Abstract
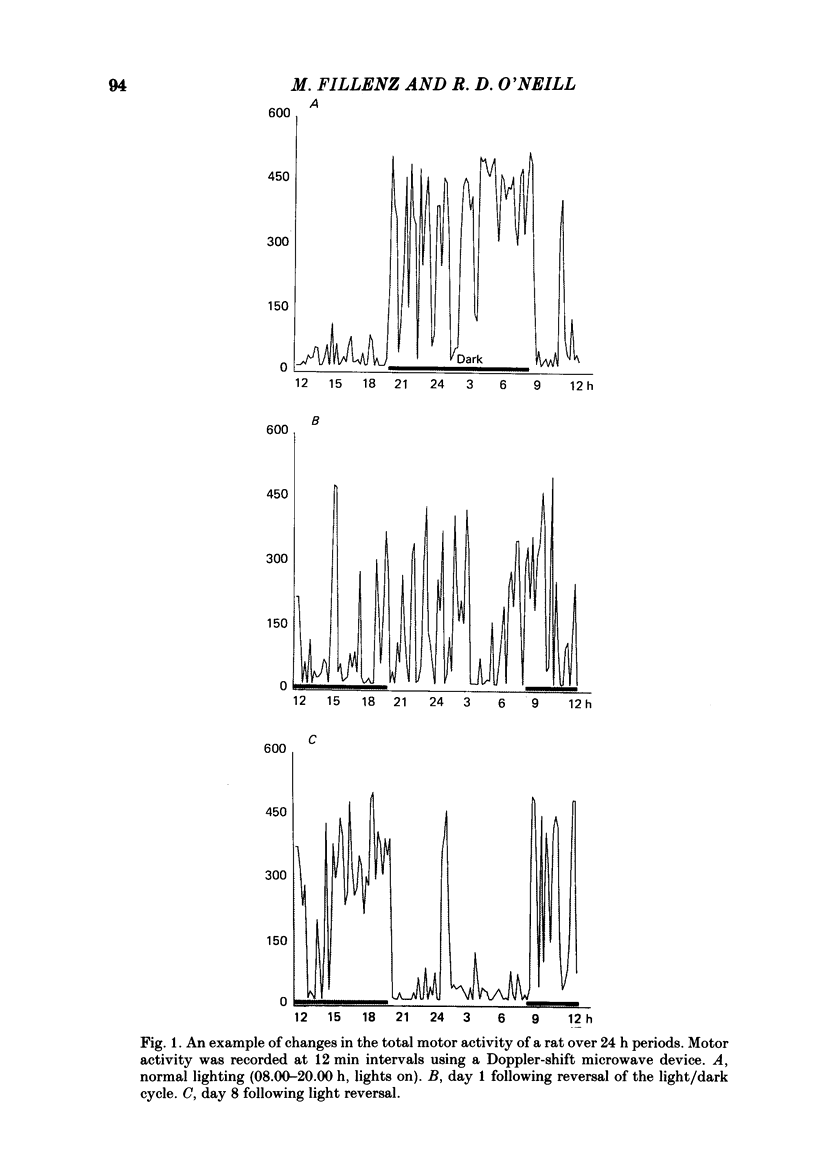
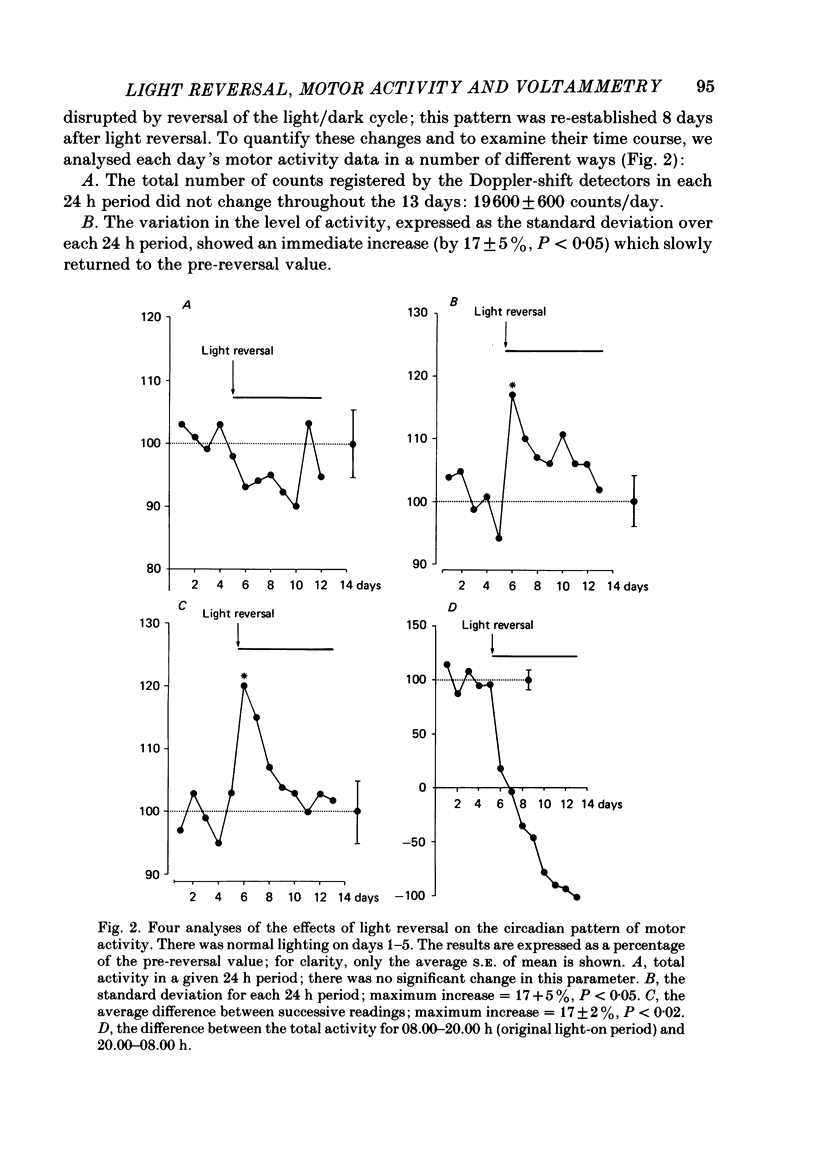
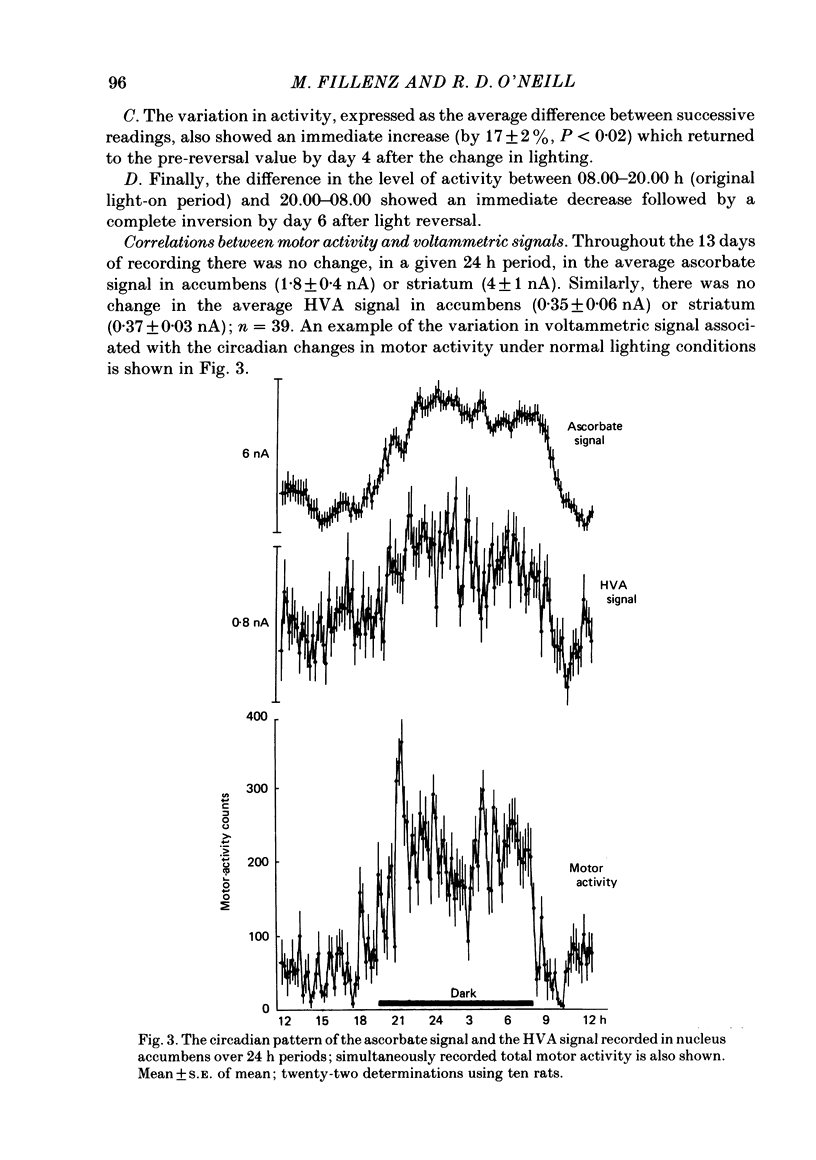
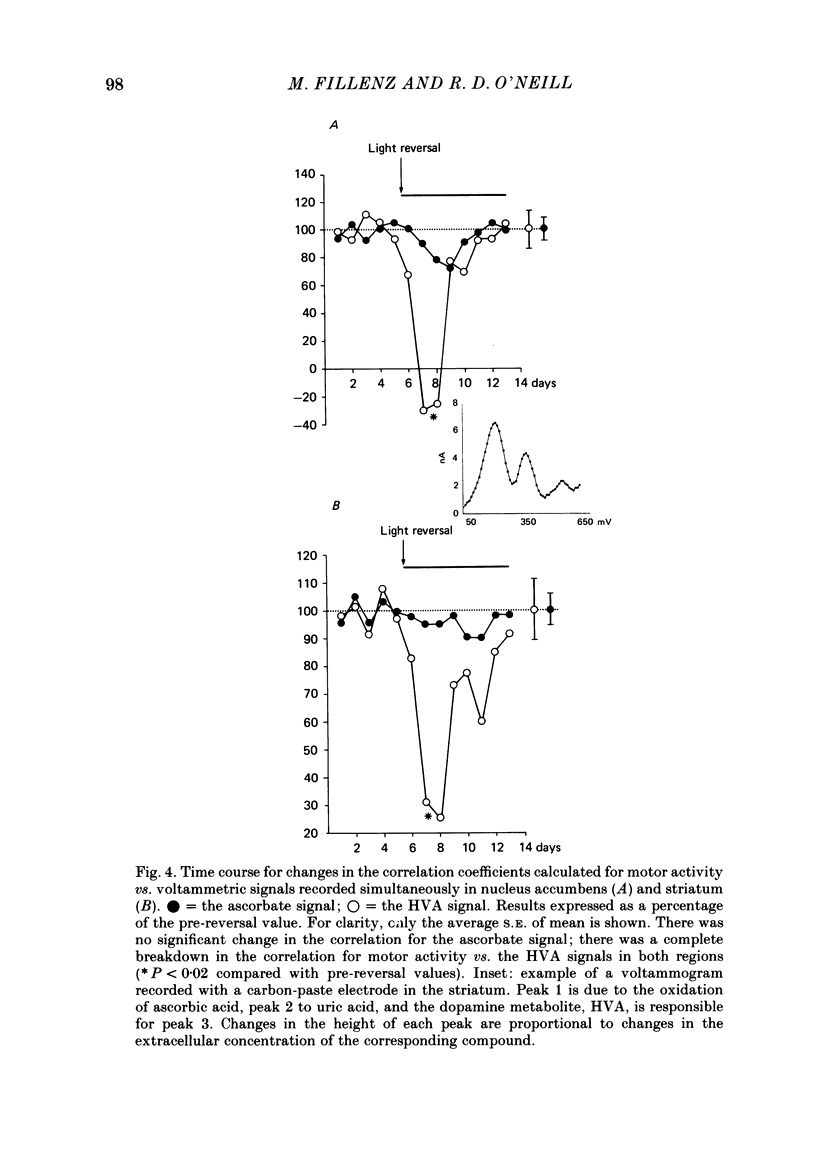
To investigate the functional relationships between the circadian changes in rat motor activity and changes in the extracellular concentration of ascorbic acid and homovanillic acid (HVA) monitored in the striatum and nucleus accumbens, reversal of the light/dark cycle was used to disturb the pattern of motor activity. Microcomputer-controlled linear sweep voltammetry with carbon-paste electrodes was used to continuously monitor circadian changes in the ascorbate signal and the HVA signal simultaneously in nucleus accumbens and striatum over a 13 day period in unrestrained rats; total motor activity for each animal was also recorded. Regression analyses were carried out on each day's data to investigate the relationships between motor activity and the two voltammetric signals. During days 1-5, the lighting was on normal 12/12 light/dark cycle and high correlations were observed. Reversal of the light/dark cycle on day 6 caused an immediate change in the pattern of motor activity and electrochemical signals; by days 7-8 after light reversal the relationships between lighting, ascorbate, HVA and motor activity were reestablished