a cell surface effector phenotype were present in mice persistently infected with FV.
Previous experiments showed that virus-specific CD8+ T cells adoptively transferred into persistently infected mice rapidly became activated and proliferated as well as when transferred into acutely infected mice (14). Thus, poor antigen presentation could not account for lack of CD8+ T-cell function in persistently infected mice. Flow cytometry revealed the expansion of activated CD8+ T cells during acute infection that was still present during chronic infection as measured by expression of the activation-associated glycoform of CD43 (8) (Fig. 1A). During both phases of infection, a large majority of the CD43+ CD8+ T cells were also positive for CD69, a very early activation marker, indicating recent engagement with antigen (data not shown).
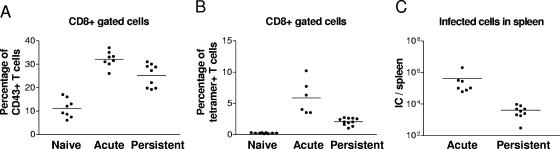
FIG. 1.
Analysis of CD8+ T cells and FV infection. A. Gated CD8+ T cells from naive acutely infected (2 weeks postinfection) and persistently infected (8 weeks postinfection) mice were stained for CD43 to detect effector T cells. The difference between the means of the group of acutely and the group of persistently infected mice is statistically significant (P = 0.0044, unpaired t test). The mean of total T-cell numbers for each group were for CD8+ T cells: Naïve = 7.8 × 106, acutely infected = 2.2 × 107, and persistently infected mice 3.2 × 107 and for CD8+ CD43+ T cells: Naïve = 8.2 × 105, acutely infected = 7.6 × 106, and persistently infected mice = 8.0 × 106. B. The percentages of virus-specific CD8+ T cells reactive with DbGagL tetramers are shown. Costaining experiments indicated that all tetramer-positive CD8+ T cells expressed the CD43 activation marker (data not shown). The difference between the means of the groups of acutely and persistently infected mice is statistically significant (P < 0.0001, unpaired t test). The mean of total DbGagL-specific T-cell numbers for each group were for tetramer-positive CD8+ T cells: Acutely infected = 1.3 × 106 and persistently infected mice = 0.8 × 105. C. Spleen virus levels (infectious centers) in acutely versus persistently infected mice. The difference between the means is statistically significant (P < 0.0001, unpaired t test). Each dot represents an individual mouse. A line indicates the mean percentage for each group. Similar results were obtained in two independent experiments.
To ensure that the activated population of CD8+ T cells reflected the properties of virus-specific CD8+ T cells, the cells were also stained with a major histocompatibility complex class I tetramer specific for cells recognizing the H-2Db-restricted F-MuLV glycosylated Gag epitope (H-2DbGagL) (44). The mean percentage of activated tetramer-positive CD8+ T cells was significantly increased in both acutely and persistently infected mice, although it was lower in persistently infected animals (Fig. 1B) consistent with their lower levels of spleen virus (Fig. 1C). All tetramer-positive cells expressed the activation marker CD43+ (Fig. 4) and the vast majority were also positive for CD69 (data not shown). These results confirmed the presence of a significant population of recently activated FV-specific CD8+ T cells in persistently infected mice and made it possible to compare functional properties of CD8+ T cells with an effector phenotype during acute and persistent FV infection.
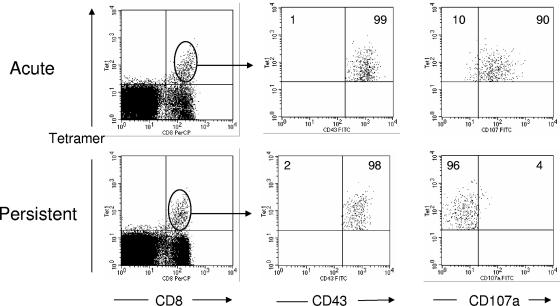
FIG. 4.
Detection of exocytosis in the Db GagL tetramer+ subset of virus-specific effector CD8+ T cells. Flow cytometry was used to detect the degranulation marker CD107a on DbGagL tetramer-positive effector CD8+ T cells. CD8+ DbGagL tetramer+ were gated and analyzed for CD43 and CD107a expression. Activation marker CD43 was used to indicate that all DbGagL tetramer-positive CD8+ T cells were of effector phenotype during both acute (2 weeks postinfection) and persistent (8 weeks postinfection) FV infection. The figures show the results of a representative mouse from each group. Mean percentages of tetramer-positive CD8+ T cells that were negative (left) or positive (right) for CD107a are given in the upper quadrants. The mean fluorescence intensities of the CD107a signals were: acute infection = 37.4 (n = 5) and persistent infection = 9.9 (n = 12). The difference between the means is statistically significant (P < 0.0001, unpaired t test). Similar results were obtained in two independent experiments.
