Abstract
In this study, we provide evidence that the double-stranded RNA-dependent protein kinase (PKR) is not required for virus-induced expression of inducible nitric oxide synthase (iNOS) or the activation of specific signaling pathways in macrophages. The infection of RAW264.7 cells with encephalomyocarditis virus (EMCV) induces iNOS expression and nitric oxide production, which are unaffected by a dominant-negative mutant of PKR. EMCV infection also activates the mitogen-activated protein kinase, cyclic AMP response element binding protein, and nuclear factor κB (NF-κB) signaling cascades at 15 to 30 min postinfection in PKR+/+ and PKR−/− macrophages. Activation of these signaling cascades does not temporally correlate with PKR activity or the accumulation of EMCV RNA, suggesting that an interaction between a structural component of the virion and the cell surface may activate macrophages. Consistent with this hypothesis, empty EMCV capsids induced comparable levels of iNOS expression, nitrite production, and activation of these signaling cascades to those induced by intact virions. These findings support the hypothesis that virion-host cell interactions are primary mediators of the PKR-independent activation of signaling pathways that participate in the macrophage antiviral response of inflammatory gene expression.
Double-stranded RNA (dsRNA), which accumulates during viral replication, is an active component of virus infection that stimulates antiviral responses within infected cells (23). The accrual of dsRNA of viral origin activates the dsRNA-dependent protein kinase (PKR), a ubiquitously expressed 65- to 68-kDa serine/threonine protein kinase that binds dsRNA and initiates various antiviral responses (14, 44). PKR is believed to be a primary regulator of antiviral activities, as it has been implicated in the inhibition of translation by phosphorylation of the eukaryotic initiation factor eIF2α, the upregulation of proinflammatory and antiviral gene expression by activation of the transcription factor nuclear factor κB (NF-κB), and apoptosis of infected cells (14, 25, 29). Notwithstanding these observations, recent reports have suggested that PKR-independent pathways also participate in the antiviral response. In particular, the role of PKR in mediating the activation of NF-κB following virus infection remains controversial. NF-κB activation requires the phosphorylation and subsequent proteasome-mediated degradation of its associated inhibitory protein κB (IκB), which when bound obscures a nuclear localization signal on NF-κB, thereby sequestering it in the cytoplasm of unstimulated cells (5). PKR was initially believed to participate in dsRNA-induced NF-κB activation either directly by phosphorylating IκB or indirectly by inducing IκB kinase activity (9, 26). Recent work by Magun and coworkers, however, has shown that the treatment of PKR−/− mouse embryonic fibroblasts (MEFs) with dsRNA or their infection with encephalomyocarditis virus (EMCV) induces IκB degradation, NF-κB nuclear localization, and DNA binding, as well as NF-κB promoter activation (21). Additionally, we have shown that dsRNA plus gamma interferon (IFN-γ) stimulates comparable levels of inducible nitric oxide synthase (iNOS) expression, nitrite production, and interleukin-1 (IL-1) expression and release by macrophages isolated from PKR−/− and PKR+/+ mice (29, 30). Taken together, these results suggest that additional PKR-independent pathways participate in the cellular response to viral infection.
The macrophage response to viral infection is characterized by the expression of proinflammatory and antiviral genes, including those for IL-1β and iNOS (17). Viral infection of both rodent and human macrophages results in the expression of iNOS and the production of nitric oxide (8, 20). The induction of iNOS expression appears to participate in the antiviral response, since the IFN-γ-mediated inhibition of viral replication by macrophages is due, in part, to the production of nitric oxide (24). Furthermore, mice with compromised iNOS activity, either due to treatment with iNOS inhibitors or by genetic deficiency (iNOS−/− mice), have increased viral titers and a higher mortality rate than wild-type mice following virus challenge (13, 28). One mechanism by which nitric oxide attenuates viral infection is through the nitrosylation and inactivation of viral proteins required for replication, such as the coxsackievirus protease 3C, which contains an NO-sensitive cysteine residue within the active site that is necessary for catalysis (35).
While the molecular mechanisms by which viral infection stimulates the expression of proinflammatory and antiviral genes by macrophages have yet to be clearly defined, it appears that independent signaling pathways regulate the transcriptional activation of specific target genes. Previous results from our laboratory have suggested that PKR is not absolutely required for dsRNA-induced iNOS or IL-1β expression by macrophages (29), supporting a PKR-independent mechanism of transcriptional regulation of these genes during the antiviral response. EMCV infection of macrophages is known to activate multiple signaling pathways, including the mitogen-activated protein (MAP) kinases and NF-κB (20, 21). Recently, we have shown that the MAP kinase extracellular signal-regulated kinase (ERK) participates in the selective regulation of IL-1β expression by macrophages during the antiviral response, as ERK inhibitors attenuated dsRNA- or virus-induced IL-1β expression while iNOS expression and nitrite production were unaffected (30). Conversely, the Ca2+-independent phospholipase A2 (iPLA2)-selective inhibitor bromoenol lactone prevents dsRNA- or EMCV-induced cyclic AMP response element binding protein (CREB) phosphorylation, iNOS expression, and nitrite production but has no effect on IL-1β expression by macrophages (31), supporting a novel role for iPLA2 in the regulation of iNOS expression by macrophages in response to virus infection. While divergent signaling pathways contribute to the regulation of iNOS and IL-1β expression during the antiviral response, NF-κB is absolutely required for the transcriptional activation of these as well as numerous other proinflammatory genes. The transcriptional regulation of cyclooxygenase 2 (COX-2) expression during a viral infection of macrophages requires NF-κB activation but does not require the activation of signaling pathways involved in the regulation of iNOS and IL-1 expression (39). These findings highlight the complexity of the transcriptional activation of antiviral genes and suggest that unique and independent signaling cascades mediate the specificity of gene expression during the antiviral response.
For this study, we have evaluated the temporal association of PKR, MAP kinase, CREB, and NF-κB activation in macrophages infected with the B variant of EMCV. Using dominant-negative PKR (dnPKR)-expressing RAW264.7 cells, we provide evidence that PKR activation is not required for iNOS expression and nitrite production by EMCV-infected macrophages. In addition, the IFN-α/β response does not appear to participate in the activation of proinflammatory gene expression by macrophages during EMCV infection, as both iNOS and IL-1β are expressed to comparable levels in the presence or absence of the IFN-α/β receptor (IFN-α/βR). We show that EMCV infection of macrophages stimulates the rapid activation of MAP kinases, CREB, and NF-κB, events that precede PKR phosphorylation by several hours. The mechanism by which EMCV infection activates these signaling pathways does not appear to be associated with an accumulation of viral RNA or other viral replication events, but rather may be due to an interaction between the virus capsid and a macrophage cell surface receptor during the initial stages of infection.
MATERIALS AND METHODS
Materials and animals.
