Abstract
Alpha/beta interferons (IFN-α/β) are key mediators of innate immunity and important modulators of adaptive immunity. The mechanisms by which IFN-α/β are induced are becoming increasingly well understood. Recent studies showed that Toll-like receptors 7 and 8 expressed by plasmacytoid dendritic cells (pDCs) mediate the endosomal recognition of incoming viral RNA genomes, a process which requires myeloid differentiation factor 88 (MyD88). Here we investigate the requirements for virus-induced IFN-α/β production in cultures of bone marrow-derived murine myeloid DCs (mDCs). Using recombinant Semliki Forest virus blocked at different steps in the viral life cycle, we show that replication-defective virus induced IFN-α/β in mDCs while fusion-defective virus did not induce IFN-α/β. The response to replication-defective virus was largely intact in MyD88−/− mDC cultures but was severely reduced in mDC cultures from mice lacking IFN regulatory factor 3. Our observations suggest that mDCs respond to incoming virus via a pathway that differs from the fusion-independent, MyD88-mediated endosomal pathway described for the induction of IFN-α/β in pDCs. We propose that events during or downstream of viral fusion, but prior to replication, can activate IFN-α/β in mDCs. Thus, mDCs may contribute to the antiviral response activated by the immune system at early time points after infection.
Alpha/beta interferons (IFN-α/β) are part of a first line of defense against viral infection and are important modulators of adaptive immunity (27, 28, 38, 39, 52, 54). The early recognition of virus and induction of IFN-α/β are critical for limiting infection by and spread of virus. Most cells have the capacity to produce IFN-α/β and to respond to a virus infection by phosphorylating IFN regulatory factor 3 (IRF-3). Activated IRF-3 is translocated into the nucleus, where it binds DNA and drives the expression of early IFN-α/β subtypes (31, 44, 70, 73, 74). Their secretion and subsequent engagement of IFN-α/β receptor (IFN-AR1) stimulate the production of several secondary response genes, including the IRF-7 gene, which is required for the transcription of additional IFN-α/β subtypes (7, 40, 61). Thus, signaling via IFN-AR1 primes the cells for increased IFN-α/β production, amplifying the antiviral response.
In addition to the classical pathway for IFN-α/β induction, several recent reports have shown that immune cells such as dendritic cells (DCs) and macrophages act as early sentinels of virus infection that are capable of producing IFN-α/β via additional mechanisms (9). For example, viruses may be recognized through specific interactions between their envelope glycoproteins and cell surface-expressed Toll-like receptors (TLRs) (11, 37) or through the recognition of viral genomes by TLRs present in the endosomal compartment (12, 21, 36, 47, 48). The latter may be a general mechanism by which viruses are recognized independent of replication and is a function ascribed to plasmacytoid DCs (pDCs), a DC subset known to produce high levels of IFN-α in response to virus (3). The constitutive expression of IRF-7 in pDCs (20, 25, 29) permits these cells to produce large amounts of IFN-α, even in the absence of priming through IFN-AR1. Specifically, it has been shown that viral single-stranded RNA (ssRNA) is a ligand for TLR7/8 (12, 21, 48) and that unmethylated viral dsDNA is a ligand for TLR9 (36, 47). The induction of IFN-α through TLR7, TLR8, and TLR9 is dependent on signaling through myeloid differentiation factor 88 (MyD88) (12, 33), which forms a complex with IRF-7 and TRAF6 (33).
Myeloid DCs (mDCs) are also capable of IFN-α/β production in response to replicating virus (13, 22, 45, 46, 55, 57). However, only a few reports (45, 46, 55) describe an ability of mDCs to produce IFN-α/β in response to virus independent of replication. The relative abundance of mDCs in vivo is higher than that of pDCs (63), and we therefore wished to investigate if mDCs are capable of early IFN-α/β induction. It is of interest to further characterize the events that lead to the first wave of IFN-α/β production in virus-exposed mDCs and to understand the cellular pathways involved in this response.
We addressed these issues by using Semliki Forest virus (SFV), a positive-strand RNA virus of the Alphavirus genus and a frequently used model virus. We have previously developed a method for generating suicidal recombinant SFV particles (rSFV), in which recombinant vector RNA is packaged using helper constructs encoding the viral structural proteins (42, 64). Cells infected with rSFV produce large amounts of vector-encoded heterologous protein, and rSFV is currently being developed as a platform for recombinant vaccines. To investigate how rSFV induces IFN-α/β production, we used viral particles with different functional properties. The wild-type SFV genome was packaged using fusion-defective envelope glycoproteins, creating a conditionally infectious virus (nrSFV) (8). Proteolytic activation of nrSFV yields a fully infectious virus (rSFV), which subsequently can be rendered replication defective through UV inactivation (UV-rSFV). This strategy allowed us to study viruses that originated from the same preparation, thus providing a well-controlled system for studying the virus-specific IFN-α/β response. Specifically, this system allowed us to address whether early viral functions could stimulate an IFN-α/β response in mDCs.
The results presented here show that UV inactivation did not abolish the ability of the virus to induce IFN-α/β in mDCs in vitro and systemically in vivo. We demonstrate that IFN-α/β induction by mDCs requires the entry of fusion-competent virus particles into a low-pH compartment. Interestingly, MyD88 is not required for this response, indicating that TLR7 is not involved. By using IRF-3−/− mDC cultures, we show a dependence on IRF-3 for early IFN-α/β induction by UV-inactivated virus. Collectively, our results demonstrate that mDCs respond to nonreplicating virus using a different pathway than that described for pDCs, adding to the current understanding of IFN-α/β induction in response to early events during viral infection.
MATERIALS AND METHODS
Cell lines and virus.
BHK-21 cells (American Type Culture Collection) were cultured in Glasgow minimal essential medium (MEM) supplemented with 5% fetal calf serum (FCS), 10 mM HEPES, 10% tryptose phosphate, 2 mM l-glutamine, penicillin, and streptomycin (Invitrogen, Carlsbad, California). Mouse embryo fibroblasts (MEFs) were obtained from 12- to 13-day-old C57BL/6 fetuses. Each batch was kept in culture for no more than 1 month. L929 cells (American Type Culture Collection) and MEFs were cultured in Dulbecco's modified Eagle's medium (Sigma, St. Louis, Mo.) supplemented with 10% FCS, l-glutamine, penicillin, and streptomycin. Single-round infectious rSFV was produced by cotransfecting pSFV-EGFP with split-helper constructs (64) or, for conditionally infectious particles (nrSFV), with pSFV-Helper-2-derived RNA (8). The ability of nrSFV to bind cells has been shown to be somewhat reduced compared to that of wild-type (wt) SFV, but this defect is small at a physiological pH (68, 75). The viruses were generated from the nrSFV stock by taking an aliquot from nrSFV and proteolytically activating these particles to generate rSFV. From the rSFV stock, an aliquot was taken out for UV inactivation to generate UV-rSFV. The particle concentration of nrSFV was determined by titrating the virus preparation after activation. We used identical volumes of nrSFV, rSFV, and UV-rSFV, and thus the physical amounts of virus particles were the same in all three preparations. Specifically, rSFV was generated by activating nrSFV at room temperature for 10 min using 200 μg/ml of α-chymotrypsin in phosphate-buffered saline (PBS) (Sigma), followed by the addition of 0.67 mg/ml aprotinin (Sigma) on ice. The activation mix (AM), containing only α-chymotrypsin and aprotinin but no virus, was used as a negative control in some experiments. UV inactivation was performed using an Amersham UV cross-linker at 2,000 μJ/cm2 for 1 min. Radiolabeled virus was generated in the presence of [35S]methionine (Amersham Biosciences, Uppsala, Sweden) as described previously (69). Wild-type SFV4 for use in the bioassay for murine IFN-α/β (muIFN-α/β; see below) was generated from the infectious cDNA clone (43).
