Abstract
To determine whether avian H5N1 influenza viruses associated with human infections in Vietnam had transmitted to pigs, we investigated serologic evidence of exposure to H5N1 influenza virus in Vietnamese pigs in 2004. Of the 3,175 pig sera tested, 8 (0.25%) were positive for avian H5N1 influenza viruses isolated in 2004 by virus neutralization assay and Western blot analysis. Experimental studies of replication and transmissibility of the 2004 Asian H5N1 viruses in pigs revealed that all viruses tested replicated in the swine respiratory tract but none were transmitted to contact pigs. Virus titers from nasal swabs peaked on day 2, and low titers were detected in the liver of two of the four pigs tested. Our findings indicate that pigs can be infected with highly lethal Asian H5N1 viruses but that these viruses are not readily transmitted between pigs under experimental conditions.
A highly pathogenic H5N1 avian influenza virus caused disease outbreaks in poultry in China and seven other east Asian countries (Cambodia, Indonesia, Japan, Korea, Laos, Thailand, and Vietnam) between late 2003 and early 2004 (12). Most of these outbreaks were confined to poultry, but the virus was transmitted to humans in at least three countries, and most of the infected humans died (33 of 51 in Vietnam, 12 of 17 in Thailand, and 1 of 1 in Cambodia). Despite the comparatively small number of human cases, this situation warrants careful monitoring. Of foremost concern is the risk that conditions in parts of Asia could give rise to an influenza pandemic (5, 8).
The precursor of the H5N1 avian influenza virus that spread across Southeast Asia in 2004 was first detected in geese in 1996 in Guangdong, China, and subsequently spread to the live poultry markets in Hong Kong and to humans in 1997 (reviewed by Sims et al. [10]).
The H5N1 variant responsible for the human deaths in Vietnam and Thailand evolved from the A/Goose/Guangdong/1/96 (H5N1) virus and was first detected in Hong Kong in November 2002 in dead egrets (Egretta garzetta), gray herons (Ardea cinerea), and Canada geese (Branta canadensis), all of which are wild aquatic birds. The isolated viruses were antigenic drift variants of H5N1 viruses isolated earlier in Hong Kong which had subsequently acquired the ability to cause lethal infection in ducks (11). The virus that spread to humans in Vietnam and Thailand was the dominant Z genotype of the H5N1 virus that is antigenically and genetically similar to A/Vietnam/1203/04 (A/Vt/1203/04) (H5N1) (5). Additionally, the H5N1 virus has been isolated and has transmitted to domestic and wild felids (4).
Several studies have shown that a small number of mammalian species, including pigs, seals, whales, mink, cats, and ferrets, are susceptible to natural infection with influenza viruses of purely avian genetic makeup. Of these species, the pig has the greatest significance for human health. Pigs coinfected with avian and human influenza viruses can serve as “mixing vessels” for viral genetic material. Such mixing can result in the emergence of a new influenza virus (7), as in the case of new swine H3N2 and H1N2 viruses identified in the United States (2, 13). More recently, avian and human H5N1 isolates from 1997 were shown to replicate to moderate titers in the upper respiratory tracts of intranasally inoculated pigs (9), and human H3N2 viruses were reported to be cocirculating with avian influenza viruses in pigs in China (6).
There is no evidence yet of continuing human-to-human transmission of the 2004 H5N1 (H5N1/04) virus, but the continued coinfection of pigs with human and bird influenza viruses could allow the emergence of a strain capable of transmission among humans. Furthermore, the recent cases of human infection by these viruses in Asia may have allowed the viruses to adapt to mammalian hosts such as humans and pigs. We therefore investigated whether the highly pathogenic H5N1 2004 isolates can naturally infect pigs, replicate in pigs, and be transmitted among pigs.
To investigate the prevalence of swine infection with the 2004 H5N1 viruses, we collected swine sera from three slaughterhouses in Vietnam. Of the 3,175 pig sera tested, 8 (0.25%) showed exposure to avian H5N1 viruses. The first positive serum sample was obtained in Hanoi in January 2004 and had a high neutralizing titer (160). Five of the eight positive sera were collected in Hanoi during April 2004 and had neutralizing titers of 40 to 160 (Table 1). One serum sample in May 2004 and one in June 2004 in Hanoi were positive for avian H5N1 virus, which was circulating in the region.