Impaired cytotoxicity in persistently infected mice.
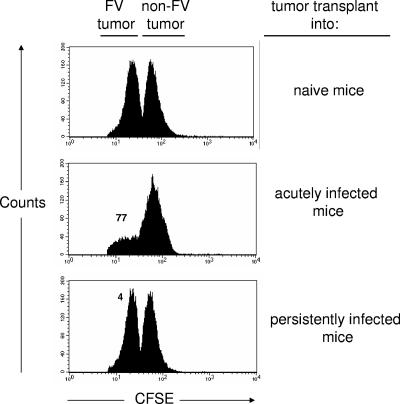
Previous results showed that CD8+ T cells from persistently infected mice were poorly cytotoxic in vitro compared to CD8+ T cells from acutely infected mice (41) (data not shown). To confirm that these results reflected the physiological situation, an in vivo cytotoxic T-lymphocyte assay was performed by injecting CFSE-labeled Friend virus-induced tumor cells (FBL-3) into naive, acutely infected, and persistently infected mice (Fig. 2). More brightly labeled Friend virus-negative tumor cells (EL4) were coinjected as controls. Twenty-four hours after transfer 77% of the FV antigen-expressing FBL-3 cells were eliminated in the acutely infected mice. By contrast, only 4% of the FBL-3 targets were killed in persistently infected mice. These results indicate a functional impairment of cytotoxicity in persistently infected mice and are consistent with previous studies showing suppressed CD8+ T-cell-mediated rejection of FBL-3 tumors in mice chronically infected with FV (24).
FIG. 2.
CD8+ T-cell killing in vivo. CFSE-labeled Friend virus-induced FBL-3 tumor cells (FV tumor) were injected subcutaneously into naïve, acutely infected (2 weeks postinfection), persistently infected mice (8 weeks postinfection). As controls CFSE-brightly labeled Friend virus negative EL-4 (non-FV tumor) tumor cells were coinjected. The tumor cells were harvested 24 h after injection and analyzed for CFSE fluorescence. The figures show the results of a representative mouse from each group (three mice per group) with the numbers representing the percentages of target cell killing.
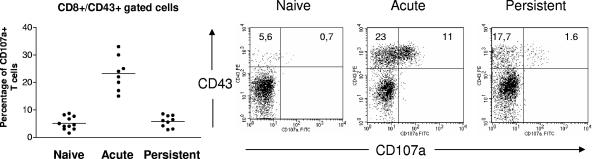
The cytotoxic activity of CD8+ T cells was further analyzed by examining cell surface expression of CD107a (LAMP-1). CD107a resides on the luminal side of the membranes of cytotoxic vesicles and becomes exposed on the cell surface as a result of exocytosis (6, 43). Thus, cell surface expression of CD107a indicates recent cytotoxic vesicle degranulation and can be used as a surrogate marker for cytotoxicity. The entire population of both activated (CD43+) CD8+ T cells and the tetramer-positive subpopulation were compared between acute and chronic FV infections. In acutely infected mice, a mean of 23.3% of the activated CD8+ T cells were positive for CD107a, while only 5.8% of the cells from chronically infected mice were positive (Fig. 3). The results were even more striking in the activated tetramer-positive CD8+ T cells, where most of the virus-specific CD8+ T cells were positive for CD107a, while almost none of the cells from persistently infected mice were positive (Fig. 4). Thus, activated CD8+ T cells from acutely infected mice had significantly higher percentages of cells showing evidence of recent degranulation than the equivalent subpopulations from chronically infected mice.
FIG. 3.
Detection of exocytosis in activated CD8+ T cells. Flow cytometry was used to detect the degranulation marker CD107a on activated CD8+ T cells. Accumulated results are shown on the left with representative flow data on the right. The difference between the means of the acutely infected (2 weeks postinfection) and persistently infected (8 weeks postinfection) mice is statistically significant (P < 0.0001, unpaired t test). Similar results were obtained in two independent experiments.
Activated virus-specific CD8+ T cells in persistent FV infection lack the key components of cytolytic granules.