Mice.
Wild-type C57BL/6 (wt), IRF-3−/−, and MyD88−/− mice (both in the C57BL/6 background) were kept and bred under pathogen-free conditions at the Microbiology and Tumor Biology Center, Karolinska Institute. The IRF-3−/− mice (58) were kindly provided by T. Taniguchi (University of Tokyo), and the MyD88−/− mice (1) had previously been provided to our department by S. Akira (Osaka University). All mice were 6 to 12 weeks old and were sex and age matched (within 3 weeks) for each experiment. The animal experiments were approved by the Committee for Animal Ethics in Stockholm, Sweden, and performed according to given guidelines.
Immunofluorescence.
The antibodies used for immunofluorescence included goat anti-IRF-3 (Santa Cruz Biotechnology) and rabbit anti-IRF-3 (Zymed Laboratories). The secondary antibodies were Cy3-conjugated donkey anti-goat and anti-mouse sera and Texas Red-conjugated donkey anti-rabbit sera (Jackson ImmunoResearch, West Grove, Pennsylvania). Cells were fixed by incubation in 4% paraformaldehyde (in PBS) for 8 to 10 min at room temperature followed by incubation in either 0.1% Triton X-100 (in PBS) for 8 to 10 min at room temperature or methanol for 8 to 10 min at −20°C. Coverslips were incubated in blocking buffer (5% horse serum [Invitrogen], 0.02% sodium azide in PBS) for 1 h at room temperature. The primary and secondary antibodies were diluted in blocking buffer and incubated with the cells for 1 to 3 h each. The secondary incubation typically contained 0.5 μg/ml Hoechst 33258 (Molecular Probes, Eugene, Oregon) for the identification of cell nuclei. The cells were mounted in vinol mounting medium, and images were captured using a Leitz DM RB fluorescence microscope with a Hamamatsu C4880 cooled charge-coupled device camera. Images were processed and compiled using Adobe Photoshop software.
Bioassay for murine IFN-α/β.
A bioassay was employed to determine the total amount of active IFN-α/β (51). Briefly, flat-bottomed 96-well plates were seeded with 1.5 × 104 L929 cells/well. The following day, twofold serially diluted samples or an IFN-α/β standard (Gu02-901-511; National Institute for Allergy and Infectious Diseases [NIAID]) was added to the cells. After overnight incubation, the cells were infected with SFV4 (350 IU/well). Mitochondrial dehydrogenase activity was assayed 2 days after infection, using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) according to the instructions of Sigma kit CGD-1. The MTT was dissolved in OPTI-MEM reduced serum medium lacking phenol red (Invitrogen) supplemented with penicillin, streptomycin, l-glutamine, and 2% FCS. The absorbance was read at 570 nm and normalized against the absorbance at 630 nm in a microplate reader (Elx 800 UV; BIO-TEK Instruments, Winooski, Vermont). The data were converted to international units (U) using muIFN-α/β as a standard (NIAID, NIH) in all assays. Samples were considered below the level of detection if the signal did not reach three-fourths of the plateau level of the standard. All samples analyzed in the bioassay were UV inactivated to abolish any residual infectivity of the viral particles used for stimulation. To confirm the specificity of the IFN-α/β signal, selected positive samples from the various stimulations (serum, mDC supernatant, and MEF supernatant) were preincubated with a neutralizing antiserum to murine IFN-α/β (G024-501-568; NIAID) or with a control serum (G025-501568; NIAID).
Preparation of bone marrow-derived mDCs.
Murine mDCs were generated as previously described (18). Briefly, bone marrow cells were isolated from femurs and tibiae in DC medium (RPMI 1640 supplemented with 10% FCS, l-glutamine, penicillin, streptomycin, 1 mM sodium pyruvate, 0.02 M HEPES, and 50 μM 2-mercaptoethanol) and red blood cell lysing buffer (Sigma). Cells were plated at 2 × 106 cells/ml in DC medium supplemented with 100 ng/ml of murine granulocyte-macrophage colony-stimulating factor (PeproTech, London, United Kingdom). On day 4, one-half of the culture medium was replaced with fresh DC medium containing granulocyte-macrophage colony-stimulating factor. The cells were harvested on day 6.
Flow cytometry.
The phenotype of mDCs was determined by staining using anti-CD11c-phycoerythrin and CD11b-allophycocyanin. In addition, CD86-fluorescein isothiocyanate or CD40-fluorescein isothiocyanate monoclonal antibodies (BD Biosciences PharMingen, San Jose, California) were used for an analysis of the surface expression of costimulatory markers after stimulation. Flow cytometric analysis was performed using a FACSCalibur instrument (BD Biosciences PharMingen).
In vitro and in vivo stimulations.
The mDCs were set up at 2 × 106 cells in 500 μl of DC medium for stimulation with pI-C (Sigma), CpG-ODN (Cybergene, Stockholm, Sweden), or viral particles as described in the figure legends. Supernatants from the cultures were harvested 6 and 24 h after the addition of the stimuli and were stored at −70°C. For in vivo stimulations, mice received the viral particles in 100 μl of PBS intravenously through the tail vein.
RESULTS
Characterization of virus preparations.
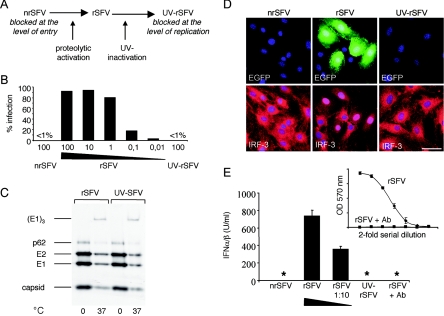
To analyze the role of different steps in the viral life cycle required for IFN-α/β induction in mDCs, we took advantage of an SFV vector system previously developed in our laboratory. This system allows the generation of fusion-defective virus particles using a helper RNA which carries a mutation in the SFV spike protein precursor, p62 (8). Activation of the fusion-competent form of SFV is dependent on the proteolytic cleavage of p62 to generate E2 and E3, an event mediated by a cellular furin-like protease during transport of the viral glycoproteins through the secretory pathway (34, 75). In the nonfusogenic virus, nrSFV, the p62 cleavage site has been mutated, rendering the spike unable to undergo the conformational changes necessary for fusion while still allowing particle assembly and budding (8, 69). Chymotrypsin treatment of nrSFV generates the fusion-competent virus, rSFV, which can subsequently be rendered replication defective through UV treatment (Fig. 1A). This system allowed us to analyze three viruses originating from the same virus stock, which thus were equivalent in terms of physical particle concentration but different in terms of their functional properties.
FIG. 1.