RAW264.7 cells, L929 cells, and Dulbecco's modified Eagle medium (DMEM) (containing 10% heat-inactivated fetal calf serum and 1× l-glutamine) were obtained from the Washington University Tissue Culture Support Center (St. Louis, MO). Vector control (pDEST27) and stably dnPKR-expressing RAW264.7 (PKR-M1) cells were maintained in G418-containing selection medium and have been described previously (29, 43). PKR−/− mice (C57BL/6J × 129/SV background) were the generous gift of Randall Kaufman and have been previously described (45). C57BL/6J wild-type mice (PKR+/+) were purchased from Harlan (Indianapolis, IN). IFN-α/βR−/− and congenic 129 [129Sv(ev)] mice were generously provided by Michel Aguet (33) and were obtained from Lynda Morrison (Saint Louis University, Saint Louis, MO) (12, 34). Mouse recombinant IFN-γ and Geneticin (G418) were obtained from Gibco-BRL (Grand Island, NY). RNase I was purchased from Promega (Madison, WI). Rabbit anti-iNOS antiserum was a generous gift of Pam Manning (Pfizer, St. Louis, MO). Rabbit anti-phospho-JNK, -phospho-ERK, and -phospho-p38 were obtained from Promega (Madison, WI). Rabbit anti-phospho-CREB was purchased from Upstate Biotechnology (Charlottesville, VA). Rabbit anti-IκBα and anti-PKR antisera were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Rabbit anti-phospho-PKR was obtained from BioSource International (Camarillo, CA). Mouse anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) was purchased from Ambion (Austin, TX). Rabbit anti-phospho-eIF2α and anti-eIF2α antisera were obtained from Stressgen Bioreagents (Victoria, British Columbia, Canada). Mouse anti-EMCV 1C/D antiserum was a generous gift of Ann Palmenberg (University of Wisconsin, Madison, WI). Rabbit anti-ERK2 antiserum was generously provided by John C. Lawrence, Jr. (University of Virginia, Charlottesville, VA). Horseradish peroxidase-conjugated donkey anti-rabbit and donkey anti-mouse antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). All other reagents were obtained from commercially available sources. The Saint Louis University Institutional Review Board has approved all animal studies contained within this report.
Virus propagation and infection.
The B variant of EMCV was a generous gift of Ji-won Yoon (University of Calgary, Calgary, Alberta, Canada) and has been previously described (4). EMCV was propagated in L929 cells, supernatants were clarified by centrifugation, and titers were determined by a plaque assay. Cell monolayers were infected with a multiplicity of infection of 1 PFU/cell by the addition of EMCV to the culture medium for the indicated times at 37°C.
Peritoneal macrophage isolation and cell culture.
Peritoneal exudate cells (PEC) were isolated from PKR-deficient C57BL/6J × 129/SV mice (PKR−/−) and C57BL/6J wild-type mice (PKR+/+) or from IFN-αβ receptor-deficient 129Sv(ev) mice (IFN-αβR−/−) and 129Sv(ev) wild-type mice (IFN-αβR+/+) by peritoneal lavage as described previously (7). We used both C57BL/6J and C57BL/6J × 129/SV mice in previous work and have observed no differences in the antiviral responses of macrophages isolated from either mouse strain. Following isolation, 4 × 105 cells/400 μl cCMRL-1066 were incubated at 37°C under an atmosphere of 95% air and 5% CO2 for 2 h prior to the initiation of experiments. RAW264.7 cells were removed from growth flasks by treatment with 0.05% trypsin-0.02% EDTA at 37°C. Cells were washed twice with DMEM, plated at the indicated concentration, and cultured for 2 to 3 h under an atmosphere of 95% air and 5% CO2 prior to the initiation of experiments.
PCR.
Total RNA was isolated from RAW264.7 cells by using a QIAGEN RNeasy kit according to the manufacturer's instructions and was used to prepare a cDNA library using the Superscript preamplification system from Gibco-BRL (Grand Island, NY) according to the manufacturer's specifications. A standard 25-μl PCR mixture was set up as previously described (3). PCR primers for EMCV have been described previously and are as follows: forward, 5′-GGA GGT GAG AAT GCT GAG AG-3′; and reverse, 5′-TTC CAG CAT AAG GAC TCC AG-3′ (PCR product size of 850 bp) (20). iNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers have been previously described (3).
Nitrite and IL-1 determination.
Nitrite production was determined from culture supernatants by mixing 50 μl of culture medium with 50 μl of Greiss reagent as previously described (15). The absorbance at 540 nm was measured, and nitrite concentrations were calculated from a sodium nitrite standard curve. IL-1 release into culture supernatants was determined by the RINm5F cell bioassay as previously described (19).
Western blot analysis.
Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Amersham) under semidry transfer conditions as previously described (18, 27). Antibody dilutions were as follows: rabbit anti-iNOS, 1:2,000; rabbit anti-ERK2, 1:2,000; mouse anti-GAPDH, 1:5,000; all other primary antibodies, 1:1,000. Horseradish peroxidase-conjugated donkey anti-mouse and donkey anti-rabbit secondary antibodies were used at 1:5,000 and 1:7,000, respectively.
RESULTS
Role of PKR in virus-induced iNOS expression and nitric oxide production by macrophages.
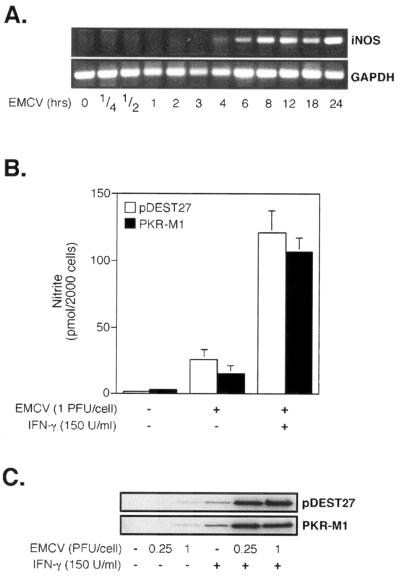
The antiviral effects of IFN-γ, particularly the inhibition of viral replication, are mediated in part by the expression of iNOS and subsequent production of nitric oxide (24). In an attempt to determine if PKR participates in the regulation of iNOS expression by macrophages during virus infection, we initiated our studies by determining the time-dependent effects of EMCV infection on the transcriptional activation of iNOS in macrophages. As shown in Fig. 1A, EMCV induces an accumulation of iNOS mRNA in RAW264.7 cells that is first detectable at 4 h postinfection (p.i.), an effect that persists for 24 h.
FIG. 1.
Effects of dnPKR expression on virus-induced nitrite production and iNOS expression by macrophages. (A) RAW264.7 cells (1 × 106/2 ml) were infected with EMCV (1 PFU/cell), RNA was isolated at the indicated times postinfection, and iNOS mRNA accumulation was determined by RT-PCR with GAPDH serving as an internal control. (B) Vector control (pDEST27)- or dnPKR-expressing RAW264.7 cells (PKR-M1; 4 × 105/400 μl DMEM) were infected with EMCV (1 PFU/cell) for 24 h at 37°C in the presence and absence of 150 U/ml IFN-γ, and nitrite production in culture supernatants was determined by a Greiss assay. (C) pDEST27- and PKR-M1-expressing RAW264.7 cells were treated with 0.25 or 1 PFU/cell of EMCV in the presence and absence of IFN-γ for 24 h, and iNOS expression was determined by Western blot analysis. The results for nitrite production represent the means ± standard errors of the means (SEM) for five independent experiments, and the results for iNOS expression and mRNA accumulation are representative of three independent experiments.