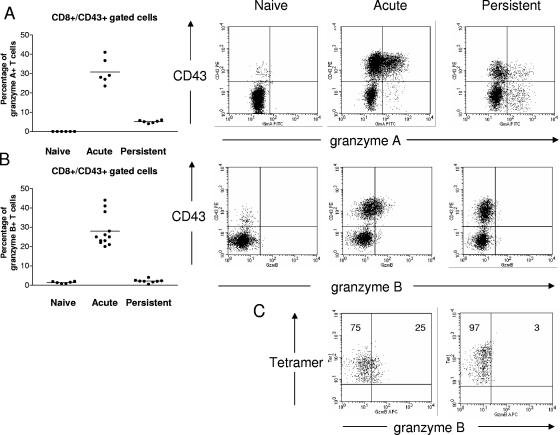
It was previously shown that FV-specific CD8+ T cells from persistently infected mice exhibited low granularity (14), suggesting that the failure of cytotoxicity and exocytosis could be related to defects in cytotoxic granule production. The expression of three key proteins stored in the specialized cytolytic granules of activated CD8+ T cells, granzyme A, granzyme B, and perforin, was measured. Flow cytometric analysis showed that the percentage of activated (CD43+) CD8+ T cells expressing granzyme A was significantly higher in acutely infected mice than in persistently infected mice (Fig. 5A). Activated CD8+ T cells from acutely infected mice also expressed granzyme B, but the cells from persistently infected mice were extremely low or negative for granzyme B (Fig. 5B).
FIG. 5.
Production of granzyme A and granzyme B in activated virus-specific CD8+ T cells. Flow cytometry was used to detect intracellular granzyme A and granzyme B in effector CD8+ T cells. Accumulated results are shown on the left with representative flow cytometry data on the right. A. Percentages of effector CD8+ T cells expressing granzyme A. The difference between the means of the acutely infected (2 weeks postinfection) and persistently infected mice (8 weeks postinfection) is statistically significant (P < 0.0001, unpaired t test). The mean fluorescence intensities of the granzyme A signals in the activated CD8+ T cells were: naïve = 45,86, acute infection = 90.15, and persistent infection = 59.14. Similar results were obtained in two independent experiments. B. Percentages of effector CD8+ T cells expressing granzyme B. The difference between the means of the acutely infected and persistently infected mice is statistically significant (P < 0.0001, unpaired t test). The mean fluorescence intensities of the granzyme B signals in the activated CD8+ T cells were: naïve = 7.45, acute infection = 21.78, and persistent infection = 9.08. Similar results were obtained in two independent experiments. C. DbGagL tetramer+ effector CD8+ T cells expressing granzyme B during different phases of infection. Activation marker CD43 was used to indicate that all DbGagL tetramer-positive CD8+ T cells were of effector phenotype during both acute and persistent FV infection (data not shown). The figures show the results of a representative mouse from each group. Mean percentages of tetramer-positive CD8+ T cells that were negative (left) or positive (right) for granzyme B are given in the upper quadrants. The mean fluorescence intensities of the granzyme B signals were: acute infection = 15.7 (n = 3) and persistent infection = 11.0 (n = 4). The difference between the groups was statistically significant (P < 0.01, unpaired t test).
Activated tetramer-positive CD8+ T cells were also analyzed to confirm that the results from the total activated population reflected the FV antigen-specific subset. Approximately 25% of the tetramer-positive CD8+ T cells from acutely infected mice were positive for granzyme B, while only about 3% of tetramer-positive CD8+ T cells from persistently infected mice were positive (Fig. 5C). In addition, the mean fluorescent intensity for granzyme B was significantly higher in cells from acutely infected mice compared to persistently infected mice.
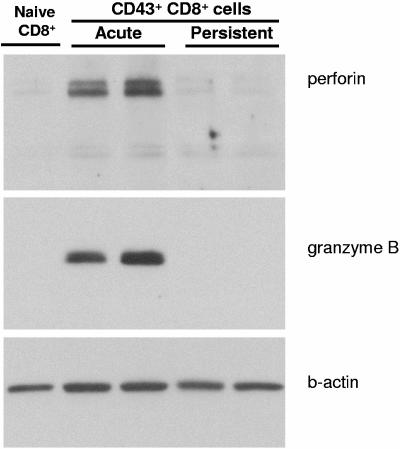
Attempts to detect intracellular perforin by flow cytometry using several different antibodies proved unsuccessful, so perforin expression was evaluated by Western blotting. This analysis revealed that CD43+CD8+ T cells isolated during persistent FV infection had undetectable perforin, whereas activated CD8+ T cells from acutely infected mice exhibited significantly increased levels of perforin protein compared to naïve CD8+ T cells (Fig. 6). The Western blot was stripped and reprobed with anti-granzyme B, confirming the flow cytometry data showing little or no expression in the activated CD8+ T cells from persistently infected mice. Thus, the lack of cytolytic molecule expression in effector CD8+ T cells from persistently infected mice suggested a severe defect in cytolytic granule formation.