UV treatment renders rSFV replication incompetent but leaves the virus fusion competent. (A) Schematic representation of the recombinant viruses used for this study. (B) Infectious titers of nrSFV, rSFV, and UV-rSFV determined on BHK-21 cells using the equivalent of 4 × 107 IU/ml (corresponding to 100 IU/cell) for all three viruses and 10-fold dilutions of rSFV (100 to 0.01 IU/cell). EGFP expression (% infection) was measured after 24 h of incubation with virus. (C) Analysis of E1 trimer formation. MEFs were incubated with [35S]methionine-labeled rSFV or UV-rSFV for 1 hour at 0°C and were then incubated at either 0°C or 37°C for a further 20 min. The cells were washed in PBS and lysed in a buffer containing 1% NP-40. The lysates were analyzed by nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. (D) MEFs were infected with equivalent amounts of nrSFV, rSFV, and UV-rSFV (corresponding to 1 IU/cell) and assayed for EGFP expression and IRF-3 localization by immunofluorescence using an anti-IRF-3 antibody. Bar, 50 μm. (E) Bioassay for IFN-α/β produced by MEFs treated with equivalent amounts of rSFV, nrSFV, and UV-rSFV (corresponding to 100 IU/cell) or with a 1:10 dilution of rSFV. *, no IFN-α/β could be detected in the sample. A sample from the rSFV-stimulated cells was preincubated with a specific anti-IFN-α/β antibody (raw data from the bioassay are shown in the inset) to show the specificity of the assay.
To determine the differences in the numbers of infectious viral particles after the different treatments, we used highly permissive BHK-21 cells as target cells for infection. nrSFV, rSFV, and UV-rSFV were titrated according to a standard protocol (32), and the titers of nrSFV and UV-rSFV were both consistently found to be reduced >103-fold compared to that of rSFV (data not shown). The titrations were repeated at least three times for each virus preparation used. To visualize the differences in infectious titers of the three forms of the virus, the numbers of infected cells expressing enhanced green fluorescent protein (EGFP) were quantified by flow cytometry (Fig. 1B). Since the production of EGFP mRNA is dependent on RNA replication, EGFP expression was used as a marker of viral infection. rSFV infected close to 100% of the cells at a multiplicity of infection (MOI) of 100, while <1% of the cells became infected when an equivalent amount of either nrSFV or UV-rSFV was used. Three percent of the cells became infected with rSFV at an MOI of 0.01, demonstrating that even a 104-fold dilution of rSFV contained more infectious units than the undiluted nrSFV or UV-rSFV virus.
UV-inactivated virus retains a fusion-competent envelope glycoprotein.
It has previously been shown that SFV can be UV inactivated under conditions that do not abolish the ability of the virus to fuse (71). To characterize the UV-inactivated virus used in this study, we analyzed the ability of the virus spike complex to undergo the conformational changes required for viral fusion. SFV fusion in low-pH vesicles is driven by conformational changes in the envelope glycoproteins, resulting in E1 trimerization and exposure of the fusion peptide of the E1 protein (34). To determine if the UV-inactivated virus maintained the ability to undergo the conformational changes required for fusion, we analyzed E1 trimer formation upon endosomal uptake of the virus. [35S]methionine-labeled virus and MEFs were incubated on ice to allow virus binding, and the cells were then shifted to 37°C to allow endocytosis of the bound virus. We found that both rSFV and UV-rSFV were capable of E1 trimer formation upon uptake into low-pH vesicles, as shown in Fig. 1C, confirming that the UV treatment used for this study had not detectably affected the function of the viral spike proteins. We also performed immunofluorescence experiments to stain endocytosed rSFV and UV-rSFV using the monoclonal antibody E1a-1, with specificity for the low-pH-induced conformation of E1 (35). This demonstrated that no E1a-1-specific signal was detectable when the virus-treated cells were incubated at 0°C, but when the cells were shifted to 37°C for 20 min, both rSFV- and UV-rSFV-treated cells stained positive with the E1a-1 monoclonal antibody (data not shown), supporting the conclusion that UV-rSFV was fusion competent.
MEFs require viral replication for induction of IFN-α/β.
It has previously been shown that SFV induces the translocation of IRF-3 and IRF-7 to the nucleus (4, 5). To characterize the ability of the three virus preparations to induce IFN-α/β, we infected low-passage-number MEFs with equal volumes of nrSFV, rSFV, and UV-rSFV. Fibroblasts have previously been shown to require viral replication for the induction of IFN-α/β in a process that is mediated by IRF-3 (5, 10, 58, 67). We confirmed that only the cultures infected with replicating virus, as shown by the expression of EGFP, exhibited nuclear localization of IRF-3 (Fig. 1D). In contrast, all cells exposed to nrSFV or UV-rSFV remained negative for EGFP and showed a cytoplasmic distribution of IRF-3. Parallel immunofluorescence assays for viral nonstructural proteins gave detectable signals only in cultures infected with rSFV (data not shown). Consistent with the distribution of IRF-3, neither nrSFV nor UV-rSFV induced IFN-α/β production in MEFs, despite the relatively high concentrations of viral particles used (Fig. 1E). This agrees with the current understanding that viral replication is required to induce IFN-α/β production in nonimmune cells (10, 67) and that this is mediated by IRF-3 binding to DNA in the nucleus (58, 74). Antisera against IFN-α/β completely neutralized the signal obtained in the IFN-α/β bioassay (Fig. 1E, inset), showing the specificity of the assay.
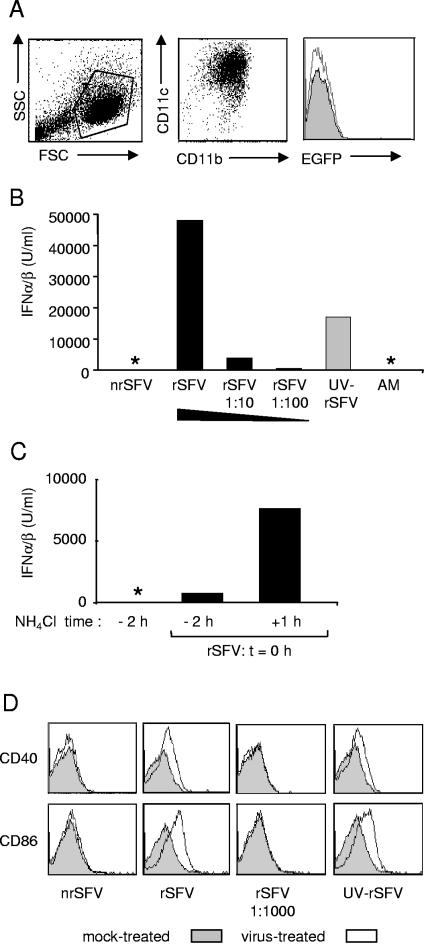
Production of IFN-α/β by mDCs is not dependent on viral replication.