In previous studies, our laboratory has shown that PKR is required for iNOS expression by RAW264.7 cells in response to the synthetic dsRNA polyinosinic-poly(C) [poly(IC)] and that this PKR dependence can be overcome by the addition of IFN-γ (29). To determine whether functional PKR is required for the induction of iNOS expression during authentic viral infection, vector control RAW264.7 cells (pDEST27) or RAW264.7 cells stably expressing a dominant-negative mutant of PKR (PKR-M1) (29) containing a K296P point mutation in the ATP binding domain were infected with EMCV in the presence and absence of IFN-γ. This well-characterized dnPKR mutant has been shown to prevent dsRNA-induced PKR activity (38, 43). EMCV stimulated the production of nitric oxide (Fig. 1B) and the expression of iNOS (Fig. 1C) to similar levels in pDEST and PKR-M1 RAW264.7 cells, while poly(IC) failed to induce nitrite production in dnPKR-expressing RAW264.7 cells (29; data not shown). In contrast to the effects of poly(IC), EMCV-induced iNOS expression by RAW264.7 cells does not appear to require the presence of functional PKR. However, similar to our previous studies with poly(IC), the levels of iNOS expression and nitrite production were significantly enhanced in the presence of IFN-γ. Importantly, EMCV plus IFN-γ-induced iNOS expression does not require PKR, suggesting that PKR-independent antiviral pathways participate in the regulation of iNOS expression by EMCV-infected RAW264.7 cells.
EMCV infection rapidly activates signaling cascades in macrophages.
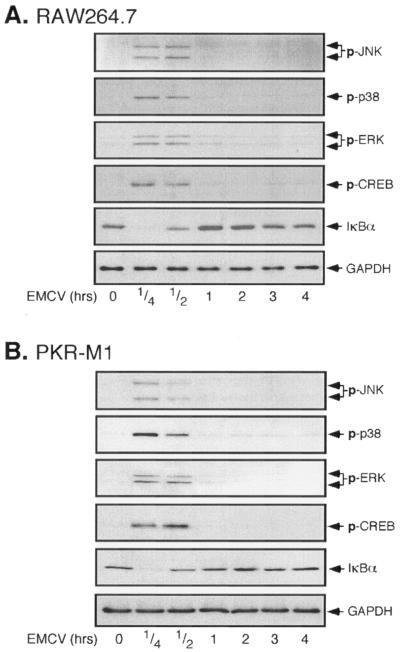
A virus infection of macrophages induces the expression of proinflammatory and antiviral genes, each of whose transcription is regulated by precise and highly orchestrated signal transduction cascades (39). To examine this regulation, the time-dependent effects of EMCV infection of RAW264.7 cells on the activation of MAP kinase signaling cascades, specifically ERK, Jun N-terminal kinase (JNK), and p38, as well as the activation of the transcription factors NF-κB and CREB, were examined (Fig. 2A). EMCV infection stimulates the rapid phosphorylation of each of the MAP kinases, which is first detectable following a 15-min incubation and is sustained for 30 min. In a similar fashion, EMCV induces CREB phosphorylation following a 15-min incubation, and this phosphorylation persists for 30 min. As an assay of NF-κB activation, the effect of EMCV infection on IκB degradation was evaluated. EMCV stimulates the nearly complete degradation of IκB following 15 min of incubation, and the levels of IκB in EMCV-treated RAW264.7 cells return to basal levels at 1 h p.i. Importantly, functional PKR does not appear to be required for the activation of each of these signaling cascades in response to EMCV infection. As shown in Fig. 2B, EMCV infection stimulates ERK, JNK, p38, and CREB phosphorylation, as well as IκB degradation, in RAW264.7 cells stably expressing dnPKR that is similar both temporally and in magnitude to the effects of EMCV on RAW264.7 cells. These findings suggest that EMCV infection activates several signaling pathways in a rapid manner and that the activation of each of these pathways occurs by mechanisms that are independent of PKR.
FIG. 2.
EMCV infection of RAW264.7 and PKR-M1 cells activates MAP kinases, CREB, and NF-κB. RAW264.7 (A) or PKR-M1 (B) cells were treated with EMCV (1 PFU/cell) for the indicated times, and the activation of MAP kinases (JNK, p38, and ERK) and CREB was determined by Western blot analysis using phospho-specific antisera. NF-κB activation was evaluated by determining the loss of IκBα by Western blot analysis. The levels of GAPDH were determined as a protein loading control. The results are representative of three independent experiments.
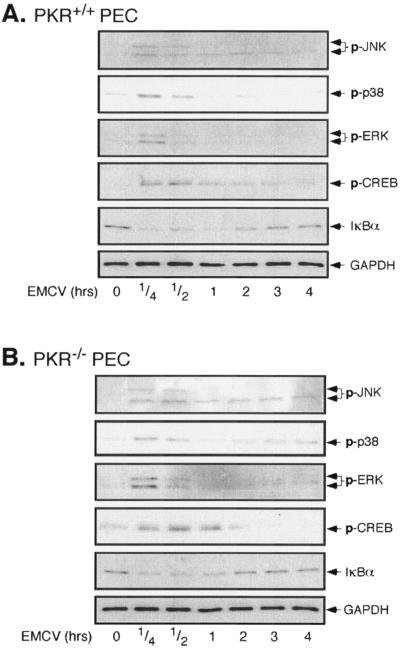
To confirm that PKR is not required for the virus-induced activation of these signaling pathways, the effects of EMCV infection on each of these target proteins was examined in peritoneal macrophages (PEC) isolated from wild-type and PKR-deficient mice. The infection of PKR+/+ (Fig. 3A) or PKR−/− (Fig. 3B) PEC with EMCV results in the phosphorylation of ERK, JNK, p38, and CREB, which occurs in a time-dependent manner immediately following infection. EMCV also stimulates a similar time-dependent degradation of IκB that is maximal at 15 to 30 min p.i., with reduced levels of IκB persisting for 1 to 2 h. Overall, these findings provide additional evidence to suggest that PKR is not required for the activation of MAP kinases, CREB, or NF-κB in macrophages in response to EMCV infection.
FIG. 3.
EMCV induces MAP kinase, CREB, and NF-κB activation in PKR+/+ and PKR−/− macrophages. Macrophages (4 × 105/400 μl cCMRL-1066) isolated from PKR+/+ (A) or PKR−/− (B) mice by peritoneal lavage were treated with EMCV (1 PFU/cell) at 37°C for the indicated times, and JNK, p38, ERK, and CREB activation was determined by Western blot analysis using phospho-specific antisera. EMCV-induced NF-κB activation was evaluated by determining the loss of IκBα by Western blot analysis. The levels of GAPDH were determined as a protein loading control. The results are representative of three independent experiments.