under the new lighting conditions. During the intervening period, however, there was a complete breakdown in some of the correlations. The findings are discussed in the light of the hypothesis that changes in brain extracellular ascorbate reflect changes in the release of excitatory amino acids, and in terms of a recent model of the role, in the control of motor activity, for cortical and mesencephalic inputs to forebrain subcortical regions.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Commissiong J. W. Monoamine metabolites: their relationship and lack of relationship to monoaminergic neuronal activity. Biochem Pharmacol. 1985 Apr 15;34(8):1127–1131. doi: 10.1016/0006-2952(85)90484-8. [DOI] [PubMed] [Google Scholar]
- Da Prada M., Keller H. H., Pieri L., Kettler R., Haefely W. E. The pharmacology of Parkinson's disease: basic aspects and recent advances. Experientia. 1984 Nov 15;40(11):1165–1172. doi: 10.1007/BF01946641. [DOI] [PubMed] [Google Scholar]
- Kuo C. H., Yonehara N., Hata F., Yoshida H. Subcellular distribution of ascorbic acid in rat brain. Jpn J Pharmacol. 1978 Oct;28(5):789–791. doi: 10.1254/jjp.28.789. [DOI] [PubMed] [Google Scholar]
- Louilot A., Gonon F., Buda M., Simon H., Le Moal M., Pujol J. F. Effects of D- and L-amphetamine on dopamine metabolism and ascorbic acid levels in nucleus accumbens and olfactory tubercle as studied by in vivo differential pulse voltammetry. Brain Res. 1985 Jun 17;336(2):253–263. doi: 10.1016/0006-8993(85)90652-3. [DOI] [PubMed] [Google Scholar]
- McGeer E. G., Staines W. A., McGeer P. L. Neurotransmitters in the basal ganglia. Can J Neurol Sci. 1984 Feb;11(1 Suppl):89–99. doi: 10.1017/s0317167100046217. [DOI] [PubMed] [Google Scholar]
- Miller J. D., Farber J., Gatz P., Roffwarg H., German D. C. Activity of mesencephalic dopamine and non-dopamine neurons across stages of sleep and walking in the rat. Brain Res. 1983 Aug 22;273(1):133–141. doi: 10.1016/0006-8993(83)91101-0. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., Kerkerian L., Dusticier N. Presynaptic dopaminergic control of high affinity glutamate uptake in the striatum. Neurosci Lett. 1983 Dec 30;43(2-3):191–196. doi: 10.1016/0304-3940(83)90186-6. [DOI] [PubMed] [Google Scholar]
- O'Neill R. D., Fillenz M., Albery W. J. The development of linear sweep voltammetry with carbon paste electrodes in vivo. J Neurosci Methods. 1983 Jul;8(3):263–273. doi: 10.1016/0165-0270(83)90039-0. [DOI] [PubMed] [Google Scholar]
- O'Neill R. D., Fillenz M. Circadian changes in extracellular ascorbate in rat cortex, accumbens, striatum and hippocampus: correlations with motor activity. Neurosci Lett. 1985 Oct 10;60(3):331–336. doi: 10.1016/0304-3940(85)90599-3. [DOI] [PubMed] [Google Scholar]