We further investigated the ability of the three different forms of virus to induce IFN-α/β in mDCs. Consistent with a previous report showing that mDCs are relatively resistant to infection by rSFV (26), we did not detect any EGFP-positive cells in mDC cultures using an MOI of 10 (Fig. 2A). We have not defined at which level rSFV infection is suppressed in mDCs other than that it is prior to the accumulation of detectable levels of EGFP reporter protein. The resistance of mDCs to infection by Sindbis virus (a related alphavirus) was reported to be due to the constitutive expression of antiviral proteins in these cells (56, 57). However, despite the lack of detectable rSFV-driven protein expression in mDCs, we found that the cells responded strongly by producing IFN-α/β (Fig. 2B). The IFN-α/β response to rSFV was dose dependent and titrated down to near the limits of detection at a 100-fold dilution of the viral particles. Interestingly, UV-rSFV stimulated IFN-α/β levels consistently above those obtained with a 10- or 100-fold dilution of rSFV (Fig. 2B). Since UV-rSFV is replication defective (Fig. 1B), these results indicated that the ability of mDCs to respond to virus was not dependent on viral RNA replication. In contrast, the fusion-defective nrSFV did not induce a detectable IFN-α/β signal. The lack of response to fusion-incompetent virus demonstrated that the response to the fusion-competent viruses was not induced by contaminants present in the virus preparation, since the functionally distinct forms of the virus originated from a single virus preparation.
FIG. 2.
mDCs produce IFN-α/β and up-regulate costimulatory markers in response to rSFV and UV-rSFV. (A) Phenotype (left and center panels) and EGFP expression 24 h after the addition of rSFV (10 IU/cell) (right panel) of representative mDC cultures. (B) IFN-α/β production by mDCs 24 h after incubation with equal amounts of nrSFV, rSFV, and UV-rSFV (corresponding to 10 IU/cell of rSFV) and with rSFV diluted 10-fold and 100-fold. Control cultures were incubated with AM (see Materials and Methods). *, no IFN-α/β could be detected in the sample. (C) NH4Cl was added to the mDC cultures either 2 h before (−2 h) or 1 h after (+1 h) the addition of rSFV (10 IU/cell), and IFN-α/β was measured in the supernatant 24 h after the addition of the virus. (D) Up-regulation of CD40 and CD86 24 h after the addition of rSFV (10 IU/cell) and equivalent amounts of nrSFV and UV-SFV or a 1:1,000 dilution of rSFV. The results shown are representative of at least three experiments using independent preparations of mDCs.
To investigate if viral fusion was required, we treated mDC cultures with NH4Cl to prevent acidification of the endosome, an event necessary for the activation of SFV fusion (34). This concentration of NH4Cl (15 mM) has been shown to inhibit SFV infection without compromising binding or endocytic uptake of the virus (19, 50). In our experiments, IFN-α/β production was markedly reduced when NH4Cl was added to the cultures prior to the addition of virus compared to that in control cultures to which NH4Cl was added after the addition of the virus (Fig. 2C). Collectively, these data showed that IFN-α/β induction in mDCs did not require replication, while the uptake of fusion-competent virus particles into a low-pH compartment was required.
As an additional measure of the ability of mDCs to respond to nrSFV, rSFV, and UV-rSFV, we analyzed the surface expression of costimulatory molecules after the incubation of mDCs with the viruses. Treatment with rSFV and UV-rSFV, but not nrSFV, resulted in an up-regulation of CD86 and CD40 (Fig. 2D). Control samples incubated with a 1,000-fold dilution of rSFV (approaching the infectious titers of nrSFV and UV-rSFV) showed no up-regulation of CD86 or CD40. Similar results were obtained for CD80 and major histocompatibility complex class II (data not shown). Together, the results in Fig. 2 show that mDCs were activated in response to rSFV and the replication-defective UV-rSFV but not in response to the fusion-defective nrSFV.
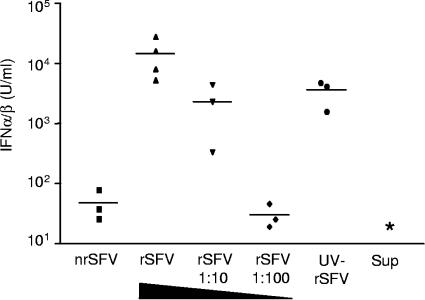
Replication-defective, but not fusion-defective, virus induces IFN-α/β production in vivo.
We next analyzed the in vivo response to the three viruses (Fig. 3). The IFN-α/β response in mice injected with rSFV was high and dose dependent (mean values of 14,370, 2,310, and 30 U/ml, respectively, for three serial 10-fold dilutions), while nrSFV induced IFN-α/β levels that were just above the limit of detection (48 U/ml). UV-rSFV induced a strong IFN-α/β response (mean value of 5,282 U/ml), similar to that induced by a 10-fold dilution of rSFV and significantly higher than that induced by a 100-fold dilution of rSFV or by nrSFV. Since the infectious titer of UV-rSFV was reduced >103-fold compared to that of rSFV (Fig. 1B), we do not consider the IFN-α/β response induced by UV-rSFV to be caused by residual replication-competent particles. Control mice injected with the chymotrypsin-aprotinin mix used for the activation of nrSFV (AM) or with mock-packaged supernatants, generated by omitting the helper RNAs from the packaging procedure, did not give rise to detectable levels of IFN-α/β. These results suggest that systemic IFN-α/β production can be induced by a replication-independent mechanism.
FIG. 3.
rSFV and UV-rSFV induce an early systemic IFN-α/β response. IFN-α/β was measured in the sera of individual wt mice 4 h after injection with rSFV (108 IU), an equivalent amount of nrSFV or UV-SFV, or a 1:10 or 1:100 dilution of rSFV. Control mice were injected with the supernatant from mock-packaged recombinant SFV RNA (Sup). *, no IFN-α/β could be detected in the sample. Data from one representative experiment of two are shown. The UV-inactivated virus induced significantly more IFN-α/β than nrSFV or a 100-fold dilution of rSFV (P < 0.05).
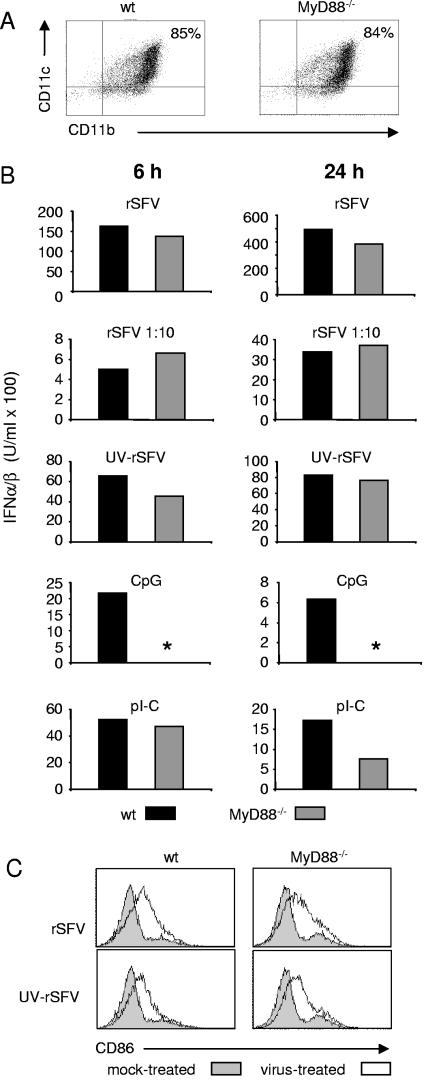
IFN-α/β production in response to replication-defective virus is not dependent on MyD88.