Virus-induced macrophage activation precedes PKR activity.
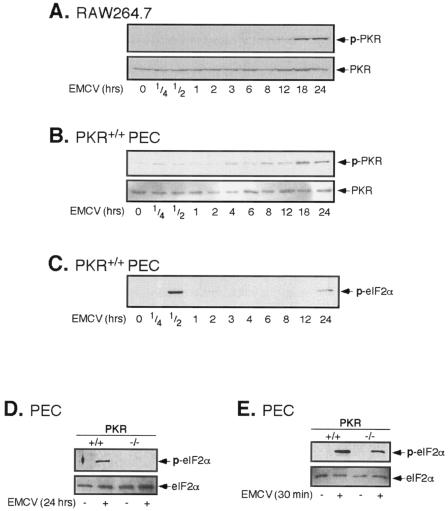
PKR, by virtue of its ability to bind dsRNA that accumulates during viral replication and to mobilize a cellular response to infection, is believed to be a primary mediator of the antiviral response. Given the kinetics with which EMCV activates each of the signaling pathways in macrophages and that this activation occurs in a similar manner in the presence or absence of functional PKR, we examined if EMCV infection activates PKR and if this activation correlates temporally with the activation of each of the signaling cascades. The infection of RAW264.7 cells with EMCV stimulates the phosphorylation and activation of PKR (Fig. 4A); however, PKR activation appears to be a late response that is first detectable at 8 h p.i. and is maximal at 18 to 24 h. Importantly, the activation of PKR does not temporally correlate with MAP kinase activation or the activation of the transcription factors CREB and NF-κB in RAW264.7 cells (Fig. 2A and B). Consistent with the effects observed in RAW264.7 cells, the infection of PEC with EMCV results in PKR phosphorylation at 8 to 24 h p.i. (24-fold). Virus-induced PKR phosphorylation appears to be indicative of PKR activity, since the phosphorylation of eIF2α, a well-characterized downstream target of PKR, correlates with PKR phosphorylation and occurs in a PKR-dependent manner following 24 h of infection of PEC with EMCV (Fig. 4C and D). Surprisingly, a virus infection of macrophages also induces robust phosphorylation of eIF2α at 30 min p.i. in a manner that neither correlates with nor requires PKR activity (Fig. 4C and E). Importantly, the induction of cellular signaling pathways (MAP kinase cascades) and transcription factor activation (CREB and NF-κB) are early responses to virus infection (15 to 30 min), while robust PKR activation is observed much later during viral infection, at 12 to 24 h p.i. Moreover, EMCV-induced iNOS mRNA accumulation (at ∼4 h p.i.) precedes PKR activation by several hours, providing additional experimental evidence that PKR does not participate in the transcriptional activation of iNOS during EMCV infection of macrophages. These findings demonstrate that EMCV infection of RAW264.7 cells and primary mouse macrophages activates PKR but that this activation does not correlate with the activation of cellular signaling pathways and transcription factors that participate in antiviral gene expression.
FIG. 4.
EMCV-induced PKR activation in RAW264.7 cells and primary macrophages. RAW264.7 cells (A) or peritoneal macrophages (B to E) were treated with EMCV (1 PFU/cell) at 37°C for the indicated times. The cells were isolated, and PKR activation was determined by a Western blot analysis of PKR phosphorylation (A and B) or eIF2α phosphorylation (C to E) using phospho-specific antisera. The total levels of PKR or eIF2α were determined by Western blot analysis to control for protein loading. The results are representative of two or three independent experiments.
The IFN-α/β response is not required for EMCV-induced proinflammatory gene expression.
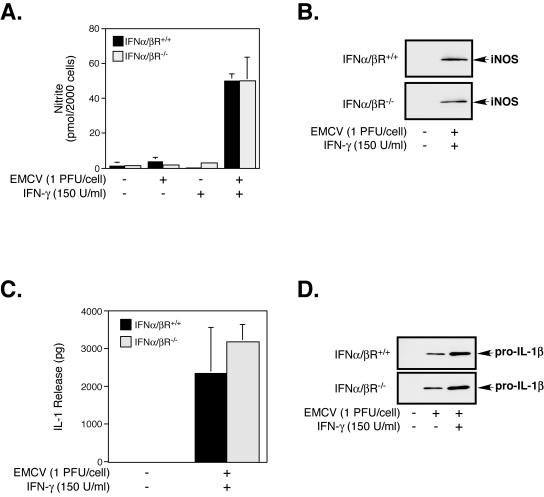
IFN-α/β plays a critical role in the host defense against invading pathogens, primarily due to the upregulation of interferon-regulated proteins such as PKR that induce an “antiviral state” and participate in the inhibition of virus replication. Although PKR is not required for the macrophage expression of iNOS in response to EMCV infection, the contribution of other interferon-regulated proteins to this antiviral response was unclear. Since IFN-β has been implicated in the regulation of iNOS expression by macrophages in response to bacterial infection (22, 42), we utilized PEC isolated from mice genetically deficient in the IFN-α/βR (IFN-α/βR−/−) to determine if the production and release of IFNs are required for EMCV-induced proinflammatory gene expression by macrophages. In the presence of IFN-γ, the infection of IFN-α/βR−/− PEC with EMCV induces the expression of iNOS and the production of nitrite, events that are indistinguishable from those of wild-type (IFN-α/βR+/+) PEC (Fig. 5A and B). An IFN-α/β-independent regulation of virus-induced antiviral gene expression does not appear to be selective for iNOS, as EMCV or EMCV plus IFN-γ induces comparable levels of IL-1 expression and release from IFN-α/βR+/+ and IFN-α/βR−/− PEC (Fig. 5C and D). Taken together, these results suggest that IFN-α/β or interferon-regulated proteins do not participate in the transcriptional activation of iNOS or IL-1 during EMCV infection of macrophages.
FIG. 5.
IFN-α/β response is not required for EMCV-induced iNOS or IL-1β expression by macrophages. Macrophages (4 × 105/400 μl cCMRL-1066) isolated from wild-type (IFN-α/βR+/+) mice or mice deficient in the IFN-α/βR (IFN-α/βR−/−) were infected with EMCV (1 PFU/cell) in the presence and absence of IFN-γ (150 U/ml) for 24 h, and nitrite production (A) and IL-1 release (C) were determined in cell culture supernatants by a Greiss assay and a RINm5F cell bioassay, respectively. The expression of iNOS (B) and IL-1β (D) following a 24-h treatment with EMCV in the presence or absence of IFN-γ was determined in cell lysates by Western blot analysis. The results for nitrite production and IL-1 release are means ± SEM for three independent experiments, and the results for iNOS and IL-1β expression are representative of three independent experiments.
MAP kinase and transcription factor activation precede EMCV replication.
