Abstract
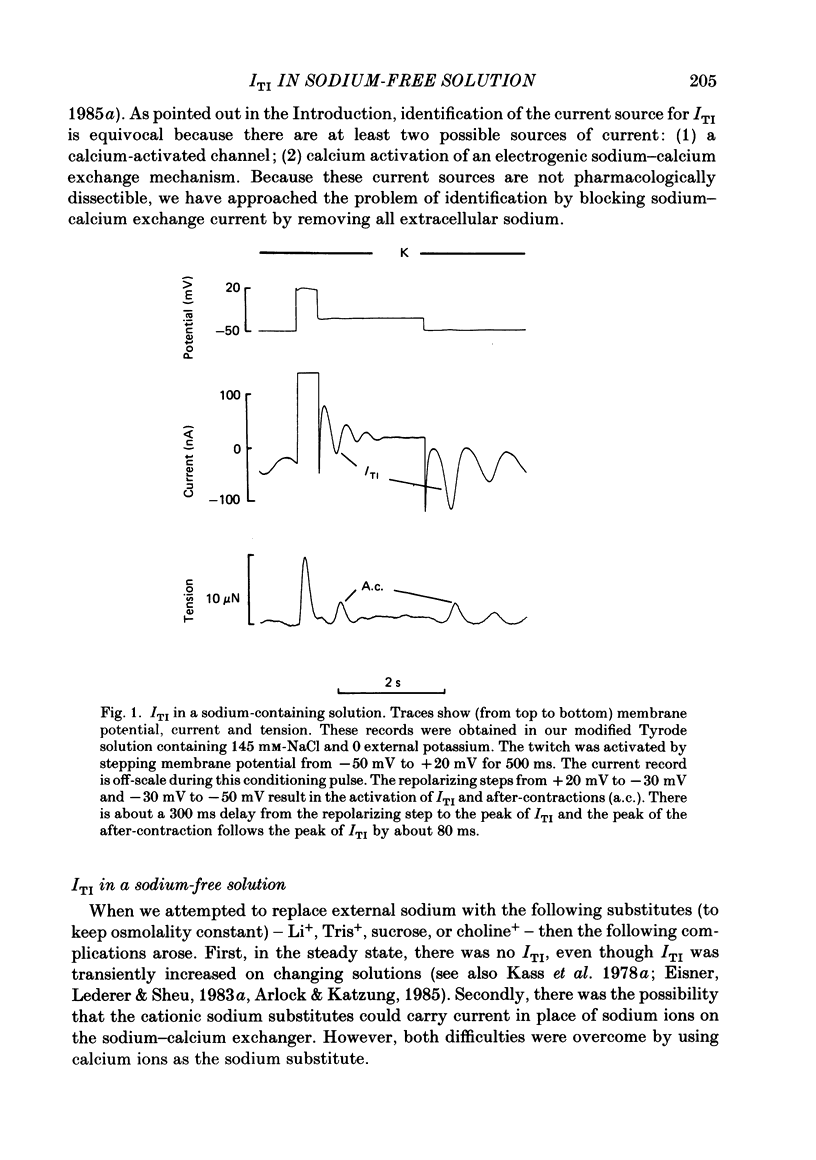
