Abstract
Many viruses and bacteriophage utilize chaperone systems for DNA replication and viral morphogenesis. We have previously shown that in the herpes simplex virus type 1 (HSV-1)-infected cell nucleus, foci enriched in the Hsp70/Hsp40 chaperone machinery are formed adjacent to viral replication compartments (A. D. Burch and S. K. Weller, J. Virol. 78:7175-7185, 2004). These foci have now been named virus-induced chaperone-enriched (VICE) foci. Since the Hsp90 chaperone machinery is known to engage the Hsp70/Hsp40 system in eukaryotes, the subcellular localization of Hsp90 in HSV-1-infected cells was analyzed. Hsp90 is found within viral replication compartments as well as in the Hsp70/Hsp40-enriched foci. Geldanamycin, an inhibitor of Hsp90, results in decreased HSV-1 yields and blocks viral DNA synthesis. Furthermore, we have found that the viral DNA polymerase is mislocalized to the cytoplasm in both infected and transfected cells in the presence of geldanamycin. Additionally, in the presence of an Hsp90 inhibitor, proteasome-dependent degradation of the viral polymerase was detected by Western blot analysis. These data identify the HSV-1 polymerase as a putative client protein of the Hsp90 chaperone system. Perturbations in this association appear to result in degradation, aberrant folding, and/or intracellular localization of the viral polymerase.
Cellular chaperone molecules are employed to maintain protein quality during times of cellular stress. We have recently proposed that the cellular chaperone and proteasomal machinery is utilized during herpes simplex virus type 1 (HSV-1) infection (3). We found that Hsp70 and Hsc70 chaperones as well as the cochaperone Hsp40 are redistributed to foci within the infected-cell nucleus. We now designate these virus-induced chaperone-enriched (VICE) foci. Ubiquitin-conjugated proteins as well as components of the 26S proteasome were also localized to these sites. The VICE foci lie adjacent to viral replication compartments—sites of viral DNA synthesis, morphogenesis, and genome encapsidation (12, 20). A subpopulation of the viral portal protein, a structural component of viral capsids, also localizes to these sites during infection and is a substrate for ubiquitination during infection (3). We propose that the virus has evolved a mechanism to sequester misfolded or modified proteins in such a way as to prevent the triggering of innate antiviral responses. For example, sequestration of certain signals may represent a mechanism to prevent the induction of apoptosis or the unfolded protein response pathway. Given that the Hsp70/Hsp40 chaperone system is known to engage the Hsp90 chaperone machine during specialized interactions with proteins (25), we asked whether Hsp90 was required during HSV-1 infection.
Hsp90 is a multifunctional, complex, and highly specialized chaperone machine that is extremely abundant in most organisms and cell types (reviewed in reference 15). In addition to traditional chaperone activities such as protein folding, Hsp90 can detain nonnative proteins for interaction with other chaperone molecules. It can facilitate the assembly of multiprotein complexes and participate in protein trafficking within the cellular milieu (19). Viral proteins have also been shown to associate with Hsp90. For instance, Hsp90 is required for full activity of the hepatitis B virus reverse transcriptase (9). A number of client proteins have been defined, including steroid hormone receptors, cellular kinases, cytoskeletal proteins, and prolyl isomerases. Hsp90 is also known to facilitate the conformational maturation of many oncogenic proteins, including Her-2, Bcr-Abl, and mutated p53 (reviewed in reference 19). The activity of Hsp90 is modulated by the cofactors with which it interacts, and it becomes “activated” during times of stress. It has been proposed that most soluble Hsp90 found in tumor tissues is “activated.” In the activated state Hsp90 is tightly coupled with other chaperones such as Hsc70/Hsp70 and cochaperones such as Hsp40, p23, and Hop, forming a multichaperone machine (11). In contrast, in normal cells, Hsp90 is not associated with these cofactors, as determined by immunoprecipitation (11). Hsp90 inhibitors, such as geldanamycin (GM) and its derivative 17-AAG, are known to bind to Hsp90 and induce the proteasomal degradation of client proteins (1). These drugs have been shown to have 100-fold-higher affinity for Hsp90 in the “activated” multichaperone complex present in cancerous tissues than for Hsp90 in normal tissues (11). This specificity may explain why Hsp90 inhibitors selectively target and induce apoptosis in tumor tissues but have modest effects in normal cells.
In this paper, the involvement of Hsp90 in HSV-1 infection was studied by determining the subcellular localization of Hsp90 in infected cells and assessing the effect of Hsp90 inhibitors on the progression of viral infection. We found that Hsp90 is localized not only to viral replication compartments but also to the previously described Hsc/Hsp70-enriched foci, now called VICE foci. Furthermore, inhibition of Hsp90 was found to inhibit viral DNA synthesis and to cause the improper localization of the HSV-1 DNA polymerase in both infected and transfected cells. We also found that the viral polymerase was degraded in a proteasome-dependent fashion when the activity of Hsp90 was inhibited. Our studies indicate that HSV-1 employs the Hsp90 chaperone system during infection and that the viral polymerase may be a client protein of Hsp90. We are struck by the observation that, in the infected cell, a subpopulation of Hsp90 chaperone molecules may be in an “activated” state similar to that found in cancer cells. Activation of Hsp90 may thus be a common cellular response to stress, whether oncogenic or viral. This study not only provides information about the basic biology of chaperone-dependent viral processes and cellular responses to stress but also demonstrates that the host-pathogen interface may represent a novel and specific antiviral target.