TABLE 1.
Serological reactivity to H5N1 influenza viruses of pig sera collected in Vietnamese slaughterhousesa
| Yr and mo | Location | No. of serum samples tested | No. of sera positive for H5 antibody (%) in neutralization tests | Neutralization titer(s) of positive sera |
|---|---|---|---|---|
| 2003 September | Hanoi | 109 | 0 | |
| 2003 October | Hanoi | 106 | 0 | |
| 2003 November | Hanoi | 91 | 0 | |
| 2003 December | Hanoi | 100 | 0 | |
| 2004 January | Hanoi | 105 | 1 (1) | 160 |
| 2004 February | Hanoi | 156 | 0 | |
| 2004 March | Hanoi | 343 | 0 | |
| 2004 April | Hanoi | 603 | 5 (0.8) | 80, 160, 160, 40, 40 |
| 2004 April | Can Tho | 1,039 | 0 | |
| 2004 April | Ho Chi Minh City | 307 | 0 | |
| 2004 May | Hanoi | 102 | 1 (1) | 80 |
| 2004 June | Hanoi | 114 | 1 (0.9) | 80 |
| Total | 3,175 | 8 (0.25) |
Sera were collected from 3,175 pigs in Vietnam during September 2003 through June 2004. Pigs were sampled in a slaughterhouse at three different locations as indicated. All sera were heat inactivated at 56°C for 30 minutes and tested in a neutralization test. Briefly, sera were screened at a dilution of 1/10 by mixing 100 50% tissue culture infective doses of MDCK cell-adapted viruses (A/Vietnam/3078/04 [H5N1]) with the antibody dilution, incubating for 1 hour at 37°C, and adding to a preformed MDCK cell monolayer. Cytopathic effect was read at 3 days when the virus back-titration confirmed the challenge virus dose to be 100 50% tissue culture infective doses. Positive and negative control sera and a virus back-titration were included. Sera positive in the screening test were retested after pretreatment with receptor-destroying enzyme derived from Vibrio cholerae (Denka Seiken, Tokyo, Japan) to remove nonspecific inhibitors. The antibody titer was determined by testing serial twofold dilutions from 1/10 to 1/640 in quadruplicate. The highest antibody dilution providing complete protection of the cell monolayer in >2 of the quadruplicate wells was regarded as the antibody titer. The sera giving positive neutralization tests were confirmed by Western blotting using H5 antigen (Fig. 1).
To further confirm the specificity of the neutralization test results, Western immunoblotting was done using baculovirus-expressed H5 antigen of A/Vt/1203/04 purified by affinity chromatography columns (Protein Sciences Corporation, Meridian, CT). One microgram of H5 protein per lane was separated on 10% sodium dodecyl sulfate-polyacrylamide gel and immunoblotted with pig sera at a dilution of 1/200. Pig sera that gave positive reactions in the H5N1 virus-neutralizing antibody test and a random selection of sera giving negative reactions were tested (Fig. 1). The immunoblotting results correlated with the results of the neutralization test. These findings suggested that the H5N1 viruses naturally infect pigs, although the incidence of such infection is low.
FIG. 1.
Baculovirus-expressed H5 antigen from A/Vietnam/1203/04 purified by affinity chromatography columns (Protein Sciences Corporation, Meridian, CT). One microgram of H5 protein per lane was separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and immunoblotted with pig sera at a dilution of 1/200. Horseradish peroxidase-conjugated anti-pig immunoglobulin (DakoCytomatron, Glostrup, Denmark) was used at a dilution of 1/10,000 as recommended by the manufacturer. All the sera giving positive neutralization test reactions and a random selection of sera giving negative neutralization test results were tested by Western blotting. A representative result is shown.
Experimental infection of pigs with H5N1/04 viruses was done in biosafety level 3 or greater (BSL3+) containment facilities. All of the viruses were recovered from nasal swabs obtained from the inoculated pigs after intranasal infection with 106 50% egg infective doses (EID50). On day 1 after inoculation, the mean virus titer of nasal swabs was ≥2.75 log10 EID50/0.1 ml. Virus was shed for at least 3 days (Table 2). Virus titers in nasal swabs peaked on day 2 after inoculation at 3.33 to 3.75 log10 EID50/0.1 ml. Mild cough and elevated body temperature were observed on days 1 through 4 in all inoculated pigs (Fig. 2). Food consumption dropped dramatically on days 1 and 2 but began increasing on day 3. Neither the human nor the avian H5N1/04 isolates were transmitted to the contact pigs (Table 2). Although the pigs inoculated with avian isolates showed slightly higher nasal swab titers than those inoculated with human isolates, pigs inoculated with the human virus (A/Vt/1203/04) showed more severe clinical signs (data not shown).