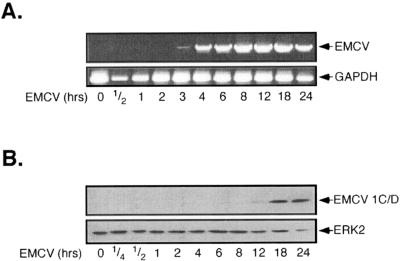
Our findings suggest that PKR does not participate in the activation of the MAP kinase cascade or in the activation of the transcription factors CREB and NF-κB following EMCV infection of macrophages; however, the mechanism by which an invading virus initiates these signal transduction cascades remains unclear. EMCV, as a member of the Picornaviridae virus family, is a small, nonenveloped, single-stranded RNA virus that exploits host-mediated translation of its ∼7.8-kb positive-stranded genome for replication. Immediately following cell surface binding and virus internalization, one of the earliest events during EMCV infection is the accumulation of single-stranded viral genomic RNA in host cells. To determine whether viral RNA accumulation correlates temporally with transcription factor and MAP kinase activation, the time-dependent appearance of EMCV RNA was evaluated in RAW264.7 cells. RAW264.7 cells were infected with EMCV, the cells were isolated, and EMCV RNA was identified by reverse transcription-PCR (RT-PCR). As shown in Fig. 6A, EMCV RNA is first detectable at 3 h, while high levels of EMCV RNA are apparent at 4 to 24 h p.i. This time course of viral RNA accumulation is consistent with findings by Hirasawa et al. (20), in which RAW264.7 cells infected with the D variant of EMCV were shown to first possess maximal levels of viral RNA at 6 h p.i. These findings temporally disassociate the accumulation of EMCV RNA (which occurs at ∼3 to 4 h p.i.) with the activation of MAP kinase signaling and transcription factor activation (which occur at ∼15 min p.i.).
FIG. 6.
EMCV RNA accumulation and protein expression in RAW264.7 cells. (A) RAW264.7 cells (1 × 106/2 ml) were treated with EMCV (1 PFU/cell) at 37°C for the indicated times, total RNA was isolated, and EMCV genomic RNA accumulation was determined by RT-PCR. GAPDH was used as an internal control for PCR. (B) RAW264.7 cells (4 × 105/400 μl) were treated with EMCV for the indicated times, cells were isolated, and viral protein expression was determined by Western blot analysis. Blots were subsequently stripped and reprobed for ERK2 as a loading control. The results for EMCV RNA accumulation and protein expression are representative of three independent experiments.
EMCV protein expression is characterized by continuous translation of the complete viral genome to generate a single full-length polypeptide that is subsequently processed through intramolecular and intermolecular cleavage; thus, all EMCV proteins are expressed simultaneously during infection and are present with equal stoichiometries. The expression of viral proteins in RAW264.7 cells was examined by Western blot analysis of EMCV 1C and 1D capsid polypeptides. Consistent with the accumulation of EMCV RNA in macrophages, the expression of these EMCV structural proteins was first detectable at 12 h, with maximal protein accumulation at 18 to 24 h p.i. (Fig. 6B). The productive infection and propagation of virus require viral protein synthesis within infected cells. While others have shown that infection of RAW264.7 cells with mengovirus, a serologically related member of the EMCV species, results in progeny virion production within 4 to 6 h (2, 32), the delay in viral protein production we observe is likely due to the decreased virulence of the B variant of EMCV. Importantly, the D variant of EMCV, which is similar to the B variant used for these studies, has been shown to activate macrophages despite nearly undetectable levels of virus protein expression and progeny virion production (20). Collectively, these findings suggest that the ability of EMCV to initiate cellular signaling through the MAP kinase cascade or to activate transcription factors does not appear to be associated with the accumulation of virus RNA or the expression of viral proteins.
Productive viral infection is not required for EMCV-induced macrophage activation.
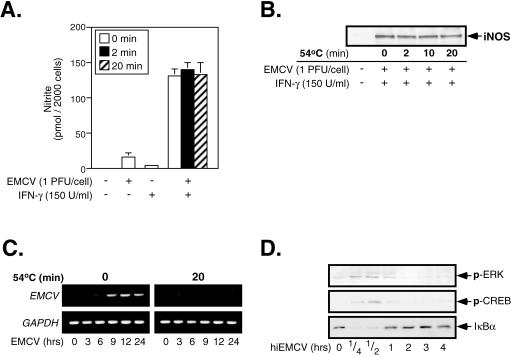
In an attempt to establish the active component of EMCV infection that induces macrophage activation, we considered the kinetics of EMCV-induced signaling and hypothesized that the initiation of these events may be receptor mediated. To this end, we sought to inactivate the replicating capacity of EMCV, thereby facilitating the study of the earliest stages of EMCV infection and their effects on macrophage activation. Previous studies by Smirnov et al. (37) have shown that heat treatment (54°C for 20 min) liberates the genomic RNA from EMCV particles and results in a nearly complete conversion of virions into empty capsid structures. To determine if empty EMCV capsids could activate the macrophage antiviral response, in the presence of IFN-γ RAW264.7 cells were infected with EMCV that had previously been heated at 54°C for 0, 2, or 20 min, and nitrite production (Fig. 7A) and iNOS expression (Fig. 7B) were determined following a 24-h infection. Importantly, the levels of nitrite production and iNOS expression induced by heat-treated virus are indistinguishable from those induced by native EMCV, suggesting that empty viral capsids are capable of activating the antiviral response of iNOS expression by macrophages. To confirm that heat treatment of EMCV sufficiently removes the genomic RNA from virus particles and prevents infection, viral RNA accumulation in macrophages was examined at 3 to 24 h p.i. As shown in Fig. 7C, macrophages infected for 3, 6, 9, 12, and 24 h with heat-inactivated EMCV (hiEMCV) did not accumulate EMCV RNA, while viral RNA was detectable in macrophages infected with the native virus. These findings suggest that heat treatment of virions at 54°C for 20 min abolishes their infectivity. Despite the loss of genomic RNA from virions following heat treatment, viral capsids retain the ability to induce signaling within macrophages. The treatment of RAW264.7 cells with hiEMCV results in the phosphorylation of ERK and CREB as well as the degradation of IκBα (Fig. 7D) in a time-dependent manner that is similar to the effects of native EMCV (Fig. 2A). Taken together, these results are consistent with the hypothesis that empty EMCV capsids possess the ability to activate macrophages and suggest that an interaction between a structural component of the virion and the macrophage contributes to the activation of the antiviral response.
FIG. 7.
Effects of heat-inactivated EMCV on RAW264.7 cells. RAW264.7 cells (4 × 105/400 μl) were treated with 150 U/ml IFN-γ and infected with EMCV that had previously been heated at 54°C for 0, 2, 10, or 20 min. Nitrite production in culture supernatants following a 24-h infection with EMCV was determined by Greiss assay (A), and iNOS expression was determined by Western blot analysis (B). RAW264.7 cells (1 × 106/2 ml) were infected with EMCV or heat-inactivated EMCV (54°C, 20 min), total RNA was isolated at the indicated times, and viral genomic RNA accumulation was determined by RT-PCR using EMCV-specific primers (C). GAPDH was used as an internal control for PCR. (D) RAW264.7 cells (4 × 105/400 μl) were treated with heat-inactivated EMCV (hiEMCV; 54°C, 20 min) for the indicated times at 37°C, and virus-induced ERK, CREB, and NF-κB activation was determined by Western blot analysis. The results are averages ± SEM for three independent experiments (A) or are representative of three independent experiments (B to D).