MATERIALS AND METHODS
Viruses, cells, reagents, and antibodies.
African green monkey kidney cells (Vero CCl81; American Type Culture Collection, Manassas, VA) were propagated and maintained as described previously (26). The KOS strain of HSV-1 was used as the wild-type virus. Virus titrations and plaque assays have been described previously (21). Transfections were performed using the Lipofectamine 2000 transfection kit (Gibco BRL) according to the manufacturer's instructions. In transfection analyses where the localization of viral proteins was analyzed, 1 μM geldanamycin was added to cells after a 3-h incubation period with the DNA/transfection reagent. The ICP8 mouse monoclonal antibody (αICP8; also called 39S) has been described by Showalter et al. (22). The UL30 mouse monoclonal antibody (αUL30; also called Mab1051c) was a kind gift from Charles W. Knopf (Deutsches Krebsforschungszentrum) and has been described previously (24). The rat monoclonal anti-Hsc70 (SPA-815), and rabbit polyclonal anti-Hsp90 (SPA-846) antibodies were purchased from StressGen (Victoria, British Columbia, Canada). Primary antibodies were used at dilutions of 1:500 (αICP8, αHsc70, and αHsp90) and 1:200 (αUL30). Secondary antibodies were purchased from Molecular Probes (Eugene, OR) and include AlexaFluor 488-conjugated goat anti-mouse, AlexaFluor 546-conjugated goat anti-rat, and AlexaFluor 647-conjugated goat anti-rabbit antibodies. These fluorophores were chosen to maximize the spectral separation of the emission wavelengths in order to limit the amount of overlapping signal in triple-labeling experiments. We found that it was necessary to use commercially available highly cross-adsorbed secondary antibodies to prevent cross-reactivity between rat and mouse primary antibodies. Geldanamycin and its derivative 17-AAG were purchased from InvivoGen (San Diego, CA). Unless otherwise noted, a concentration of 1 μM of the Hsp90 inhibitors was used in the studies reported in this paper.
Immunofluorescence confocal microscopy.
Cells were prepared for immunofluorescence microscopy as described by Burch and Weller (3). Fluorescence microscopy was performed using the Zeiss LSM 410 confocal microscope with a 100× objective. Adobe Photoshop 5.0 was used for image preparation for figures.
Western and Southern blot analysis and PFGE.
Western blot analysis was performed as previously described (3). Quantification was performed using ImageQuant software (Amersham Biosciences). Southern blot analysis and pulsed-field gel electrophoresis (PFGE) were performed as described by Martinez et al. (15). The probe used for Southern blot analysis was generated using the Random Prime DNA labeling kit (Invitrogen).
RESULTS
Hsp90 is concentrated within viral replication compartments and VICE foci.
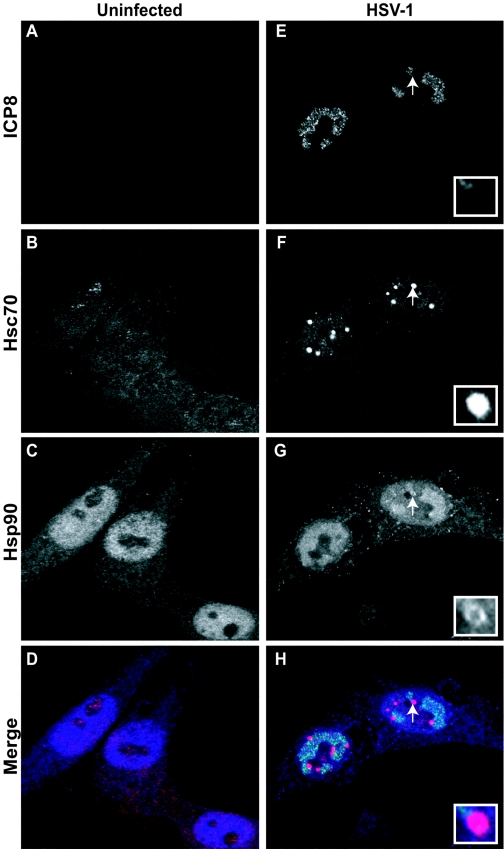
HSV-1 DNA replication and virus assembly occur in large globular domains (called replication compartments) that are formed in the nucleus of the infected cell (12, 20). We have previously shown that Hsc/Hsp70 and Hsp40 molecules are sequestered into foci adjacent to viral replication compartments and that a subpopulation of the viral portal protein UL6 that may be misfolded or modified is found at these sites (3). Since it is well documented that the Hsp90 chaperone system can coordinate with Hsc70, Hsp70, and Hsp40 to perform alternative/specialized functions during the response to stress, we examined the subcellular localization of Hsp90 molecules in uninfected and HSV-1-infected cells (Fig. 1A to H). Cells were prepared for immunofluorescence microscopy as described in Materials and Methods. Fixed cells were triple labeled with antibodies recognizing the viral ICP8 protein and cellular Hsc70 and Hsp90 proteins. As expected, no ICP8 staining was detected in the uninfected cells (Fig. 1A). The staining pattern for Hsc70 in uninfected cells was faint in both the nuclear and cytoplasmic compartments, with some concentration in the nucleolus (Fig. 1B). Hsp90 staining in these cells is predominantly diffuse nuclear staining; however, some diffuse staining was also observed in the cytoplasm (Fig. 1C). This result is consistent with previous reports indicating that Hsp90 is both cytoplasmic and nuclear, although in our hands more Hsp90 is seen in the nucleus compared to previous reports showing predominantly cytoplasmic staining (16). This discrepancy may be related to tissue variability or slight differences in fixation or permeabilization. The merged image of uninfected cells is shown in Fig. 1D. In HSV-1-infected cells, replication compartments can be readily detected using an antibody specific to the viral single-stranded DNA binding protein ICP8 (Fig. 1E). As previously shown, Hsc70 accumulates at VICE foci adjacent to replication compartments (Fig. 1F). Interestingly, in the infected cell, Hsp90 molecules could be detected within viral replication compartments and at the VICE foci outside of replication compartments, as well as faintly in the cytoplasm (Fig. 1G). It can be seen that colocalization of Hsp90 at the VICE foci was not complete; instead, Hsp90 staining appeared to surround the VICE foci (Fig. 1G, inset). In the merged image, colocalization between Hsc70 and Hsp90 at the foci is observed as magenta (Fig. 1H). Dual-labeling experiments were used to confirm the presence of Hsp90 in viral replication compartments and VICE foci (data not shown). These results indicate that molecules of the mammalian chaperone Hsp90 in the infected-cell nucleus are localized to viral replication compartments as well as the previously described VICE foci that lie adjacent to viral replication compartments.