To determine if the MyD88-dependent pathway described for pDCs (12, 21) was responsible for the induction of IFN-α/β in mDCs in response to rSFV and UV-rSFV, we set up mDC cultures from MyD88−/− mice. The phenotypes of wt and MyD88−/− mDCs were examined by analyzing the cell surface expression of CD11b and CD11c, and the cultures were found to be comparable (Fig. 4A). As wt mDCs, MyD88−/− mDCs did not support productive rSFV infection, as determined by virus-driven EGFP expression (data not shown). In order to study the induction of IFN-α/β in response to virus, we measured the IFN-α/β levels in supernatants of mDC cultures 6 and 24 h after the addition of rSFV, a 10-fold dilution of rSFV, or UV-rSFV. Neither rSFV nor UV-rSFV required MyD88 for the induction of IFN-α/β (Fig. 4B). The induction of IFN-α/β by CpG was strictly dependent on MyD88, consistent with previous reports (22, 33), while the response to pI-C was not. When the mDC cultures were stained for CD86 expression, we found that the exposure of wt and MyD88−/− mDCs to rSFV and UV-rSFV resulted in similar levels of up-regulation (Fig. 4C). This supported the conclusion that UV-rSFV was recognized by the MyD88−/− mDCs and stimulated maturation.
FIG. 4.
IFN-α/β production and activation of mDCs in response to rSFV and UV-rSFV are intact in the absence of MyD88. (A) Phenotypes of representative mDC cultures generated from wt and MyD88−/− mice. (B) IFN-α/β produced by mDCs generated from wt and MyD88−/− mice. mDCs were incubated with rSFV (25 IU/cell), a 1:10 dilution of rSFV (2.5 IU/cell), UV-rSFV (a volume equivalent to 25 IU/cell of rSFV), CpG (1 μM), or pI-C (50 μg/ml) for 6 or 24 h. *, no IFN-α/β could be detected in the sample. (C) Expression of CD86 on wt and MyD88−/− mDC surfaces 24 h after the addition of rSFV. The results shown are representative of at least three independent preparations of mDCs.
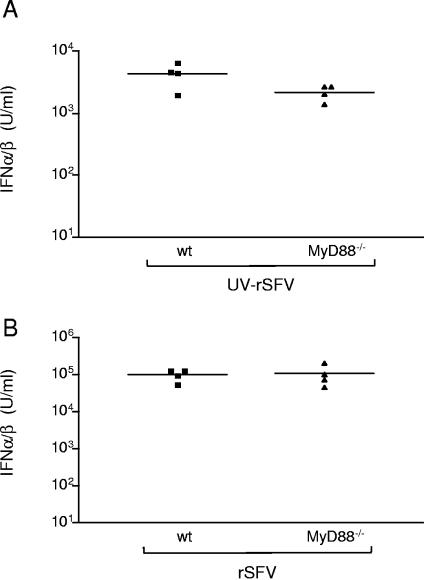
The results obtained in vitro led us to investigate if the early systemic IFN-α/β responses induced by rSFV and UV-rSFV in vivo were dependent on MyD88. As shown in Fig. 5, MyD88 was not required for the response to either rSFV or UV-rSFV. However, there was a reduction (not significant) in the IFN-α/β levels obtained with MyD88−/− mice in response to UV-rSFV compared to those obtained with wt mice (the average for wt mice was 4,451 U/ml and that for MyD88−/− mice was 2,231 U/ml) (Fig. 5A). In agreement with data obtained using other RNA viruses (24, 76), no such reduction was observed in sera from MyD88−/− mice injected with rSFV compared to sera from wt mice (Fig. 5B). Based on these observations, we concluded that the induction of IFN-α/β by UV-rSFV and rSFV is not dependent on MyD88-mediated signaling.
FIG. 5.
rSFV induces early systemic IFN-α/β production in mice lacking MyD88. (A) IFN-α/β levels in sera from individual wt and MyD88−/− mice 4 h after injection with UV-rSFV (equivalent of 108 IU). (B) IFN-α/β levels in sera from individual wt and MyD88−/− mice 6 h after injection with rSFV (107 IU). Data from one representative experiment of two experiments using at least four mice per group are shown.
IFN-α/β production by mDCs in response to replication-defective virus is mediated by IRF-3.
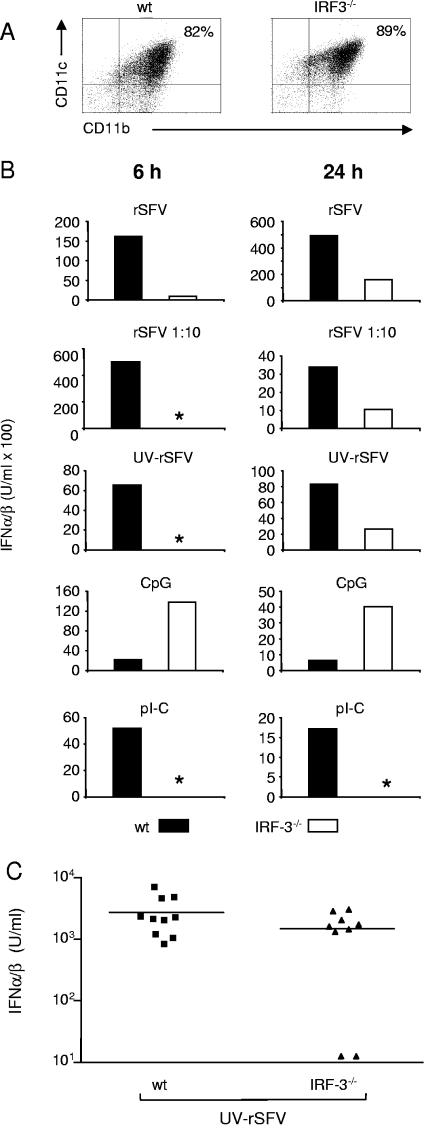
The induction of IFN-α/β in mDCs required the uptake of fusion-competent virus into low-pH endosomes (Fig. 2). We hypothesized that the recognition of virus prior to replication occurred in the cytosol during or after viral entry. It has been shown that UV-inactivated virus can activate the transcription of antiviral genes via IRF-3 in MEFs (10). We therefore investigated if IRF-3 was required for IFN-α/β induction by rSFV and UV-rSFV in mDCs. mDC cultures were generated from wt and IRF-3−/− mice, and their phenotypes were analyzed and found to be comparable (Fig. 6A). As the wt and MyD88−/− mDCs, IRF-3−/− mDCs did not support rSFV-driven reporter protein synthesis (data not shown). When the early induction of IFN-α/β in IRF-3−/− mDCs was investigated, we found it to be severely impaired compared to the IFN-α/β levels obtained in wt mDC cultures in response to both rSFV and UV-rSFV (Fig. 6B, left panels). After 6 h of incubation, rSFV induced considerably lower levels of IFN-α/β in IRF-3−/− mDCs than in wt mDCs, and no IFN-α/β was detected in response to UV-rSFV in the IRF-3 mDCs at this time point. When IFN-α/β production was measured after 24 h of stimulation, both rSFV and UV-rSFV induced a detectable response in IRF-3−/− mDCs, but the response was consistently lower than the response obtained in control wt cultures (Fig. 6B, right panels). It is likely that IRF-7 or other IRFs compensate for IRF-3 at the later time point (24, 40). As expected, pI-C did not induce IFN-α/β in the IRF-3−/− cultures (73), but the IRF-3 cultures responded surprisingly well to CpG in repeated experiments. We currently have no explanation for this, but it may reflect the slightly higher percentage of CD11c and CD11b double-positive cells in the IRF-3−/− mDC cultures than in wt mDC cultures that we typically observed (Fig. 6A). IRF-3−/− mice responded to UV-inactivated rSFV by producing IFN-α/β in vivo (Fig. 6C). The response in the IRF-3−/− mice was somewhat more variable, but there was no significant difference in the IFN-α/β responses between the group means for wt and IRF-3−/− mice (the average for wt mice was 2,724 U/ml and that for IRF-3−/− mice was 1,493 U/ml). This finding is in agreement with recently published data showing that the in vivo IFN-α response to viruses measured 12 h after administration is largely intact in IRF-3−/− mice (24).