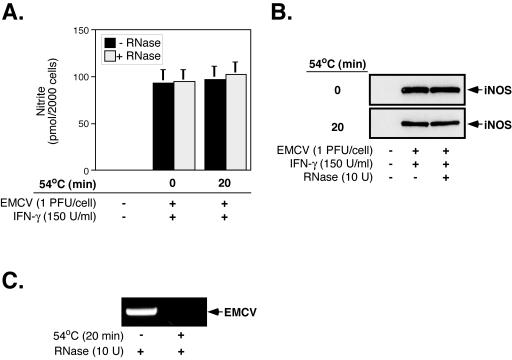
While these data suggest that the removal of viral RNA from EMCV virions does not prevent virus-induced iNOS expression, the contribution of empty EMCV capsids to macrophage activation independent of the liberated viral RNA could not be determined with certainty, as macrophages treated with heat-inactivated virus were exposed not only to EMCV capsids but also to free EMCV RNA. To determine the effects of EMCV capsids on macrophage activation and iNOS expression, viral RNA released from virions was degraded by the inclusion of a heat-stable RNase during heat inactivation. Importantly, to reduce the possibility that small RNA fragments generated by RNase degradation could activate macrophages, we chose an RNase that indiscriminately degrades RNA into single ribonucleotides in a sequence-independent manner. The addition of RNase to unheated virus preparations has no effect on virus-induced iNOS expression, since EMCV-induced nitrite production and iNOS expression occur to similar levels in the presence or absence of RNase (Fig. 8A and B). Furthermore, under conditions in which viral RNA isolated from heated virus preparations is no longer detectable by RT-PCR (Fig. 8C), EMCV induces iNOS expression and nitrite production to levels that are comparable to those induced by both native and heat-inactivated viruses (Fig. 8A and B). These results suggest that viral RNA liberated from virions during heat inactivation is not responsible for EMCV-induced iNOS expression and are consistent with the hypothesis that virus capsids are capable of inducing the antiviral response of iNOS expression by macrophages.
FIG. 8.
EMCV RNA is not required for virus-induced iNOS expression by RAW264.7 cells. EMCV was heat inactivated (54°C, 20 min) in the presence or absence of RNase (10 U) and added to RAW264.7 cells for 24 h in the presence of IFN-γ (150 U/ml). Nitrite production in culture supernatants was assessed by a Greiss assay (A), and iNOS expression in cell lysates was determined by Western blot analysis (B). (C) After the treatment of native or heat-inactivated virus preparations with RNase, RNAs were isolated, and the presence of EMCV RNA was determined by RT-PCR. The results represent means ± SEM for three independent experiments (A) or are representative of three independent experiments (B and C).
DISCUSSION
In the present study, we provide evidence to support the hypothesis that virus infection activates signaling pathways in addition to PKR and that these pathways participate in the macrophage response to viral infection. PKR is believed to be a primary mediator of antiviral activities within infected cells due to its abilities to recognize the accumulation of dsRNA and to mobilize a response to viral infection. dsRNA has been shown to activate two opposing antiviral strategies, i.e., a cell death pathway (apoptosis) (11, 36) and a survival pathway characterized by the production of proinflammatory cytokines such as interferons and interleukins (16, 17). As a critical regulatory component of the transcriptional activation of proinflammatory and antiviral genes, NF-κB may be one downstream target of PKR that mediates antiviral activities. Indeed, the central role of NF-κB during the macrophage antiviral response is evidenced by its requirement for the transcription of numerous proinflammatory genes (17, 40).
Based on initial studies using MEFs isolated from wild-type and PKR−/− mice, PKR was believed to be required for dsRNA-induced NF-κB activation (9). More recently, however, treatment with dsRNA or infection with EMCV has been shown to stimulate NF-κB activation in PKR−/− MEFs, suggesting that NF-κB activity in response to viral infection does not absolutely require PKR (21). Consistent with the PKR-independent activation of NF-κB, previous results from our laboratory as well as those shown herein indicate that dsRNA or EMCV induces NF-κB activation in macrophages despite an overexpression of dnPKR mutants or the genetic absence of PKR (29). Furthermore, the expression of NF-κB-dependent genes such as those encoding iNOS, IL-1β, and COX-2 in response to dsRNA plus IFN-γ occurs to similar levels in the presence or absence of functional PKR (29, 40).
The inducible expression of inflammatory genes during the antiviral response requires the precise and highly orchestrated activation of signaling pathways required for their transcription. The MAP kinases appear to be one signaling pathway involved in the selective regulation of specific inflammatory genes in response to dsRNA. We have shown that ERK is required for IL-1β expression and that p38 participates in COX-2 expression by macrophages (30, 39). Additionally, we have shown that the transcription factor CREB is one downstream target of a novel phospholipid signaling cascade that regulates iNOS expression (31). Since recent evidence suggests that PKR-independent pathways participate in antiviral activities within infected cells, we sought to determine if the signaling pathways involved in the dsRNA-induced transcriptional activation of proinflammatory and antiviral genes were also activated by authentic viral infection and if PKR participates in the regulation of these signaling cascades during infection. We show here that EMCV infection of RAW264.7 cells or PEC results in the rapid activation of the MAP kinases as well as the transcription factors CREB and NF-κB. The time-dependent activation of these pathways in dnPKR-expressing RAW264.7 cells or PKR−/− PEC is indistinguishable from that observed in vector control RAW264.7 cells or PKR+/+ PEC, suggesting that PKR does not participate in the regulation and activation of these signaling pathways. These results are reminiscent of studies performed by Magun and coworkers suggesting that EMCV-induced activation of the MAP kinase p38 is independent of PKR (21). These conclusions must be tempered, however, by the possibility that other “PKR-like” proteins may compensate for PKR in dnPKR-expressing RAW264.7 cells as well as by recent evidence that the PKR−/− mice used for this study may be incomplete knockouts (6). However, EMCV-induced MAP kinase, CREB, and NF-κB activation precedes PKR phosphorylation by more than 8 h, further supporting a PKR-independent mechanism of activation of these pathways. EMCV-induced PKR activity appears to be a late response of macrophages to viral infection (at 24 h p.i.), as evidenced by a temporal correlation between PKR phosphorylation and phosphorylation of the PKR substrate eIF2α. Surprisingly, the phosphorylation of eIF2α is also evident early during virus infection (at 30 min p.i.), and this event appears to be independent of PKR. While it is known that multiple eIF2α kinases exist, the mechanism by which virus infection may induce eIF2α phosphorylation in a PKR-independent manner is currently unknown.