FIG. 6.
Western blots for perforin and granzyme B. CD8+CD43+ T cells were purified from spleens of FV-infected mice at 2 weeks postinfection (acute) and 8 weeks postinfection (persistent), and analyzed by Western blot using anti-perforin and anti-granzyme B antibodies. Protein lysates of CD8+ T cells from naïve mice were used as a negative control, and immunoblotting with anti-β-actin was used to quantify the protein content in each lane. The two bands for perforin are the inactive precursor protein and the activated protein that has been proteolytically cleaved (49).
Defects in perforin and granzyme expression were mainly mediated at the posttranscriptional level.
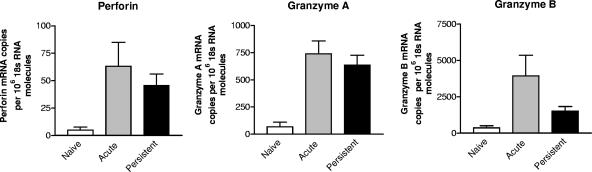
To determine the level at which downregulation of perforin, granzyme A, and granzyme B occurred in the CD43+CD8+ T cells from persistently infected mice, mRNA from sorted cells was quantitated by real-time PCR analysis. Interestingly, there were no significant differences in perforin or granzyme A mRNA expression in cells from acutely and persistently infected mice (Fig. 7). Thus, their dramatic differences in protein expression must have been due to posttranscriptional mechanisms. For granzyme B, mRNA levels were significantly lower in activated CD8+ T cells from persistently infected mice. Although mRNA levels were low, they were still significantly higher than in nonactivated cells from naive mice. Thus, low granzyme B levels may have been due to both transcriptional and posttranscriptional control mechanisms.
FIG. 7.
mRNA levels for cytotoxic molecules in activated CD8+ T cells. Levels of transcripts for perforin, granzyme A, and granzyme B from effector (CD43+) CD8+ T cells isolated from acutely (2 weeks postinfection) and persistently (8 weeks postinfection) infected mice were compared by quantitative real-time PCR. The 18s RNA was used as internal standard. The mean and standard deviation of six mice per group are shown. The difference between the groups of acutely and persistently infected mice were only significant for granzyme B (P = 0.043) but not for perforin (P = 0.93) or granzyme A (P = 0.54). Statistic analysis was done by Mann-Whitney test. Similar results were obtained in two independent experiments.
DISCUSSION
The current study provides an explanation for the lack of CD8+ T-cell control of virus during persistent FV infections (21). FV-specific CD8+ T cells expressing activation markers could be detected in persistently infected mice, but they were functionally impaired in their ability to produce lytic granules and thus could not use the cytolytic pathway to kill infected cells. Previous studies in which FV-specific CD8+ T cells were adoptively transferred into chronically infected mice revealed that the transferred cells rapidly up-regulated activation markers and proliferated. This result indicated strong antigenic stimulation, but the cells had an impaired ability to produce gamma interferon and were unable to reduce virus levels (14). Furthermore, the transferred cells were low in granularity, suggesting a defect in cytolytic granule formation.
Evidence from CD4+/CD8+ T-cell cotransfer experiments indicated that CD4+ regulatory T cells from persistently infected mice rapidly suppressed the effector functions of transferred CD8+ T cells. Presumably, the endogenous population of virus-specific CD8+ T cells studied in the current experiments would be under the same immunosuppressive pressure by CD4+ regulatory T cells, likely contributing to their loss of effector functions. Additionally, it has been shown in the lymphocytic choriomeningitis virus model that chronic stimulation by antigen can lead to exhaustion of CD8+ T-cell effector functions such as interleukin-2, tumor necrosis factor alpha, and gamma interferon secretion (52). Since virus-specific CD8+ T cells in mice persistently infected with FV are also subject to chronic antigenic stimulation, exhaustion may also play a role in their dysfunction.