FIG. 1.
Subcellular localization of viral and cellular chaperones in the HSV-1-infected cell. (A to D) Uninfected cells; (E to H) HSV-1-infected cells. Shown are staining profiles for the viral single-stranded DNA binding protein ICP8 (A and E) (green in merged image), for the cellular chaperone Hsc70 (B and F) (red in merged image), and for Hsp90 (shown in blue in panels C and G) (blue in merged image), as well as merged images of triple-labeled cells (D and H). White arrows and insets in panels F through H show the localization pattern of Hsp90 at the VICE foci.
Hsp90-specific inhibitors inhibit HSV-1 infection at very early times.
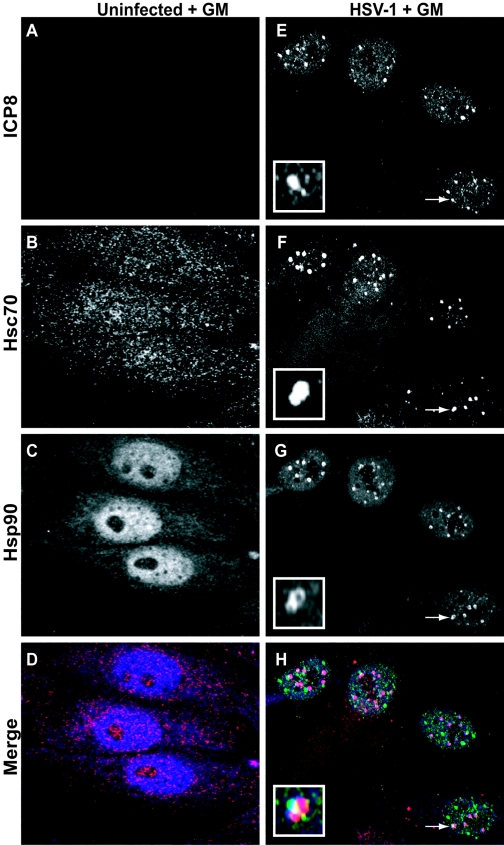
The naturally occurring antibiotic GM is a highly specific inhibitor of Hsp90 which promotes the degradation of Hsp90 “client” proteins (1, 17, 28). To determine if the presence of geldanamycin affected the progression of HSV-1 infection, we analyzed infected cells using confocal immunofluorescence microscopy. These results are shown in Fig. 2A to H. Geldanamycin did not appear to have a dramatic effect on the localization of Hsc70 or Hsp90 in uninfected cells (Fig. 2B and C, respectively). In infected cells treated with GM, Hsc70 staining was observed in the cytoplasm and more strongly within the VICE foci (Fig. 2F). Interestingly, in HSV-1-infected cells that were treated with GM, no replication compartments were observed (Fig. 2E). Instead, staining for the viral protein ICP8 was observed in a punctate pattern within the nucleus reminiscent of early “prereplicative” sites that are formed in HSV-infected cells in which DNA synthesis is impaired (4, 5, 20). The foci seen in Fig. 2E are reminiscent of stage III foci (or prereplicative sites) reported by Burkham et al. (4). It was observed that the ICP8 foci were often adjacent to or colocalizing with the chaperone-enriched VICE foci; however, the significance of this juxtaposition remains to be determined (Fig. 2E to H and insets). The appearance of VICE foci even in the absence of viral replication compartments was expected, because we have previously shown that chaperone redistribution during infection is independent of DNA synthesis and dependent only on the expression of the viral immediate-early protein ICP0 (3). In infected cells treated with GM, Hsp90 staining was predominantly concentrated within the nucleus and included punctate domains which colocalized with Hsc70 (VICE foci) (Fig. 2G and H). Taken together, these results indicate that when the activity of Hsp90 is inhibited, HSV-1 infection is arrested at very early times at or before DNA synthesis.