FIG. 6.
IRF3−/− mDCs are defective in early IFN-α/β production in response to rSFV and UV-rSFV. (A) Phenotypes of representative mDC cultures generated from wt and IRF-3−/− mice. (B) mDCs were incubated with rSFV (25 IU/cell), a 1:10 dilution of rSFV (2.5 IU/cell), UV-rSFV (a volume equivalent to 25 IU/cell of rSFV), CpG (1 μM), or pI-C (50 μg/ml) for 6 or 24 h. *, no IFN-α/β could be detected in the sample. The results shown are representative of at least three independent preparations of mDCs. (C) IFN-α/β levels in sera from individual wt and IRF-3−/− mice 4 h after injection with UV-rSFV (equivalent of 108 IU).
DISCUSSION
DCs are central to the detection of pathogens and the activation of innate and adaptive immunity. All DC types, including pDCs and mDCs, have the capacity to produce IFN-α/β in response to replicating virus (13, 22, 25, 46). In addition, the ability of pDCs to act as potent IFN-α producers in response to nonreplicating virus has recently been established (12, 21). However, it is less well known if mDCs are capable of inducing IFN-α/β in response to events prior to the initiation of viral replication in a manner similar to that described for pDCs. The ability of specialized immune cells to detect virus at early time points may be critical since this allows an antiviral response to be initiated prior to the potential production of viral proteins that act as antagonists of antiviral signaling pathways. Furthermore, IFN-α/β induced by the early recognition of virus will lead to a general up-regulation of IRF-7 in uninfected cells, and this will determine the amplitude of the IFN-α/β response by these cells once they encounter virus. The central role of IRF-7 in IFN-α/β production in response to a viral infection has recently been demonstrated in a series of experiments using mice deficient in IRF-7 (24).
In this work, we have studied the viral functions and cellular pathways required for the production of IFN-α/β by bone marrow-derived mDCs. The relative abundance of mDCs is higher than that of pDCs, and we therefore investigated if mDCs are also capable of early IFN-α/β production in response to incoming virus particles. Our results showed that both replication-competent and UV-inactivated viruses induced IFN-α/β in murine mDC cultures. In addition, both viruses induced an early IFN-α/β response systemically in vivo. We have not investigated the cellular sources of IFN-α/β in vivo, but it is possible that mDCs contribute to the production of systemic IFN-α/β at early time points after virus administration. Furthermore, we found that a fusion-defective virus did not induce an appreciable response in mDCs or in vivo. In contrast to those of the fusion-defective nrSFV, the envelope glycoproteins of the UV-inactivated virus used for this study retained the ability to undergo the low-pH-induced conformational changes necessary for viral fusion. The pretreatment of mDC cultures with NH4Cl to prevent endosomal acidification and SFV fusion abolished the IFN-α/β induction, demonstrating that uptake into low-pH endosomes is required for the response. Since the concentration of NH4Cl used here has been shown to not interfere with the binding and cellular uptake of SFV (19, 50), this also shows that interactions with cell surface receptors were not sufficient to induce a response, as suggested for other viruses (11, 37, 51).
Our data demonstrated that MyD88 was not required for the IFN-α/β induction by UV-inactivated rSFV, suggesting that mDCs did not respond via the TLR7-dependent pathway described for pDCs (12, 21, 48). IFN-α/β induction by TLR7 has been suggested to be independent of viral fusion (48) and to result from DC sampling of the environment and a nonspecific uptake of virus into low-pH endosomes, where viral ssRNA and TLR7 interact after the proteolytic degradation of viral particles (12). However, if this mechanism were responsible for the IFN-α/β induction in mDCs, then fusion-defective virus would also be expected to induce IFN-α/β production, but this was not observed (Fig. 2). Our results support the conclusions that a TLR7/MyD88-independent pathway can mediate IFN-α/β induction in response to nonreplicating virus in mDCs and that events dependent on viral fusion but independent of replication are sufficient to activate IFN-α/β production by mDCs.
Viral fusion has previously been implicated as a stimulus for DC activation (16, 53), and here we extend these findings by showing that a fusion-competent, UV-inactivated virus induces IFN-α/β in mDCs. Previous work has shown that UV-inactivated Sindbis virus induces apoptosis in CHO cells and that this response is mediated by viral entry (30), thus further implicating early events during virus infection in the stimulation of signaling events. It has been shown that a UV dose that reduced the infectious titer of SFV by several orders of magnitude did not abolish viral fusogenicity, while a harsher UV treatment did (71). In agreement with this, the UV treatment used for this study did not interfere with the ability of the virus to undergo low-pH-induced conformational changes such as E1 trimer formation, while a stronger UV treatment used for control experiments resulted in cross-linking of the viral proteins such that they could not be separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The cross-linked virus did not induce IFN-α/β production or up-regulate costimulatory markers on mDCs (data not shown). This underscores the importance of characterizing the virus after UV treatment and other inactivation methods, because the degree of treatment can affect the viral functions to a great extent, as also discussed by others (22, 49).
Our results demonstrate that IRF-3 is required for the early induction of IFN-α/β by mDCs, possibly suggesting a role for TLR3 in mediating this response, since TLR3 signals via IRF-3 (14). However, although synthetic dsRNA (pI-C) is a ligand for TLR3 (2), TLR3 has been shown to be dispensable for IFN-α/β induction by mDCs in response to cell-free virus (23, 46). It is possible that rather than acting as a primary receptor for incoming viruses, TLR3 plays a role in DC activation later during infection, for example, by recognizing dsRNA replication intermediates originating from dying infected cells (41, 60). Although the generation of some dsRNA intermediates cannot be excluded, they should not be generated in abundance by the UV-inactivated virus, and we therefore consider it unlikely that TLR3 plays a role in the IFN-α/β response to replication-defective virus studied here. Nevertheless, the genomic RNA of UV-inactivated virus could contain dsRNA structures, and their potential role remains to be investigated.
It is well established that IRF-3 plays an important role in the induction of IFN-α/β in response to replicating virus (20, 58, 66). Only recently has it been shown that IRF-3 mediates antiviral activity in response to viral entry events (10). Collins et al. demonstrated that several genes involved in the antiviral defense were up-regulated in MEFs in an IRF-3-dependent manner, although UV-inactivated viruses did not induce IFN-α/β in these cells (10). Consistent with the results of Collins et al., MEFs did not produce IFN-α/β in response to UV-inactivated virus in our study. However, we found that mDCs had the ability to produce IFN-α/β when exposed to UV-inactivated virus and that the early induction required IRF-3. The different abilities of mDCs and MEFs to produce IFN-α/β in response to UV-inactivated virus could be explained by differences in signal transduction pathways between these cell types. For example, the differential expression of kinases responsible for phosphorylating IRF-3 (15, 62) or other molecules acting upstream of IRF-3 (6, 72) could account for the results. It has recently been shown that dsRNA can be recognized by the cytosolic DexD/H box helicase RIG-I, which mediates IFN-α/β production in an IRF-3-dependent manner (72). Interestingly, RIG-I has also been shown to recognize secondary structures in the untranslated termini of the hepatitis C virus genome (17, 65). It is therefore possible that secondary RNA structures in the genomes of other viruses also could be recognized by RIG-I and lead to the induction of an antiviral response prior to replication. The molecular basis of the ability of mDCs to produce IFN-α/β in response to early infection events is the focus of our on-going investigations.