Loss of cytotoxic T-lymphocyte killing mediated by regulatory T cells has previously been noted (11, 28, 30, 48, 54), but little is known about why cytolytic activity is lost or what types of mechanisms induce loss. One in vitro study of human cytotoxic T-lymphocyte demonstrated that CD4+CD25+ regulatory T cells suppressed perforin and granzyme B mRNA transcription (7). This result contrasts with our finding that inhibition was predominantly at the posttranscriptional level, but that difference could be due to in vitro versus direct ex vivo experiments. More consistent with our findings, a study of dysfunctional cytotoxic T-lymphocyte function in human immunodeficiency virus-infected humans showed that perforin protein expression was down-regulated posttranscriptionally (45).
Functional impairment of CD8+ T cells has been described for patients infected with human immunodeficiency virus and in patients with hepatitis C virus (4, 17, 23, 31, 33, 37, 50). Both viruses are genetically highly variable and escape from CD8+ T-cell responses by mutations in T-cell epitopes has been discussed as a major reason for the loss of CD8+ T-cell activity in chronic infected carriers (35, 36, 40). However, other evidence suggests that several viral epitopes of human immunodeficiency virus and hepatitis C virus are recognized by CD8+ T cells throughout the chronic phase of infection (16, 27, 53) and that viral loads in infected patients are more associated with functional properties of CD8+ T cells than with viral mutations (16, 23, 31, 33). Interestingly, in both human immunodeficiency virus and hepatitis C virus infection CD4+ regulatory T cells have been described to suppress antiviral T-cell responses (1, 2, 3, 26, 32, 51). Thus, impairment of CD8+ T-cell function via regulatory T cells may be a mechanism contributing to virus escape and the establishment of chronic infections.
It is likely that suppression of cytotoxic T-lymphocyte function has evolved as a mechanism to control the immunopathological damage that can occur as a consequence of cytolytic killing by CD8+ T cells. For example, in the mouse model for hepatitis B infection CD8+ T cells exhibit virus control via gamma interferon and tumor necrosis factor alpha production without causing the liver tissue destruction that would result from active cytolysis (18). In human immunodeficiency virus infections, the down-regulation of perforin in virus-specific CD8+ T cells from gut-associated lymphoid tissue may be a mechanism to protect the integrity of the rectal mucosa from cytotoxic T-lymphocyte activity (45). The role of regulatory T cells in dampening cytolytic activity may have evolved as a control over immunopathology (34, 42). For example, it has been shown that the immunopathological damage caused by herpes simplex virus infections of the eye is significantly more severe in the absence of regulatory T cells (47). Thus, suppression of cytolytic activity during persistent infections may represent a host compromise between the immunopathological damage that could accompany complete elimination of infection, and the pathological damage associated with the persistent virus. For most persistent viruses the compromise is probably beneficial to the host, but for notable exceptions such as hepatitis C virus and human immunodeficiency virus, the consequences can be lethal.
We have now identified broad impairment of CD8+ T-cell effector functions during persistent FV infection, including reduced gamma interferon secretion (14) and reductions in all three major cytotoxic molecules: perforin, granzyme A, and granzyme B. It may be necessary to down-regulate all three cytotoxic molecules because previous results indicated that deficiency in one or even two of them was not sufficient to prevent CD8+ T-cell function in FV infection (55). The finding that the down-regulation of granzyme B appeared to be in part at the level of transcription while the regulation of both perforin and granzyme A appeared posttranscriptional indicates complex regulatory mechanisms. The elucidation of these mechanisms could lead to new therapeutic approaches to specifically reactivate CD8+ T-cell functions for the reduction or clearance of chronic viral infections.
Acknowledgments
This work was supported by a grant to U.D. from the Deutsche Forschungsgemeinschaft (Di 714/6-2 and Di 714/8-1), by the IFORES program of the Universitaetsklinikum Essen, and by the intramural research program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
We thank Markus Simon (Max-Plank-Institut fuer Immunbiology, Freiburg, Germany) for providing the anti-granzyme A antibodies and the plasmid vectors expressing the three different cytotoxic molecules. The plasmid expressing 18S was a gift from Nico Birkner and Marinus Lamers (Max-Plank-Institut fuer Immunbiology, Freiburg, Germany). We are grateful to Koen Schepers and Ton Schumacher (The Netherlands Cancer Institute, Amsterdam, The Netherlands) for providing major histocompatibility complex class I tetramers. We thank Klaus Lennartz (Zellbiologie, Universitaetsklinikum Essen, Germany) for sorting T cells by flow cytometry.