FIG. 2.
Effect of geldanamycin on the subcellular localization of viral and cellular chaperones in the HSV-1-infected cell. (A to D) Uninfected cells treated with GM; (E to H) HSV-1-infected GM-treated cells. Shown are staining profiles for the viral single-stranded DNA binding protein ICP8 (A and E) (green in merged image), for the cellular chaperone Hsc70 (B and F) (red in merged image), and for Hsp90 (C and G) (blue in merged image), as well as merged images of triple-labeled GM-treated cells (D and H). White arrows in panels E to H indicate an ICP8 focus that is adjacent to and colocalized with a chaperone-enriched focus. This is shown enlarged within the inset in each panel.
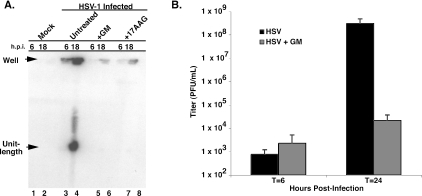
Because only prereplicative sites were observed in the presence of the Hsp90 inhibitor, we hypothesized that DNA synthesis may be impaired. During HSV infection, viral DNA is replicated by a mechanism that is thought to involve both recombination and replication (29). These processes result in larger than-unit-length DNA molecules that are then cleaved into unit-length molecules during the DNA cleavage and packaging reaction. Replication intermediates can be analyzed using pulsed-field gel electrophoresis and Southern blot analysis. Replicating DNA (composed of greater-than-unit-length, branched molecules) does not enter the gel, staying behind in the well (“well” DNA), whereas unit-length linear molecules of 152 kb are capable of migrating into the gel (7, 15). Viral DNA from HSV-1-infected cells in the presence and absence of GM was analyzed by PFGE and Southern blot analysis (Fig. 3A). In untreated infected cells, a modest accumulation of “well” DNA molecules is observed at 6 h (Fig. 3A, lane 3). At 18 h postinfection, both “well” and unit-length DNA can be detected (Fig. 3A, lane 4). On the other hand, very little “well” DNA is observed at 6 h postinfection in infected cells treated with GM or its derivative 17-AAG (Fig. 3A, lanes 5 and 7, respectively). Furthermore, in the presence of GM or 17-AAG, at 18 h postinfection only a small amount of replicating, or “well,” DNA is observed, indicating that DNA synthesis is impaired (Fig. 3A, lanes 6 and 8). As expected, no viral DNA was detected in uninfected cells harvested at 6 and 18 h postinfection (Fig. 3A, lanes 1 and 2, respectively). We also analyzed the effect of GM on total viral yields (Fig. 3B). Consistent with the results of Li et al. (13), we found that virus yield was reduced in a dose-dependent manner (data not shown). In cells treated with 1 μM GM, we consistently observed a several-log-unit reduction in the amount of virus produced after 24 h (Fig. 3B). Collectively, these results indicate that Hsp90 is needed for efficient viral DNA replication and production of progeny virus.
FIG. 3.
Infected cells treated with geldanamycin show reduced viral DNA production and virus yield. (A) Viral DNA synthesis is impaired in cells treated with Hsp90 inhibitors. Total viral DNA isolated from infected cells at 6 and 18 h postinfection was subjected to PFGE and Southern blotting. Samples include uninfected cells (lanes 1 and 2), HSV-1-infected cells (lanes 3 and 4), HSV-1-infected cells treated with GM (lanes 5 and 6), and HSV-1-infected cells treated with a derivative of GM, 17AAG (lanes 7 and 8). Newly replicated viral DNA molecules (“Well”) are large, probably branched molecules that fail to enter the pulsed-field gel. Unit-length molecules (152 kb) are readily observed at 18 h postinfection in wild-type HSV-1 infection (lane 4). DNA synthesis is severely impaired in cells treated with either GM or 17AAG (lanes 5 to 8). No signal is observed in the mock-infected samples. (B) Virus yield is reduced in cells treated with geldanamycin. HSV-1-infected Vero cells were incubated in the absence (black bar) or presence (dark gray bar) of 1.0 μM geldanamycin for 6 or 24 h. Infections were performed in triplicate. Virus titers were determined by plaque assay as described in Materials and Methods.
Treatment of HSV-1-infected cells with geldanamycin results in the aberrant localization and degradation of the viral polymerase UL30.