TABLE 2.
Nasal excretion of H5N1 avian influenza in pigsb
| Day | Swab viral titer (log10 EID50/0.1 ml) (SD)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| A/Vt/1203/04
|
A/Ck/Vt/C-58/04
|
A/Dk/Th/D4AT/04
|
A/Gs/Th/G7CS/04
|
|||||
| Inoculated | Contact | Inoculated | Contact | Inoculated | Contact | Inoculated | Contact | |
| −1 | −a | − | − | − | − | − | − | − |
| 1 | 2.75 (0.5) | − | 3.75 | − | 2.75 | − | 3.75 | − |
| 2 | 3.33 (0.3) | − | 3.75 | − | 3.33 | − | 3.75 | − |
| 3 | 1.33 (0.3) | − | 2.33 | − | 1.75 | − | 2.75 | − |
| 4 | − | − | 0.75 | − | − | − | 01.33 | − |
| 5 | − | − | − | − | − | − | − | − |
| 6 | − | − | − | − | − | − | − | − |
−, no detectable virus.
The viruses used in this study were isolated in embryonated chicken eggs as described previously (7). We selected one human and one avian isolate from Vietnam (A/Vt/1203/04 and A/Ck/Vt/C-58/04) and two avian isolates from Thailand (A/Dk/Th/D4AT/04 and A/Gs/Th/G7CS/04). Sequence analysis revealed the four viruses to have 99.1% to 99.8% homology in their hemagglutinin genes (data not shown). Yorkshire white weanling pigs (approximately 4 weeks old) that were determined to be free of detectable influenza virus by serologic testing were inoculated intranasally with 3.3 × 106 EID50 of virus in a volume of 1.0 ml (divided between two plastic syringes for separate inoculation of each nostril). An uninoculated littermate was housed in an isolator with each inoculated pig to test for pig-to-pig transmission of virus. Because of the small size of the isolators in our BSL3+ facility, we attempted to obtain the maximum possible information by using four individual, albeit genetically very similar (5), viruses to infect small numbers of animals. In most cases, only one pig was inoculated with each virus. However, the human virus (A/Vt/1203/04) group was retested separately to confirm our results. The pigs’ temperatures and food consumption were recorded daily, beginning 2 days before inoculation and ending on day 6 after inoculation (end of study). Each nostril was swabbed daily, and virus was titrated in embryonated chicken eggs. All viruses and animal experiments were handled in a BSL3+ facility approved by the U.S. Department of Agriculture, and the research staff wore fitted HEPA filter masks.
FIG. 2.
Pigs inoculated with the human virus (A/Vt/1203/04) showed initial drops in body weight (2 days) and body temperature (3 days). Infection details are given in the footnotes to Table 2.
To investigate the tissue distribution of virus, we collected samples of tonsil, trachea, serum, lung, liver, intestine, spleen, and kidney from pigs on day 6 after inoculation. To prevent cross-contamination, different sterile instruments were used for collecting each tissue. The tonsil, trachea, and lung were positive for virus in most infected pigs (Table 3). Therefore, the H5N1 viruses can persist in pigs for at least 6 days after inoculation. The sera from all inoculated and contact pigs were negative by hemagglutinin inhibition assay. (The pigs were killed on day 6.) The livers of pigs inoculated with A/Vt/1203/04 and A/Duck/Thailand/D4AT/04 (A/Dk/Th/D4AT/04) were positive for virus (0.33 to 0.75 log10 EID50 per gram of tissue). Therefore, these viruses accumulated in the liver despite undetectable viremia. No virus was recovered from intestine, spleen, or kidney in any pig. Lung tissue collected on day 6 showed that A/Chicken/Vietnam/C-58/04 (A/Ck/Vt/C-58/04) and A/Goose/Thailand/G7CS/04 (A/Gs/Th/G7CS/04) caused moderate interstitial pneumonia (Fig. 3), whereas A/Vt/1203/04 and A/Dk/Th/D4AT/04 caused less severe gross lung damage (data not shown).