REFERENCES
- 1.Aandahl, E. M., J. Michaelsson, W. J. Moretto, F. M. Hecht, and D. F. Nixon. 2004. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 78:2454-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accapezzato, D., V. Francavilla, M. Paroli, M. Casciaro, L. V. Chircu, A. Cividini, S. Abrignani, M. U. Mondelli, and V. Barnaba. 2004. Hepatic expansion of a virus-specific regulatory CD8+ T-cell population in chronic hepatitis C virus infection. J. Clin. Investig. 113:963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson, J., A. Boasso, J. Nilsson, R. Zhang, N. J. Shire, S. Lindback, G. M. Shearer, and C. A. Chougnet. 2005. Cutting edge: The prevalence of regulatory T cells in lymphoid tissue is correlated with viral load in HIV-infected patients. J. Immunol. 174:3143-3147. [DOI] [PubMed] [Google Scholar]
- 4.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, et al. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barber, D. L., E. J. Wherry, and R. Ahmed. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27-31. [DOI] [PubMed] [Google Scholar]
- 6.Betts, M. R., J. M. Brenchley, D. A. Price, De S. C. Rosa, D. C. Douek, M. Roederer, and R. A. Koup. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 281:65-78, 2003. [DOI] [PubMed]
- 7.Camara, N. O., F. Sebille, and R. I. Lechler. 2003. Human CD4+CD25+ regulatory cells have marked and sustained effects on CD8+ T-cell activation. Eur. J. Immunol. 33:3473-3483. [DOI] [PubMed] [Google Scholar]
- 8.Carlow, D. A., B. Ardman, and H. J. Ziltener. 1999. A novel CD8 T-cell-restricted CD45RB epitope shared by CD43 is differentially affected by glycosylation. J. Immunol. 163:1441-1448. [PubMed] [Google Scholar]
- 9.Chen, W., H. Qin, B. Chesebro, and M. A. Cheever. 1996. Identification of a Gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 70:7773-7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesebro, B., M. Bloom, K. Wehrly, and J. Nishio. 1979. Persistence of infectious Friend virus in spleens of mice after spontaneous recovery from virus-induced erythroleukemia. J. Virol. 32:832-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai, Z., Q. Li, Y. Wang, G. Gao, L. S. Diggs, G. Tellides, and F. G. Lakkis. 2004. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J. Clin. Investig. 113:310-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dittmer, U., D. M. Brooks, and K. J. Hasenkrug. 1998. Characterization of a live-attenuated retroviral vaccine demonstrates protection via immune mechanisms. J. Virol. 72:6554-6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittmer, U., and K. J. Hasenkrug. 2001. Cellular and molecular mechanisms of vaccine-induced protection against retroviral infections. Curr. Mol. Med. 1:431-436. [DOI] [PubMed] [Google Scholar]
- 14.Dittmer, U., H. He, R. J. Messer, et al. 2004. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection. Immunity 20:293-303. [DOI] [PubMed] [Google Scholar]
- 15.Dittmer, U., R. Messer, B. Race, I. M. Stromnes, and K. J. Hasenkrug. 2002. Essential roles for CD8+ T cells and interferon gamma in protection of mice against retrovirus-induced immunosuppression. J. Virol. 76:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draenert, R., C. L.Verrill, and Y. Tang. 2004. Persistent recognition of autologous virus by high-avidity CD8 T cells in chronic, progressive human immunodeficiency virus type 1 infection. J. Virol. 78:630-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruener, N. H., F. Lechner, M. C. Jung. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidotti, L. G., T. Ishikawa, M. V. Hobbs, B. Matzke, R. Schreiber, and F. V. Chisari. 1996. Intracellular inactivation of the hepatitis B virus by cytotoxic T lymphocytes. Immunity 4:25-36. [DOI] [PubMed] [Google Scholar]