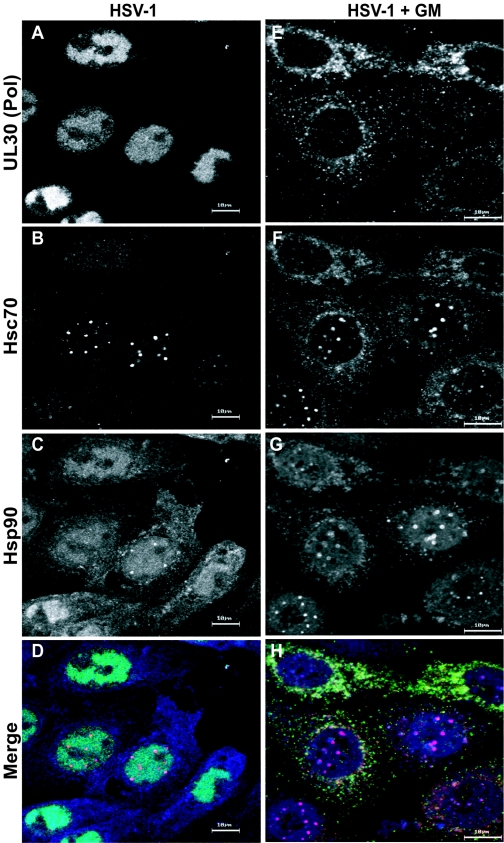
Using [35S]methionine labeling, we analyzed the overall effect of GM on viral protein synthesis. We found that the synthesis of immediate-early and early proteins was comparable in untreated and treated cells, but synthesis of late proteins such as VP5, whose induction is stimulated by DNA synthesis, was reduced (data not shown). Since Hsp90 inhibitors block HSV-1 prior to productive DNA synthesis and several of the known client proteins for Hsp90 are polymerases, we were interested in whether the viral polymerase (UL30) was affected in GM-treated infected cells. Using an antibody specific to the HSV-1 viral polymerase, we analyzed its subcellular localization in cells treated with GM. As previously demonstrated, in HSV-1-infected cells, the viral polymerase UL30 is observed completely within viral replication compartments (24) (Fig. 4A). As shown above, in HSV-1-infected cells, Hsc70 accumulates within foci juxtaposed to viral replication compartments (Fig. 4B) and Hsp90 is observed within the replication compartments and the VICE foci (Fig. 4C). The merged image is shown in Fig. 4D. In contrast, the localization of the viral polymerase is dramatically affected by GM treatment: the viral polymerase is detected entirely in the cytoplasm (Fig. 4E). Some Hsc70 and Hsp90 staining is observed in the cytoplasm in cells treated with GM and appears to colocalize with the polymerase (Fig. 4F and G, respectively). The merged image of these cells is shown in Fig. 4H. Thus, we conclude that when the activity of Hsp90 is inhibited, the viral polymerase is not able to localize to the nucleus in infected cells.
FIG. 4.
The viral polymerase is mislocalized in infected cells treated with the Hsp90 inhibitor geldanamycin. (A to D) Untreated HSV-1-infected cells; (E to H) HSV-1-infected cells treated with GM. Shown are staining profiles for the viral DNA polymerase UL30 (A and E) (green in the merged image), for the cellular chaperone Hsc70 (B and F) (red in the merged image), and for Hsp90 (C and G) (blue in the merged image), as well as merged images of triple-labeled cells (D and H). Bars, 10 μm.
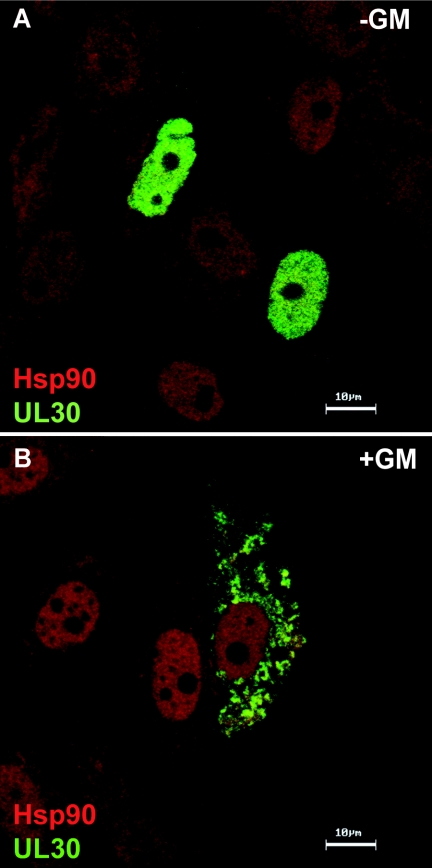
It has been shown previously that the viral polymerase can target to the nucleus in the absence of other viral proteins (11). We were interested in whether treatment of cells with the Hsp90 inhibitor affected this localization pattern. Vero cells were transfected with a plasmid expressing the viral polymerase gene and were prepared for immunofluorescence microscopy as described in Materials and Methods (Fig. 5). As previously observed, the localization of Hsp90 is similar (diffuse nuclear staining with some cytoplasmic staining) in untreated and geldanamycin-treated cells (Fig. 5A and B). Consistent with previous reports, in untreated cells (Fig. 5A), the staining pattern for the viral polymerase is restricted to the nuclei of transfected cells. On the other hand, in cells treated with geldanamycin, the viral polymerase remains in the cytoplasm. This result suggests that inhibition of the Hsp90 chaperone machinery results in the cytoplasmic mislocalization of the viral polymerase in transfected cells in the absence of other viral proteins. Treatment of transfected cells with geldanamycin did not affect the nuclear localization of the viral ICP8 protein (data not shown), indicating that transport in general was not impaired.
FIG. 5.
Hsp90 inhibitors alter the localization of the viral polymerase in transfected cells. Cells were transfected with a plasmid expressing the viral polymerase (UL30) and then prepared for immunofluorescence confocal microscopy as described in Materials and Methods. Cells were stained with a monoclonal antibody specific to the viral polymerase (green) and an Hsp90 polyclonal antibody (red). Merged images of untreated and treated cells are shown in panels A and B, respectively. In untreated cells, the viral polymerase localizes to the nucleus (A) as previously described (11). In cells treated with the inhibitor of Hsp90, staining for the viral polymerase is observed within the cytoplasm (B), which is consistent with its localization in infected cells treated with geldanamycin.