TABLE 3.
Tissue titers of H5N1 viruses in pigs on day 6 after inoculation
| Virus | Tissue titer (Log10 EID50/g of wet tissue)
|
||||
|---|---|---|---|---|---|
| Spinal cord | Tonsil | Trachea | Lung | Liverb | |
| A/Vt/1203/04 | −a | 0.5 | 0.5 | 0.5 | 0.33 |
| A/Ck/Vt/C-58/04 | − | − | 0.75 | 0.75 | − |
| A/Dk/Th/D4AT/04 | − | 0.5 | 0.5 | 0.75 | 0.75 |
| A/Gs/Th/G7CS/04 | − | 0.75 | 0.75 | 0.33 | − |
−, no detectable virus.
Intestine, spleen, kidney, and blood were also tested, and virus was not detected in these tissues.
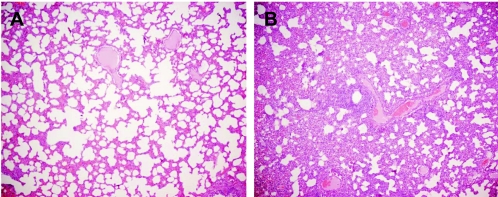
FIG. 3.
Hematoxylin- and eosin-stained sections of lung from an uninoculated pig (A) and a pig infected with A/Vt/1203/04 virus (B). Lung tissues were collected 6 days after inoculation with 3.3 × 106 EID50 of infective virus. In the infected lung tissue (B), the alveoli, interstitial septa, and perivascular spaces are extensively infiltrated by a mixture of inflammatory cells. Magnification, ×4.
In view of the ability of pigs to act as intermediate hosts for human influenza viruses, we considered it urgent to determine whether the circulating H5N1 virus could be transmitted among pigs. Our use of four different, albeit genetically very similar (5), viruses to infect small numbers of animals reduced the statistical power of the experiments, but it increased the possibility of detecting differences in transmissibility between different isolates of the same subtype of virus. None of the four viruses tested transmitted from the infected pig to the contact pig. The experimental infection with the human virus (A/Vt/1203/04 [H5N1]) was repeated with the same results. Therefore, pigs support the replication of the H5N1 virus to modest titers, and the virus is not easily transmissible among pigs under experimental conditions.
We found serological evidence of avian H5N1 influenza virus infection in a very small proportion of pigs in Vietnam. All four of the tested H5N1 viruses isolated in Vietnam and Thailand in 2004 replicated in the inoculated pigs, as another H5 strain was reported to do (3). The virus titers in the nasal swabs were modest, and the viruses induced modest clinical signs that differed with the virus strain, as we have also observed in ferrets (1). We observed virus replication in the respiratory organs of animals inoculated with each of the four viruses, and virus was found in the livers of pigs inoculated with A/Vt/1203/04 and A/Dk/Th/D4AT/04. Neither the human nor the avian H5N1 isolates tested were transmitted to contact animals. It is not clear whether transmission would occur under field conditions (with bacterial coinfection and environmental stresses).
In view of the magnitude of the H5N1 influenza virus outbreak in birds, the small number of human cases to date suggests that H5N1 virus is not easily transmitted from birds to humans at present. Two mechanisms could rapidly improve their transmissibility: (i) the exchange of gene segments by reassortment in humans or pigs simultaneously infected with H5N1 and a circulating human influenza virus that is efficiently transmissible among humans and (ii) mutation of residues in the receptor binding site or other viral proteins during infection of a human or pig.
This study provides preliminary information about the potential ability of pigs to act as intermediate hosts for the H5N1 viruses that recently emerged in Asia. Although we observed no transmission between pigs, the susceptibility of pigs to infection with these viruses suggests that they could serve as intermediate hosts and thereby facilitate the spread of the current strain of H5N1 influenza virus in Asia.
Acknowledgments
These studies were supported by grant AI95357 from the National Institute of Allergy and Infectious Disease and by the American Lebanese Syrian Associated Charities (ALSAC).
We thank Jennifer Humberd and Jon P. Seiler for technical assistance, Carol Walsh for manuscript preparation, and Sharon Naron for editorial assistance.