- 19.Hasenkrug, K. J. 1999. Lymphocyte deficiencies increase susceptibility to Friend virus-induced erythroleukemia in Fv-2 genetically resistant mice. J. Virol. 73:6468-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasenkrug, K. J., D. M. Brooks, and B. Chesebro. 1995. Passive immunotherapy for retroviral disease: influence of major histocompatibility complex type and T-cell responsiveness. Proc. Natl. Acad. Sci. USA 92:10492-10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasenkrug, K. J., D. M. Brooks, and U. Dittmer. 1998. Critical role for CD4+ T cells in controlling retrovirus replication and spread in persistently infected mice. J. Virol. 72:6559-6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasenkrug, K. J., and U. Dittmer. 2000. The role of CD4 and CD8 T cells in recovery and protection from retroviral Infection: lessons from the friend virus model. Virology 272:244-249. [DOI] [PubMed] [Google Scholar]
- 23.Hess, C., M. Altfeld, and S. Y. Thomas. 2004. HIV-1 specific CD8+ T cells with an effector phenotype and control of viral replication. Lancet 363:863-866. [DOI] [PubMed] [Google Scholar]
- 24.Iwashiro, M., R. J. Messer, K. E. Peterson, I. M. Stromnes, T. Sugie, and K. J. Hasenkrug. 2001a. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc. Natl. Acad. Sci. USA 98:9226-9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwashiro, M., Peterson, K., Messer, R. J., Stromnes, I. M., and Hasenkrug, K. J. 2001b. CD4+ T cells and gamma interferon in the long-term control of persistent friend retrovirus infection. J. Virol. 75(1):52-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinter, A. L., M. Hennessey, A. Bell, S. Kern, Y. Lin, M. Daucher, M. Planta, M. McGlaughlin, R. Jackson, S. F. Ziegler, and A. S. Fauci. 2004. CD25+CD4+ regulatory T cells from the peripheral blood of Asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. jem20032069. [DOI] [PMC free article] [PubMed]
- 27.Koziel, M. J., Dudley, D., Wong, J. T., Dienstag, J., Houghton, M., Ralston, R., and Walker, B. D. 1992. Intrahepatic cytotoxic T lymphocytes specific for hepatitis C virus in persons with chronic hepatitis. J. Immunol. 149(10):3339-3344. [PubMed] [Google Scholar]
- 28.Kursar, M., K. Bonhagen, J. Fensterle, A. Kohler, R. Hurwitz, T. Kamradt, S. H. Kaufmann, and H. W. Mittrucker. 2002. Regulatory CD4+CD25+ T cells restrict memory CD8+ T cell responses. J. Exp. Med. 196:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lilly, F., and R. A. Steeves. 1973. B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV). Virology 55:363-370. [DOI] [PubMed] [Google Scholar]
- 30.Lin, C. Y., Graca, L., S. Cobbold, P., and H. Waldmann. 2002. Dominant transplantation tolerance impairs CD8+ T-cell function but not expansion. Nat. Immunol. 3:1208-1213. [DOI] [PubMed] [Google Scholar]
- 31.Lucas, M., Vargas-Cuero, A. L., G. M. Lauer, E. Barnes, C. B. Willberg, N. Semmo, B. D. Walker, R. Phillips, and P. Klenerman. 2004. Pervasive influence of hepatitis C virus on the phenotype of antiviral CD8+ T cells. J. Immunol. 172:1744-1753. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald, A. J., M. Duffy, M. T. Brady, S. McKiernan, W. Hall, J. Hegarty, M. Curry, and K. H. Mills. 2002. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J. Infect. Dis. 185:720-727. [DOI] [PubMed] [Google Scholar]
- 33.Migueles, S. A., A. C. Laborico, W. L. Shupert, et al. 2002. HIV-specific CD8+ T-cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 34.Mills, K. H. 2004. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 4:841-855. [DOI] [PubMed] [Google Scholar]
- 35.Moorman, J. P., M. Joo, and Y. S. Hahn. 2001. Evasion of host immune surveillance by hepatitis C virus: potential roles in viral persistence. Arch. Immunol. Ther. Exp. (Warsz) 49:189-194. [PubMed] [Google Scholar]
- 36.O'Connor, D., T. Friedrich, A. Hughes, T. M. Allen, and D. Watkins. 2001. Understanding cytotoxic T-lymphocyte escape during simian immunodeficiency virus infection. Immunol. Rev. 183:115-126. [DOI] [PubMed] [Google Scholar]
- 37.Par, G., D. Rukavina, E. R. Podack, M. Horanyi, Szekeres-Bartho, J., G. Hegedus, M. Paal, L. Szereday, G., Mozsik, and A. Par. 2002. Decrease in CD3-negative-CD8dim+ and Vdelta2/Vgamma9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J. Hepatol. 37:514-522. [DOI] [PubMed] [Google Scholar]