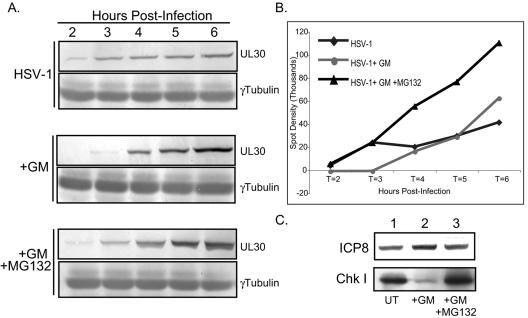
The interaction between Hsp90 and a client protein often stabilizes and protects the target protein from degradation by the proteasome. When the activity of Hsp90 is blocked with geldanamycin, often the client protein is released from Hsp90 and degraded by the proteasome (2, 17). The degradation of a protein in the presence of an Hsp90 inhibitor supports the contention that that protein is a bona fide client of the Hsp90 chaperone machine. We analyzed the fate of the viral polymerase during infection of cells treated with geldanamycin by Western blot analysis. In untreated cells, the viral polymerase can be detected at 2 h postinfection, and its levels increase during the early hours of infection (Fig. 6A, top). When cells are treated with GM, the levels of the viral polymerase at 2 and 3 h are dramatically reduced (Fig. 6A, center). The levels of the viral polymerase remain at wild-type amounts in cells treated with both GM and the proteasome inhibitor MG132 (Fig. 6A, bottom). This trend is also reflected when the density of the data from the Western blots is quantified (Fig. 6B). GM treatment does not affect the levels of other viral proteins such as ICP8 (Fig. 6C) or the scaffolding protein VP22a (A. Nellissery, J. Nellissery, R. Szczepaniak, A. D. Burch, and S. K. Weller, unpublished data). As a positive control, the GM-dependent degradation of Chk I, a cellular protein known to be a client protein of Hsp90 (2), was monitored by Western blot analysis (Fig. 6C). Western blots of gamma tubulin were used as controls for loading. Interestingly, in geldanamycin-treated cells, the viral polymerase could be detected at 4, 5, and 6 h postinfection by Western blotting (Fig. 6A, center) and immunofluorescence studies (Fig. 4E). Thus, it is possible that degradation is incomplete at these time points. Collectively, these results suggest that the HSV-1 polymerase is likely a bona fide client protein of the Hsp90 chaperone system and that when this interaction is inhibited, the polymerase is degraded via the cellular proteasome.
FIG. 6.
The HSV-1 viral polymerase UL30 is degraded in geldanaymicin-treated cells. (A) Western blot analysis of the viral polymerase UL30 and γ-tubulin in HSV-1-infected cells that were either left untreated, treated with GM alone, or treated with both GM and the proteasome inhibitor MG132. (B) Quantification of the spot densities from the Western blot analysis for which results are shown in panel A. (C) Western blot analysis of the viral ICP8 protein and Chk I, a known client protein of the Hsp90 chaperone, in untreated (lane 1), GM-treated (land 2), and GM-plus-MG132-treated (lane 3) cells.
DISCUSSION
In this report, we provide evidence that the mammalian chaperone Hsp90 is reorganized during HSV infection. Some Hsp90 is observed in replication compartments, which are sites of viral DNA synthesis, virus assembly, and DNA packaging (12, 20), and some is localized in the previously described VICE foci adjacent to replication compartments that are enriched for Hsc70, Hsp70, and Hsp40 (3). In addition, in the presence of Hsp90 inhibitors, viral DNA synthesis is impaired and viral DNA polymerase, UL30, is aberrantly localized to the cytoplasm in infected and transfected cells. Furthermore, the viral polymerase is degraded in a proteasome-dependent fashion in GM-treated cells, indicating that it is a bona fide client protein of Hsp90. Collectively, these results suggest that (i) Hsp90 is required for viral infection, (ii) some Hsp90 may be in an “activated” state similar to that seen in cancer cells, in which it associates with other chaperone molecules, and (iii) the Hsp90 chaperone machinery may be involved in interactions with the viral polymerase required for proper localization to the nucleus and/or proper function.
Our studies suggest that there may be several states of Hsp90 in HSV-1-infected cells: (i) Hsp90 within replication compartments, (ii) Hsp90 at the Hsc70-enriched VICE foci, and (iii) cytoplasmic Hsp90. The role of Hsp90 within the replication compartment is unclear, but it is tempting to speculate that this specialized chaperone machinery may actively participate in the DNA replication process, as has been seen with other viral systems. Most notably, Hsp90 has been shown to participate in essential interactions with the duck hepatitis B virus reverse transcriptase in vivo and in vitro (8-10). Hepatitis B virus reverse transcription begins with a protein-priming step. It has been shown that interactions with Hsp90 are required to help the viral polymerase achieve a specific conformation required for the initial protein-priming step. Based on studies with the Hsp90 inhibitor GM, we propose that Hsp90 is required for proper localization and stability of the viral polymerase. It is possible, however, that Hsp90 also plays an active role in the reactions carried out by HSV-1 polymerase in replication compartments.