Since our experimental results suggested that PKR does not participate in the activation of the MAP kinase, CREB, and NF-κB pathways, we examined the mechanism by which EMCV infection activates macrophages. While IFN-α/β and interferon-regulated proteins play a critical role in the host defense against viral infection and participate in the establishment of an antiviral response, they do not appear to be required for proinflammatory gene expression by EMCV-infected macrophages, since EMCV induces comparable levels of iNOS and IL-1 expression in the presence or absence of the IFN-α/β receptor.
EMCV relies on the host-mediated translation of its mRNA-sense genome for the production of viral proteins, and therefore one early event during viral replication is the introduction of genomic viral RNA into the host cell cytoplasm. Since the accumulation of EMCV RNA at 2 to 3 h p.i. and the expression of viral proteins at 8 h p.i. fail to correlate temporally with the activation of these signaling pathways, it is unlikely that viral RNA or RNA replication is responsible for macrophage activation. Interestingly, the D variant of EMCV is known to activate but not replicate in macrophages, and the apparent delay in both viral RNA accumulation and protein expression compared to those in RAW264.7 cells infected with wild-type EMCV-R is potentially due to the inability of the B variant of EMCV to establish a productive infection in these cells (20).
Specific molecular interactions between an invading virus and cell surface determinants on host cells not only confer cell and tissue tropism but also can initiate alterations in cellular function that are important for successful viral replication. The association of virus particles with the cell surface during the initial stages of infection has been shown to activate various signaling pathways. The binding of human cytomegalovirus to Toll-like receptor 2 can activate NF-κB (10), while a replication-defective adenovirus has been shown to stimulate MAP kinase signaling in A549 cells as early as 10 min p.i. (41). The kinetics with which EMCV infection activates signal transduction cascades within macrophages suggests that a virus-receptor interaction may participate in the activation of these pathways. We hypothesized, therefore, that a replication-deficient virus would retain its ability to activate macrophages. When EMCV was subjected to heating, a condition that converts infectious virus particles into empty capsid structures (37), iNOS expression, nitrite production, and ERK and CREB phosphorylation, as well as IκB degradation, were induced in macrophages in a similar manner to that induced by native EMCV, despite an inability of the heated virus to introduce viral RNA into host cells and initiate a productive infection. These results suggest that an interaction between a structural component of the virion and a cell surface receptor may initiate these signaling cascades. Caution must be used when interpreting these results, however, as the viral RNA liberated from virions during the heat-mediated conversion into empty capsid structures may contribute to the activation of the antiviral response. While it is plausible that viral RNA could interact with cell surface receptors such as Toll-like receptor 3, which is capable of binding dsRNA and mediating the downstream activation of NF-κB (1), purified EMCV RNA fails to stimulate nitrite production by macrophages in the presence of IFN-γ (J. M. Moran and J. A. Corbett, unpublished data), suggesting that viral RNA is not sufficient for virus-induced activation of the antiviral activities we observed. Furthermore, despite undetectable levels of viral RNA after RNase treatment, heat-inactivated virus preparations maintain the ability to activate the antiviral response of iNOS expression by macrophages.
In summary, these studies provide evidence that additional antiviral signaling pathways independent of PKR participate in macrophage activation in response to viral infection, since (i) EMCV-induced MAP kinase, CREB, and NF-κB signaling precedes PKR phosphorylation, (ii) the activation of these signaling pathways by EMCV infection is unaffected by the overexpression of dnPKR or the genetic absence of PKR, and (iii) iNOS expression and nitric oxide production occur to similar levels in vector control and dnPKR-expressing RAW264.7 cells infected with EMCV. The mechanism by which these antiviral signaling pathways are activated does not appear to require viral RNA accumulation or viral protein expression, but rather may be associated with an interaction between a structural determinant of the virion and a macrophage cell surface receptor. These findings highlight the complexity of interactions between an invading virus and the host cell response and suggest that PKR-independent mechanisms contribute to the antiviral response.
Acknowledgments
We thank Colleen Bratcher for expert technical assistance and Ji-won Yoon for providing EMCV. We also thank Abdul Waheed for expert technical advice and assistance with the manuscript and Ann Palmenberg for the EMCV antibody and helpful discussions concerning EMCV.
This work was supported by National Institutes of Health grants DK-52194 and AI-44458 (J.A.C.).
REFERENCES
- 1.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Aminev, A. G., S. P. Amineva, and A. C. Palmenberg. 2003. Encephalomyocarditis virus (EMCV) proteins 2A and 3BCD localize to nuclei and inhibit cellular mRNA transcription but not rRNA transcription. Virus Res. 95:59-73. [DOI] [PubMed] [Google Scholar]
- 3.Arnush, M., A. L. Scarim, M. R. Heitmeier, C. B. Kelly, and J. A. Corbett. 1998. Potential role of resident islet macrophage activation in the initiation of autoimmune diabetes. J. Immunol. 160:2684-2691. [PubMed] [Google Scholar]
- 4.Bae, Y. S., H. M. Eun, and J. W. Yoon. 1989. Genomic differences between the diabetogenic and nondiabetogenic variants of encephalomyocarditis virus. Virology 170:282-287. [DOI] [PubMed] [Google Scholar]
- 5.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 6.Baltzis, D., S. Li, and A. E. Koromilas. 2002. Functional characterization of PKR gene products expressed in cells from mice with a targeted deletion of the N terminus or C terminus domain of PKR. J. Biol. Chem. 277:38364-38372. [DOI] [PubMed] [Google Scholar]
- 7.Beckerman, K. P., H. W. Rogers, J. A. Corbett, R. D. Schreiber, M. L. McDaniel, and E. R. Unanue. 1993. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J. Immunol. 150:888-895. [PubMed] [Google Scholar]
- 8.Bukrinsky, M. I., H. S. Nottet, H. Schmidtmayerova, L. Dubrovsky, C. R. Flanagan, M. E. Mullins, S. A. Lipton, and H. E. Gendelman. 1995. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J. Exp. Med. 181:735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chu, W. M., D. Ostertag, Z. W. Li, L. Chang, Y. Chen, Y. Hu, B. Williams, J. Perrault, and M. Karin. 1999. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity 11:721-731. [DOI] [PubMed] [Google Scholar]
- 10.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Der, S. D., Y. L. Yang, C. Weissmann, and B. R. Williams. 1997. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:3279-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duerst, R. J., and L. A. Morrison. 2004. Herpes simplex virus 2 virion host shutoff protein interferes with type I interferon production and responsiveness. Virology 322:158-167. [DOI] [PubMed] [Google Scholar]