In conclusion, we showed that mDCs produce high levels of IFN-α/β in response to UV-inactivated virus but not in response to fusion-defective virus. This response was not dependent on the MyD88-dependent pathway described for pDCs but was dependent on IRF-3. Recent studies showed that MyD88 signals via IRF-7/5 and TRAF6 in a pathway that does not involve IRF-3 (12, 33, 59), and it is therefore likely that MyD88 and IRF-3 mediate distinct nonoverlapping pathways for IFN-α/β induction in DCs. Several previous studies have demonstrated the ability of IFN-α/β to stimulate adaptive immune responses (38, 39, 52, 54). The ability of mDCs to produce IFN-α/β in response to viral entry events may therefore have implications for the immunogenicity of virus-based vaccines. The results presented here add to the current understanding about early antiviral activity in specialized immune cells and show that IFN-α/β production in response to replication-inactivated virus is not solely a function of pDCs. It is possible that pDCs and mDCs complement each other in the early antiviral response by surveying different anatomical compartments or by responding to different pathogens or to different components of pathogens early after infection. Such IFN-α/β production may be critical for an early amplification of IRF-7, RIG-I, and other IFN-α/β-inducible proteins, allowing the host to mount a strong antiviral response before virally encoded antagonists are produced.
Acknowledgments
We thank the personnel at the MTC animal facility at the Karolinska Institutet for expert technical assistance.
This study was supported by the Swedish Research Council and the European Union 5th Framework Programme.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144-1150. [DOI] [PubMed] [Google Scholar]
- 4.Baigent, S. J., S. Goodbourn, and J. W. McCauley. 2004. Differential activation of interferon regulatory factors-3 and -7 by non-cytopathogenic and cytopathogenic bovine viral diarrhoea virus. Vet. Immunol. Immunopathol. 100:135-144. [DOI] [PubMed] [Google Scholar]
- 5.Baigent, S. J., G. Zhang, M. D. Fray, H. Flick-Smith, S. Goodbourn, and J. W. McCauley. 2002. Inhibition of beta interferon transcription by noncytopathogenic bovine viral diarrhea virus is through an interferon regulatory factor 3-dependent mechanism. J. Virol. 76:8979-8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balachandran, S., E. Thomas, and G. N. Barber. 2004. A FADD-dependent innate immune mechanism in mammalian cells. Nature 432:401-405. [DOI] [PubMed] [Google Scholar]
- 7.Barnes, B., B. Lubyova, and P. M. Pitha. 2002. On the role of IRF in host defense. J. Interferon Cytokine Res. 22:59-71. [DOI] [PubMed] [Google Scholar]
- 8.Berglund, P., M. Sjoberg, H. Garoff, G. J. Atkins, B. J. Sheahan, and P. Liljeström. 1993. Semliki Forest virus expression system: production of conditionally infectious recombinant particles. Biotechnology (New York) 11:916-920. [DOI] [PubMed] [Google Scholar]
- 9.Boehme, K. W., and T. Compton. 2004. Innate sensing of viruses by Toll-like receptors. J. Virol. 78:7867-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins, S. E., R. S. Noyce, and K. L. Mossman. 2004. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J. Virol. 78:1706-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and E. S. C. Reis. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 13.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 14.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251-263. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 16.Fonteneau, J. F., M. Larsson, A. S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y. J. Liu, J. D. Lifson, D. R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 78:5223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foy, E., K. Li, R. Sumpter, Jr., Y. M. Loo, C. L. Johnson, C. Wang, P. M. Fish, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc. Natl. Acad. Sci. USA 102:2986-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X. L. Xu, G. Trinchieri, A. O'Garra, and Y. J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glomb-Reinmund, S., and M. Kielian. 1998. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology 248:372-381. [DOI] [PubMed] [Google Scholar]
- 20.Hata, N., M. Sato, A. Takaoka, M. Asagiri, N. Tanaka, and T. Taniguchi. 2001. Constitutive IFN-alpha/beta signal for efficient IFN-alpha/beta gene induction by virus. Biochem. Biophys. Res. Commun. 285:518-525. [DOI] [PubMed] [Google Scholar]
- 21.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 22.Hochrein, H., B. Schlatter, M. O'Keeffe, C. Wagner, F. Schmitz, M. Schiemann, S. Bauer, M. Suter, and H. Wagner. 2004. Herpes simplex virus type-1 induces IFN-{alpha} production via Toll-like receptor 9-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 101:11416-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 25.Hornung, V., J. Schlender, M. Guenthner-Biller, S. Rothenfusser, S. Endres, K. K. Conzelmann, and G. Hartmann. 2004. Replication-dependent potent IFN-alpha induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173:5935-5943. [DOI] [PubMed] [Google Scholar]
- 26.Huckriede, A., L. Bungener, M. Holtrop, J. de Vries, B. L. Waarts, T. Daemen, and J. Wilschut. 2004. Induction of cytotoxic T lymphocyte activity by immunization with recombinant Semliki Forest virus: indications for cross-priming. Vaccine 22:1104-1113. [DOI] [PubMed] [Google Scholar]
- 27.Isaacs, A., and J. Lindenmann. 1957. Virus interference. I. The interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:258-267. [PubMed] [Google Scholar]
- 28.Isaacs, A., J. Lindenmann, and R. C. Valentine. 1957. Virus interference. II. Some properties of interferon. Proc. R. Soc. Lond. B Biol. Sci. 147:268-273.13465721 [Google Scholar]
- 29.Izaguirre, A., B. J. Barnes, S. Amrute, W. S. Yeow, N. Megjugorac, J. Dai, D. Feng, E. Chung, P. M. Pitha, and P. Fitzgerald-Bocarsly. 2003. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74:1125-1138. [DOI] [PubMed] [Google Scholar]
- 30.Jan, J. T., and D. E. Griffin. 1999. Induction of apoptosis by Sindbis virus occurs at cell entry and does not require virus replication. J. Virol. 73:10296-10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juang, Y. T., W. Lowther, M. Kellum, W. C. Au, R. Lin, J. Hiscott, and P. M. Pitha. 1998. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc. Natl. Acad. Sci. USA 95:9837-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karlsson, G. B., and P. Liljeström. 2003. Live viral vectors: Semliki Forest virus. Methods Mol. Med. 87:69-82. [DOI] [PubMed] [Google Scholar]
- 33.Kawai, T., S. Sato, K. J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, O. Takeuchi, and S. Akira. 2004. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat. Immunol. 5:1061-1068. [DOI] [PubMed] [Google Scholar]
- 34.Kielian, M. 1995. Membrane fusion and the alphavirus life cycle. Adv. Virus Res. 45:113-151. [DOI] [PubMed] [Google Scholar]