A subpopulation of Hsp90 was also detected outside of viral replication compartments at VICE foci that costain with the cellular Hsc70 chaperone (Fig. 1H). These sites are probably analogous to sites we previously described that are enriched not only for chaperone and cochaperone molecules but also for ubiquitinated proteins and components of the 26S proteasome (3). Furthermore, we have observed that specific viral proteins are found at this site. A subpopulation of the viral portal proteins (UL6) localizes to these sites during infection (3). Additionally, UL6 was shown to be a substrate for ubiquitination during infection. Interestingly, cellular proteins such as endogenous hyperphosphorylated replication protein A, a marker for DNA damage, are also detected at these sites at early times during infection (D. E. Wilkinson and S. K. Weller, unpublished data). We speculate that these sites act to sequester misfolded or otherwise deleterious proteins away from replication compartments. The localization of Hsp90 at these foci in the HSV-1-infected cell is suggestive and may indicate that it is in an “activated” conformation. Additional experiments are required to confirm this notion. Hsp90 coupled with Hsc/Hsp70 is known to target proteins for proteasomal degradation (1, 18). Thus, we speculate that Hsp90 found at the VICE foci in the HSV-1-infected cell may perform a similar role and could participate in the targeted degradation of viral or cellular proteins. This process could serve as an elegant regulatory mechanism to clear the viral replication compartment of unfolded, modified, or otherwise undesirable proteins, perhaps in an effort to delay apoptosis. It is possible that this may be a mechanism shared by several viral systems. In adenovirus-infected cells, specific components of the cellular DNA damage machinery, which are known to be inactivated during infection, are localized in foci juxtaposed to replication centers (23). It is possible that this is a mechanism to sequester these components away from sites of DNA replication. It would be interesting to determine whether these sites are analogous to the chaperone-enriched VICE foci observed in HSV-1-infected cells.
Understanding of the many complex roles of Hsp90 has been aided by the discovery and development of small molecules that specifically target and inhibit the Hsp90 chaperone machinery. Geldanamycin is a naturally occurring molecule that specifically binds the ADP/ATP-binding cleft of Hsp90 and destabilizes its interaction with client proteins. We have confirmed the results of Li et al. showing that geldanamycin inhibits HSV-1 replication in tissue culture (13). Our results suggest that the viral polymerase may be a client protein of the Hsp90 chaperone machine and that geldanamycin inhibits HSV DNA replication by blocking Hsp90-polymerase interactions required for nuclear targeting. The Hsp90 chaperone machinery is known to be involved in the trafficking and localization of specific proteins. For example, Hsp90-directed translocation of the glucocorticoid hormone receptor (GR) into the nucleus requires hormone and can be blocked by geldanamycin (6, 27). This led to the proposal that Hsp90 interacts with GR to help it achieve a conformation necessary for nuclear import. It has been shown that the HSV-1 polymerase possesses signal sequences required for nuclear localization (14). Interactions with Hsp90 chaperone molecules may alter the conformation of the viral polymerase to expose the nuclear localization signal. Alternatively, it is possible that Hsp90 directly escorts the polymerase into the nucleus and functions there to enhance polymerase activity. The localization of Hsp90 in the replication compartments may favor the latter possibility. It is possible that Hsp90 not only participates in nuclear import but also enhances polymerase activity. Additional experiments are needed to test these hypotheses.
Our studies reveal an unexpected requirement for the Hsp90 chaperone machinery during HSV-1 infection. This work provides information about the host-pathogen interface and may reveal new targets for antiviral therapies. We are struck by the observation that geldanamycin and its analogs can specifically kill tumor cells, and geldanamycin is under investigation as an anticancer treatment (21). The finding that this compound also has antiviral properties may indicate that the response of cells to various types of stress, whether cancer or viral infection, shares common pathways. This unexpected relationship between cancer and viral infection underscores the value of using viruses to study complex cellular processes.
Acknowledgments
We are grateful to all of the members of the Weller laboratory for helpful discussions and constructive input. We are especially thankful to the laboratory of Pramod Srivastava at UCHC Center for Immunotherapy for providing initial aliquots of various chaperone antibodies used during these studies. We also thank Zihai Li and Antoine Menoret at the UCHC Center for Immunotherapy for helpful discussions related to this work.
This work was supported by NIH grant F32 AI50336 to A.D.B. and NIH grants AI37549 and AI21747 to S.K.W.