- 13.Flodstrom, M., M. S. Horwitz, A. Maday, D. Balakrishna, E. Rodriguez, and N. Sarvetnick. 2001. A critical role for inducible nitric oxide synthase in host survival following coxsackievirus B4 infection. Virology 281:205-215. [DOI] [PubMed] [Google Scholar]
- 14.Gale, M., Jr., and M. G. Katze. 1998. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol. Ther. 78:29-46. [DOI] [PubMed] [Google Scholar]
- 15.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 16.Heitmeier, M. R., M. Arnush, A. L. Scarim, and J. A. Corbett. 2001. Pancreatic β-cell damage mediated by β-cell production of interleukin-1. A novel mechanism for virus-induced diabetes. J. Biol. Chem. 276:11151-11158. [DOI] [PubMed] [Google Scholar]
- 17.Heitmeier, M. R., A. L. Scarim, and J. A. Corbett. 1998. Double-stranded RNA-induced inducible nitric-oxide synthase expression and interleukin-1 release by murine macrophages requires NF-κB activation. J. Biol. Chem. 273:15301-15307. [DOI] [PubMed] [Google Scholar]
- 18.Heitmeier, M. R., A. L. Scarim, and J. A. Corbett. 1997. Interferon-γ increases the sensitivity of islets of Langerhans for inducible nitric-oxide synthase expression induced by interleukin 1. J. Biol. Chem. 272:13697-13704. [DOI] [PubMed] [Google Scholar]
- 19.Hill, J. R., J. A. Corbett, A. C. Baldwin, and M. L. McDaniel. 1996. Nitric oxide production by the rat insulinoma cell line, RINm5F, is specific for IL-1: a spectrophotometric IL-1 bioassay. Anal. Biochem. 236:14-19. [DOI] [PubMed] [Google Scholar]
- 20.Hirasawa, K., H. S. Jun, H. S. Han, M. L. Zhang, M. D. Hollenberg, and J. W. Yoon. 1999. Prevention of encephalomyocarditis virus-induced diabetes in mice by inhibition of the tyrosine kinase signaling pathway and subsequent suppression of nitric oxide production in macrophages. J. Virol. 73:8541-8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iordanov, M. S., J. Wong, J. C. Bell, and B. E. Magun. 2001. Activation of NF-κB by double-stranded RNA (dsRNA) in the absence of protein kinase R and RNase L demonstrates the existence of two separate dsRNA-triggered antiviral programs. Mol. Cell. Biol. 21:61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs, A. T., and L. J. Ignarro. 2001. Lipopolysaccharide-induced expression of interferon-β mediates the timing of inducible nitric-oxide synthase induction in RAW 264.7 macrophages. J. Biol. Chem. 276:47950-47957. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 24.Karupiah, G., Q. W. Xie, R. M. Buller, C. Nathan, C. Duarte, and J. D. MacMicking. 1993. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science 261:1445-1448. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman, R. J. 1999. Double-stranded RNA-activated protein kinase mediates virus-induced apoptosis: a new role for an old actor. Proc. Natl. Acad. Sci. USA 96:11693-11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar, A., J. Haque, J. Lacoste, J. Hiscott, and B. R. Williams. 1994. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating IκB. Proc. Natl. Acad. Sci. USA 91:6288-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 28.Lowenstein, C. J., S. L. Hill, A. Lafond-Walker, J. Wu, G. Allen, M. Landavere, N. R. Rose, and A. Herskowitz. 1996. Nitric oxide inhibits viral replication in murine myocarditis. J. Clin. Investig. 97:1837-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggi, L. B., Jr., M. R. Heitmeier, D. Scheuner, R. J. Kaufman, R. M. Buller, and J. A. Corbett. 2000. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J. 19:3630-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maggi, L. B., Jr., J. M. Moran, R. M. Buller, and J. A. Corbett. 2003. ERK activation is required for double-stranded RNA- and virus-induced interleukin-1 expression by macrophages. J. Biol. Chem. 278:16683-16689. [DOI] [PubMed] [Google Scholar]
- 31.Maggi, L. B., Jr., J. M. Moran, A. L. Scarim, D. A. Ford, J. W. Yoon, J. McHowat, R. M. Buller, and J. A. Corbett. 2002. Novel role for calcium-independent phospholipase A2 in the macrophage antiviral response of inducible nitric-oxide synthase expression. J. Biol. Chem. 277:38449-38455. [DOI] [PubMed] [Google Scholar]
- 32.Martin, L. R., Z. C. Neal, M. S. McBride, and A. C. Palmenberg. 2000. Mengovirus and encephalomyocarditis virus poly(C) tract lengths can affect virus growth in murine cell culture. J. Virol. 74:3074-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 34.Murphy, J. A., R. J. Duerst, T. J. Smith, and L. A. Morrison. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates α/β interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 77:9337-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saura, M., C. Zaragoza, A. McMillan, R. A. Quick, C. Hohenadl, J. M. Lowenstein, and C. J. Lowenstein. 1999. An antiviral mechanism of nitric oxide: inhibition of a viral protease. Immunity 10:21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scarim, A. L., M. Arnush, L. A. Blair, J. Concepcion, M. R. Heitmeier, D. Scheuner, R. J. Kaufman, J. Ryerse, R. M. Buller, and J. A. Corbett. 2001. Mechanisms of β-cell death in response to double-stranded (ds) RNA and interferon-γ: dsRNA-dependent protein kinase apoptosis and nitric oxide-dependent necrosis. Am. J. Pathol. 159:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smirnov, Y. A., M. P. Rodrigues-Molto, and M. T. Famadas. 1983. Protein-RNA interaction in encephalomyocarditis virus as revealed by UV light-induced covalent linkages. J. Virol. 45:1048-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava, S. P., K. U. Kumar, and R. J. Kaufman. 1998. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 273:2416-2423. [DOI] [PubMed] [Google Scholar]
- 39.Steer, S. A., and J. A. Corbett. 2003. The role and regulation of COX-2 during viral infection. Viral Immunol. 16:447-460. [DOI] [PubMed] [Google Scholar]
- 40.Steer, S. A., J. M. Moran, L. B. Maggi, Jr., R. M. Buller, H. Perlman, and J. A. Corbett. 2003. Regulation of cyclooxygenase-2 expression by macrophages in response to double-stranded RNA and viral infection. J. Immunol. 170:1070-1076. [DOI] [PubMed] [Google Scholar]
- 41.Tamanini, A., R. Rolfini, E. Nicolis, P. Melotti, and G. Cabrini. 2003. MAP kinases and NF-κB collaborate to induce ICAM-1 gene expression in the early phase of adenovirus infection. Virology 307:228-242. [DOI] [PubMed] [Google Scholar]
- 42.Utaisincharoen, P., N. Anuntagool, K. Limposuwan, P. Chaisuriya, and S. Sirisinha. 2003. Involvement of β interferon in enhancing inducible nitric oxide synthase production and antimicrobial activity of Burkholderia pseudomallei-infected macrophages. Infect. Immun. 71:3053-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu, S., and R. J. Kaufman. 1996. Double-stranded (ds) RNA binding and not dimerization correlates with the activation of the dsRNA-dependent protein kinase (PKR). J. Biol. Chem. 271:1756-1763. [DOI] [PubMed] [Google Scholar]
- 44.Wu, S., and R. J. Kaufman. 1997. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J. Biol. Chem. 272:1291-1296. [DOI] [PubMed] [Google Scholar]
- 45.Yang, Y. L., L. F. Reis, J. Pavlovic, A. Aguzzi, R. Schafer, A. Kumar, B. R. Williams, M. Aguet, and C. Weissmann. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14:6095-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]