REFERENCES
- 1.Govorkova, E. A., J. E. Rehg, S. Krauss, H.-L. Yen, Y. Guan, M. Peiris, T. D. Nguyen, T. H. Hanh, P. Puthavathana, H. T. Long, C. Buranathai, W. Lim, R. G. Webster, and E. Hoffmann. 2005. Lethality to ferrets of H5N1 influenza viruses isolated from humans and poultry in 2004. J. Virol. 79:2191-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karasin, A. I., C. W. Olsen, and G. A. Anderson. 2000. Genetic characterization of an H1N2 influenza virus isolated from a pig in Indiana. J. Clin. Microbiol. 38:2453-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kida, H., T. Ito, J. Yasuda, Y. Shimizu, C. Itakura, K. F. Shortridge, Y. Kawaoka, and R. G. Webster. 1994. Potential for transmission of avian influenza viruses to pigs. J. Gen. Virol. 75:2183-2188. [DOI] [PubMed] [Google Scholar]
- 4.Kuiken, T., G. Rimmelzwaan, D. Van Aiel, G. Van Amerongen, M. Baars, R. Fouchier, and A. Osterhaus. 2004. Avian H5N1 influenza in cats. Science 306:241. [DOI] [PubMed] [Google Scholar]
- 5.Li, K. S., Y. Guan, J. Wang, G. J. Smith, K. M. Xu, L. Duan, A. P. Rahardjo, P. Puthavathana, C. Buranathai, T. D. Nguyen, A. T. Estoepangestie, A. Chaisingh, P. Auewarakul, H. T. Long, N. T. Hanh, R. J. Webby, L. L Poon, H. Chen, K. F. Shortridge, K. Y. Yuen, R. G. Webster, and J. S. Peiris. 2004. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature 430:209-213. [DOI] [PubMed] [Google Scholar]
- 6.Peiris, J. S., Y. Guan, D. Markwell, P. Ghose, R. G. Webster, and K. F. Shortridge. 2001. Cocirculation of avian H9N2 and contemporary “human” H3N2 influenza A viruses in pigs in southeastern China: potential for genetic reassortment? J. Virol. 75:9679-9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scholtissek, C. 1990. Pigs as ‘Mixing Vessels’ for the creation of new pandemic influenza A viruses. Med. Principles Pract. 2:65-71. [Google Scholar]
- 8.Shortridge, K. F., W. K. Butterfield, R. G. Webster, and C. H. Campbell. 1977. Isolation and characterization of influenza A viruses from avian species in Hong Kong. Bull. W. H. O. 55:15-20. [PMC free article] [PubMed] [Google Scholar]
- 9.Shortridge, K. F., N. N. Zhou, Y. Guan, P. Gao, T. Ito, Y. Kawaoka, S. Kodihalli, S. Krauss, D. Markwell, K. G. Murti, M. Norwood, D. Senne, L. Sims, A. Takada, and R. G. Webster. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331-342. [DOI] [PubMed] [Google Scholar]
- 10.Sims, L. D., T. M. Ellis, K. K. Liu, K. Dyrting, H. Wong, M. Peiris, Y. Guan, and K. F. Shortridge. 2003. Avian influenza in Hong Kong 1997-2002. Avian Dis. 47(Suppl. 3):832-838. [DOI] [PubMed] [Google Scholar]
- 11.Sturm-Ramirez, K. M., T. Ellis, B. Bousfield, L. Bissett, K. Dyrting, J. E. Rehg, L. Poon, Y. Guan, M. Peiris, and R. G. Webster. 2004. Reemerging H5N1 influenza viruses in Hong Kong in 2002 are highly pathogenic to ducks. J. Virol. 78:4892-48901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wkly. Epidemiol. Rec. 2004. Avian influenza A (H5N1)—situation on 4 February 2004. Wkly. Epidemiol. Rec. 79:53-54. [PubMed] [Google Scholar]
- 13.Zhou, N. N., D. A. Senne, J. S. Landgraf, S. L. Swenson, G. Erickson, K. Rossow, L. Liu, K. J. Yoon, S. Krauss, and R. G. Webster. 2000. Emergence of H3N2 reassortant influenza A viruses in North American pigs. Vet. Microbiol. 74:47-58. [DOI] [PubMed] [Google Scholar]