- 35.Kielian, M., S. Jungerwirth, K. U. Sayad, and S. DeCandido. 1990. Biosynthesis, maturation, and acid activation of the Semliki Forest virus fusion protein. J. Virol. 64:4614-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2003. Herpes simplex virus type 1 (HSV-1) activates murine natural interferon-producing cells (IPC) through Toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 37.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 38.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 39.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461-470. [DOI] [PubMed] [Google Scholar]
- 40.Levy, D. E., I. Marie, E. Smith, and A. Prakash. 2002. Enhancement and diversification of IFN induction by IRF-7-mediated positive feedback. J. Interferon Cytokine Res. 22:87-93. [DOI] [PubMed] [Google Scholar]
- 41.Levy, D. E., and I. J. Marie. 2004. RIGging an antiviral defense—it's in the CARDs. Nat. Immunol. 5:699-701. [DOI] [PubMed] [Google Scholar]
- 42.Liljeström, P., and H. Garoff. 1991. A new generation of animal cell expression vectors based on the Semliki Forest virus replicon. Biotechnology (New York) 9:1356-1361. [DOI] [PubMed] [Google Scholar]
- 43.Liljeström, P., S. Lusa, D. Huylebroeck, and H. Garoff. 1991. In vitro mutagenesis of a full-length cDNA clone of Semliki Forest virus: the small 6,000-molecular-weight membrane protein modulates virus release. J. Virol. 65:4107-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez, C. B., A. Garcia-Sastre, B. R. Williams, and T. M. Moran. 2003. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 46.Lopez, C. B., B. Moltedo, L. Alexopoulou, L. Bonifaz, R. A. Flavell, and T. M. Moran. 2004. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J. Immunol. 173:6882-6889. [DOI] [PubMed] [Google Scholar]
- 47.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malmgaard, L., J. Melchjorsen, A. G. Bowie, S. C. Mogensen, and S. R. Paludan. 2004. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 173:6890-6898. [DOI] [PubMed] [Google Scholar]
- 50.Marsh, M., and A. Helenius. 1980. Adsorptive endocytosis of Semliki Forest virus. J. Mol. Biol. 142:439-454. [DOI] [PubMed] [Google Scholar]
- 51.Miller, J. L., and E. Margot Anders. 2003. Virus-cell interactions in the induction of type 1 interferon by influenza virus in mouse spleen cells. J. Gen. Virol. 84:193-202. [DOI] [PubMed] [Google Scholar]
- 52.Montoya, M., G. Schiavoni, F. Mattei, I. Gresser, F. Belardelli, P. Borrow, and D. F. Tough. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99:3263-3271. [DOI] [PubMed] [Google Scholar]
- 53.Netterwald, J. R., T. R. Jones, W. J. Britt, S. J. Yang, I. P. McCrone, and H. Zhu. 2004. Postattachment events associated with viral entry are necessary for induction of interferon-stimulated genes by human cytomegalovirus. J. Virol. 78:6688-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proietti, E., L. Bracci, S. Puzelli, T. Di Pucchio, P. Sestili, E. De Vincenzi, M. Venditti, I. Capone, I. Seif, E. De Maeyer, D. Tough, I. Donatelli, and F. Belardelli. 2002. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J. Immunol. 169:375-383. [DOI] [PubMed] [Google Scholar]
- 55.Rong, Q., T. S. Alexander, G. K. Koski, and K. S. Rosenthal. 2003. Multiple mechanisms for HSV-1 induction of interferon alpha production by peripheral blood mononuclear cells. Arch. Virol. 148:329-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryman, K. D., K. C. Meier, E. M. Nangle, S. L. Ragsdale, N. L. Korneeva, R. E. Rhoads, M. R. Macdonald, and W. B. Klimstra. 2005. Sindbis virus translation is inhibited by a PKR/RNase L-independent effector induced by alpha/beta interferon priming of dendritic cells. J. Virol. 79:1487-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ryman, K. D., L. J. White, R. E. Johnston, and W. B. Klimstra. 2002. Effects of PKR/RNase L-dependent and alternative antiviral pathways on alphavirus replication and pathogenesis. Viral Immunol. 15:53-76. [DOI] [PubMed] [Google Scholar]
- 58.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 59.Schoenemeyer, A., B. J. Barnes, M. E. Mancl, E. Latz, N. Goutagny, P. M. Pitha, K. A. Fitzgerald, and D. T. Golenbock. 2005. The interferon regulatory factor, IRF5, is a central mediator of Toll-like receptor 7 signaling. J. Biol. Chem. 280:17005-17012. [DOI] [PubMed] [Google Scholar]
- 60.Schulz, O., S. S. Diebold, M. Chen, T. I. Naslund, M. A. Nolte, L. Alexopoulou, Y. T. Azuma, R. A. Flavell, P. Liljeström, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887-892. [DOI] [PubMed] [Google Scholar]
- 61.Servant, M. J., B. tenOever, and R. Lin. 2002. Overlapping and distinct mechanisms regulating IRF-3 and IRF-7 function. J. Interferon Cytokine Res. 22:49-58. [DOI] [PubMed] [Google Scholar]
- 62.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 63.Shortman, K., and Y. J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151-161. [DOI] [PubMed] [Google Scholar]
- 64.Smerdou, C., and P. Liljeström. 1999. Two-helper RNA system for production of recombinant Semliki Forest virus particles. J. Virol. 73:1092-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sumpter, R., Jr., Y. M. Loo, E. Foy, K. Li, M. Yoneyama, T. Fujita, S. M. Lemon, and M. Gale, Jr. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79:2689-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.tenOever, B. R., M. J. Servant, N. Grandvaux, R. Lin, and J. Hiscott. 2002. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J. Virol. 76:3659-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.tenOever, B. R., S. Sharma, W. Zou, Q. Sun, N. Grandvaux, I. Julkunen, H. Hemmi, M. Yamamoto, S. Akira, W. C. Yeh, R. Lin, and J. Hiscott. 2004. Activation of TBK1 and IKKvarepsilon kinases by vesicular stomatitis virus infection and the role of viral ribonucleoprotein in the development of interferon antiviral immunity. J. Virol. 78:10636-10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tubulekas, I., and P. Liljeström. 1998. Suppressors of cleavage-site mutations in the p62 envelope protein of Semliki Forest virus reveal dynamics in spike structure and function. J. Virol. 72:2825-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wahlberg, J. M., R. Bron, J. Wilschut, and H. Garoff. 1992. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J. Virol. 66:7309-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weaver, B. K., K. P. Kumar, and N. C. Reich. 1998. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol. Cell. Biol. 18:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 73.Yoneyama, M., W. Suhara, and T. Fujita. 2002. Control of IRF-3 activation by phosphorylation. J. Interferon Cytokine Res. 22:73-76. [DOI] [PubMed] [Google Scholar]
- 74.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang, X., M. Fugere, R. Day, and M. Kielian. 2003. Furin processing and proteolytic activation of Semliki Forest virus. J. Virol. 77:2981-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou, S., E. A. Kurt-Jones, L. Mandell, A. Cerny, M. Chan, D. T. Golenbock, and R. W. Finberg. 2005. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 35:822-830. [DOI] [PubMed] [Google Scholar]