- 38.Peterson, K. E., M. Iwashiro, K. J. Hasenkrug, and B. Chesebro. 2000. Major histocompatibility complex class I gene controls the generation of gamma interferon-producing CD4+ and CD8+ T cells important for recovery from friend retrovirus-induced leukemia. J. Virol. 74:5363-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peterson, K. E., I. Stromnes, R. Messer, K. Hasenkrug, and B. Chesebro. 2002. Novel role of CD8+ T cells and major histocompatibility complex class I genes in the generation of protective CD4+ Th1 responses during retrovirus infection in mice. J. Virol. 76:7942-7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyerl, F. W., D. H. Barouch, and N. L. Letvin. 2004. Structural constraints on viral escape from HIV- and SIV-specific cytotoxic T-lymphocytes. Viral Immunol. 17:144-151. [DOI] [PubMed] [Google Scholar]
- 41.Robertson, M. N., Spangrude, G. J., Hasenkrug, K., Perry, L., Nishio, J., Wehrly, K., and Chesebro, B. 1992. Role and specificity of T-cell subsets in spontaneous recovery from Friend virus-induced leukemia in mice. J. Virol. 66:3271-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rouse, B. T., and S. Suvas. 2004. Regulatory cells and infectious agents: detentes cordiale and contraire. J. Immunol. 173:2211-2215. [DOI] [PubMed] [Google Scholar]
- 43.Rubio, V., T. B. Stuge, N. Singh, M. R. Betts, J. S. Weber, M. Roederer, and P. P. Lee. 2003. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat. Med. 9:1377-1382. [DOI] [PubMed] [Google Scholar]
- 44.Schepers, K., M. Toebes, G. Sotthewes, Vyth-Dreese, F. A., T. A. Dellemijn, C. J. Melief, F. Ossendorp, and T. N. Schumacher. 2002. Differential kinetics of antigen-specific CD4+ and CD8+ T-cell responses in the regression of retrovirus-induced sarcomas. J. Immunol. 169:3191-3199. [DOI] [PubMed] [Google Scholar]
- 45.Shacklett, B. L., C. A. Cox, M. F. Quigley, C. Kreis, N. H. Stollman, M. A. Jacobson, J. Andersson, J. K. Sandberg, and D. F. Nixon. 2004. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J. Immunol. 173:641-648. [DOI] [PubMed] [Google Scholar]
- 46.Stromnes, I. M., U. Dittmer, T. N. Schumacher, K. Schepers, R. J. Messer, L. H. Evans, K. E. Peterson, B. Race, and K. J. Hasenkrug. 2002. Temporal effects of gamma interferon deficiency on the course of Friend retrovirus infection in mice. J. Virol. 76:2225-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suvas, S., A. K. Azkur, B. S. Kim, U. Kumaraguru, and B. T. Rouse. 2004. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J. Immunol. 172:4123-4132. [DOI] [PubMed] [Google Scholar]
- 48.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T-cell responses. J. Exp. Med. 198:889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uellner, R., M. Zvelebil, J. Hopkins, et al. 1997. Perforin is activated by a proteolytic cleavage during biosynthesis which reveals a phospholipid-binding C2 domain. EMBO J. 16:7287-7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urbani, S., C. Boni, G. Missale, G. Elia, C. Cavallo, M. Massari, G. Raimondo, and C. Ferrari. 2002. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J. Virol. 76:12423-12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss, L., Donkova-Petrini, V., L. Caccavelli, M. Balbo, C. Carbonneil, and Y. Levy. 2004. Human immunodeficiency virus-driven expansion of CD4+CD25+ regulatory T cells which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood 104:3249-3256. [DOI] [PubMed]
- 52.Wherry, E. J., J. N. Blattman, K. Murali-Krishna R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong, D. K., D. D. Dudley, P. B. Dohrenwend, G. M. Lauer, R. T. Chung, D. L. Thomas, and B. D. Walker. 2001. Detection of diverse hepatitis C virus (HCV)-specific cytotoxic T lymphocytes in peripheral blood of infected persons by screening for responses to all translated proteins of HCV. J. Virol. 75(3):1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, Y., Huang, C. T., Huang, X., and Pardoll, D. M. 2004. Persistent Toll-like receptor signals are required for reversal of regulatory T-cell-mediated CD8 tolerance. Nat Immunol. 5(5):508-515. [DOI] [PubMed] [Google Scholar]
- 55.Zelinskyy, G., S. Balkow, M. M. Simon, and U. Dittmer. 2004. Independent roles of perforin, granzymes and Fas in the control of Friend retrovirus infection. Virology 330:365-374. [DOI] [PubMed]