REFERENCES
- 1.An, W. G., T. W. Schulte, and L. M. Neckers. 2000. The heat shock protein 90 antagonist geldanamycin alters chaperone association with p210bcr-abl and v-src proteins before their degradation by the proteasome. Cell Growth Differ. 11:355-360. [PubMed] [Google Scholar]
- 2.Arlander, S. J., A. K. Eapen, B. T. Vroman, R. J. McDonald, D. O. Toft, and L. M. Karnitz. 2003. Hsp90 inhibition depletes Chk1 and sensitizes tumor cells to replication stress. J. Biol. Chem. 278:52572-52577. [DOI] [PubMed] [Google Scholar]
- 3.Burch, A. D., and S. K. Weller. 2004. Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection. J. Virol. 78:7175-7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkham, J., D. M. Coen, and S. K. Weller. 1998. ND10 protein PML is recruited to herpes simplex virus type 1 prereplicative sites and replication compartments in the presence of viral DNA polymerase. J. Virol. 72:10100-10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrington-Lawrence, S. D., and S. K. Weller. 2003. Recruitment of polymerase to herpes simplex virus type 1 replication foci in cells expressing mutant primase (UL52) proteins. J. Virol. 77:4237-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czar, M. J., M. D. Galigniana, A. M. Silverstein, and W. B. Pratt. 1997. Geldanamycin, a heat shock protein 90-binding benzoquinone ansamycin, inhibits steroid-dependent translocation of the glucocorticoid receptor from the cytoplasm to the nucleus. Biochemistry 36:7776-7785. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein, J. N., and S. K. Weller. 1998. In vitro processing of herpes simplex virus type 1 DNA replication intermediates by the viral alkaline nuclease, UL12. J. Virol. 72:8772-8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu, J., and D. Anselmo. 2000. In vitro reconstitution of a functional duck hepatitis B virus reverse transcriptase: posttranslational activation by Hsp90. J. Virol. 74:11447-11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu, J., D. Toft, D. Anselmo, and X. Wang. 2002. In vitro reconstitution of functional hepadnavirus reverse transcriptase with cellular chaperone proteins. J. Virol. 76:269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu, J., D. O. Toft, and C. Seeger. 1997. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 16:59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamal, A., L. Thao, J. Sensintaffar, L. Zhang, M. F. Boehm, L. C. Fritz, and F. J. Burrows. 2003. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407-410. [DOI] [PubMed] [Google Scholar]
- 12.Lamberti, C., and S. K. Weller. 1998. The herpes simplex virus type 1 cleavage/packaging protein, UL32, is involved in efficient localization of capsids to replication compartments. J. Virol. 72:2463-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li, Y. H., P. Z. Tao, Y. Z. Liu, and J. D. Jiang. 2004. Geldanamycin, a ligand of heat shock protein 90, inhibits the replication of herpes simplex virus type 1 in vitro. Antimicrob. Agents Chemother. 48:867-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loregian, A., E. Piaia, E. Cancellotti, E. Papini, H. S. Marsden, and G. Palu. 2000. The catalytic subunit of herpes simplex virus type 1 DNA polymerase contains a nuclear localization signal in the UL42-binding region. Virology 273:139-148. [DOI] [PubMed] [Google Scholar]
- 15.Martinez, R., R. T. Sarisky, P. C. Weber, and S. K. Weller. 1996. Herpes simplex virus type 1 alkaline nuclease is required for efficient processing of viral DNA replication intermediates. J. Virol. 70:2075-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng, X., J. Devin, W. P. Sullivan, D. Toft, E. E. Baulieu, and M. G. Catelli. 1996. Mutational analysis of Hsp90 alpha dimerization and subcellular localization: dimer disruption does not impede “in vivo” interaction with estrogen receptor. J. Cell Sci. 109:1677-1687. [DOI] [PubMed] [Google Scholar]
- 17.Mimnaugh, E. G., C. Chavany, and L. Neckers. 1996. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J. Biol. Chem. 271:22796-22801. [DOI] [PubMed] [Google Scholar]
- 18.Neckers, L. 2002. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 8:S55-S61. [DOI] [PubMed] [Google Scholar]
- 19.Neckers, L., and S. P. Ivy. 2003. Heat shock protein 90. Curr. Opin. Oncol. 15:419-424. [DOI] [PubMed] [Google Scholar]
- 20.Quinlan, M. P., L. B. Chen, and D. M. Knipe. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36:857-868. [DOI] [PubMed] [Google Scholar]
- 21.Sausville, E. A., J. E. Tomaszewski, and P. Ivy. 2003. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr. Cancer Drug Targets 3:377-383. [DOI] [PubMed] [Google Scholar]
- 22.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stracker, T. H., C. T. Carson, and M. D. Weitzman. 2002. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418:348-352. [DOI] [PubMed] [Google Scholar]
- 24.Strick, R., J. Hansen, R. Bracht, D. Komitowski, and C. W. Knopf. 1997. Epitope mapping and functional characterization of monoclonal antibodies specific for herpes simplex virus type I DNA polymerase. Intervirology 40:41-49. [DOI] [PubMed] [Google Scholar]
- 25.Wegele, H., L. Muller, and J. Buchner. 2004. Hsp70 and Hsp90—a relay team for protein folding. Rev. Physiol. Biochem. Pharmacol. 151:1-44. [DOI] [PubMed] [Google Scholar]
- 26.Weller, S. K., A. Spadaro, J. E. Schaffer, A. W. Murray, A. M. Maxam, and P. A. Schaffer. 1985. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol. Cell. Biol. 5:930-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitesell, L., and P. Cook. 1996. Stable and specific binding of heat shock protein 90 by geldanamycin disrupts glucocorticoid receptor function in intact cells. Mol. Endocrinol. 10:705-712. [DOI] [PubMed] [Google Scholar]
- 28.Whitesell, L., P. D. Sutphin, E. J. Pulcini, J. D. Martinez, and P. H. Cook. 1998. The physical association of multiple molecular chaperone proteins with mutant p53 is altered by geldanamycin, an hsp90-binding agent. Mol. Cell. Biol. 18:1517-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson, D. E., and S. K. Weller. 2003. The role of DNA recombination in herpes simplex virus DNA replication. IUBMB Life 55:451-458. [DOI] [PubMed] [Google Scholar]