- O'Neill R. D., Fillenz M. Detection of homovanillic acid in vivo using microcomputer-controlled voltammetry: simultaneous monitoring of rat motor activity and striatal dopamine release. Neuroscience. 1985 Mar;14(3):753–763. doi: 10.1016/0306-4522(85)90140-x. [DOI] [PubMed] [Google Scholar]
- O'Neill R. D., Fillenz M., Grünewald R. A., Bloomfield M. R., Albery W. J., Jamieson C. M., Williams J. H., Gray J. A. Voltammetric carbon paste electrodes monitor uric acid and not 5-HIAA at the 5-hydroxyindole potential in the rat brain. Neurosci Lett. 1984 Mar 9;45(1):39–46. doi: 10.1016/0304-3940(84)90326-4. [DOI] [PubMed] [Google Scholar]
- O'Neill R. D., Fillenz M. Simultaneous monitoring of dopamine release in rat frontal cortex, nucleus accumbens and striatum: effect of drugs, circadian changes and correlations with motor activity. Neuroscience. 1985 Sep;16(1):49–55. doi: 10.1016/0306-4522(85)90046-6. [DOI] [PubMed] [Google Scholar]
- O'Neill R. D., Fillenz M., Sundstrom L., Rawlins J. N. Voltammetrically monitored brain ascorbate as an index of excitatory amino acid release in the unrestrained rat. Neurosci Lett. 1984 Dec 21;52(3):227–233. doi: 10.1016/0304-3940(84)90166-6. [DOI] [PubMed] [Google Scholar]
- O'Neill R. D., Grunewald R. A., Fillenz M., Albery W. J. The effect of unilateral cortical lesions on the circadian changes in rat striatal ascorbate and homovanillic acid levels measured in vivo using voltammetry. Neurosci Lett. 1983 Nov 21;42(1):105–110. doi: 10.1016/0304-3940(83)90430-5. [DOI] [PubMed] [Google Scholar]
- O'Neill R. D., Grünewald R. A., Fillenz M., Albery W. J. Linear sweep voltammetry with carbon paste electrodes in the rat striatum. Neuroscience. 1982;7(8):1945–1954. doi: 10.1016/0306-4522(82)90009-4. [DOI] [PubMed] [Google Scholar]
- Penney J. B., Jr, Young A. B. Speculations on the functional anatomy of basal ganglia disorders. Annu Rev Neurosci. 1983;6:73–94. doi: 10.1146/annurev.ne.06.030183.000445. [DOI] [PubMed] [Google Scholar]
- Trulson M. E., Preussler D. W., Howell G. A. Activity of substantia nigra units across the sleep-waking cycle in freely moving cats. Neurosci Lett. 1981 Oct 23;26(2):183–188. doi: 10.1016/0304-3940(81)90346-3. [DOI] [PubMed] [Google Scholar]
- Walaas I. Biochemical evidence for overlapping neocortical and allocortical glutamate projections to the nucleus accumbens and rostral caudatoputamen in the rat brain. Neuroscience. 1981;6(3):399–405. doi: 10.1016/0306-4522(81)90132-9. [DOI] [PubMed] [Google Scholar]