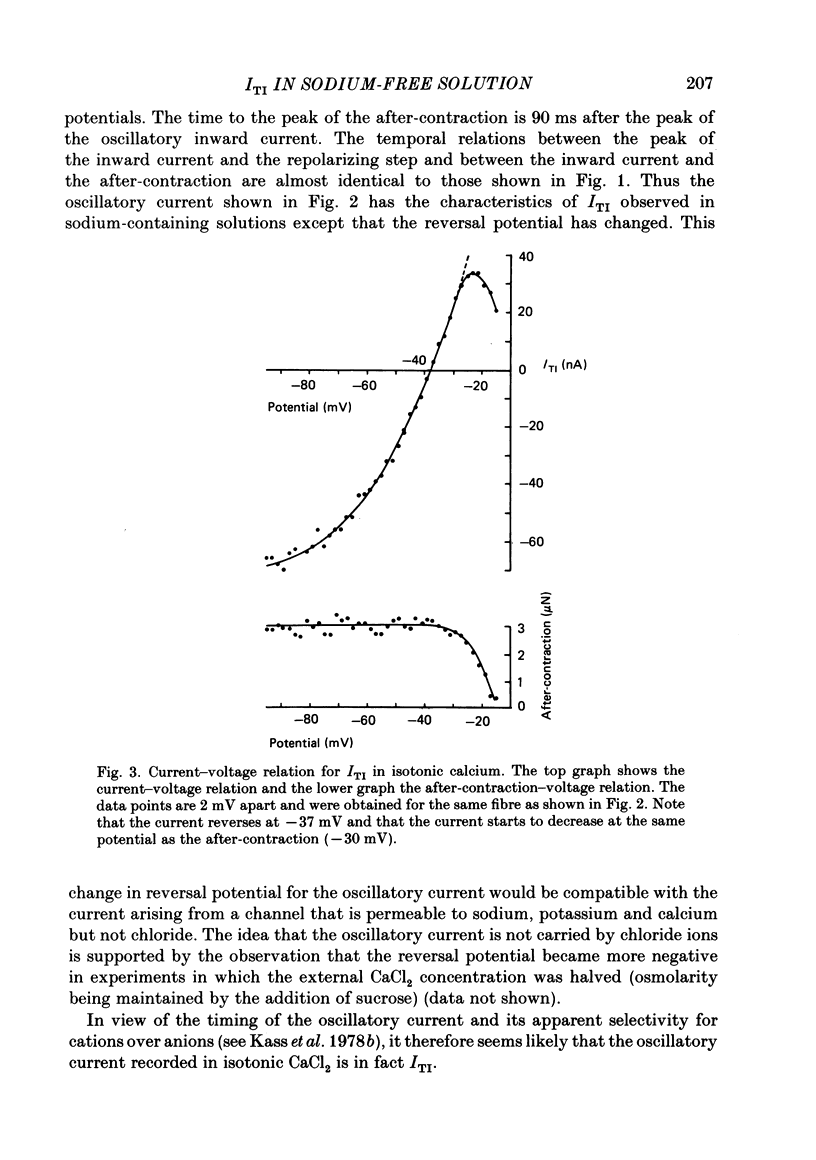
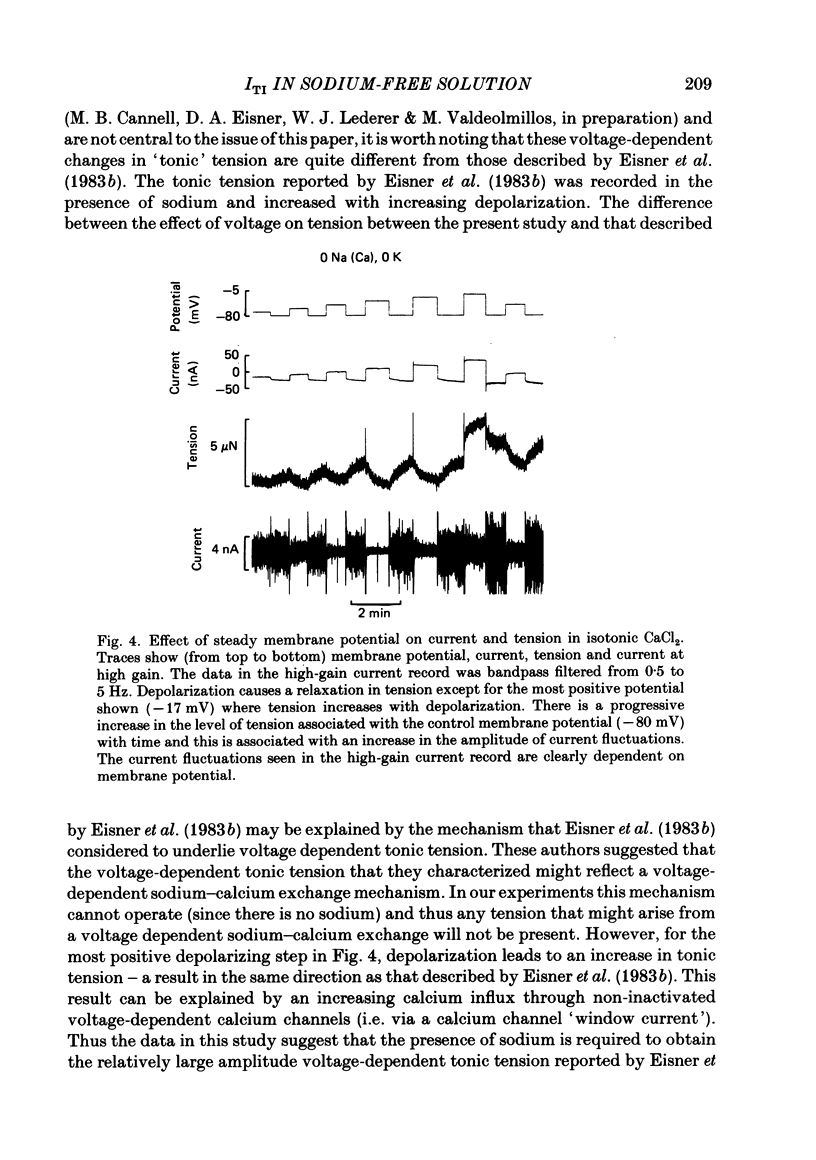
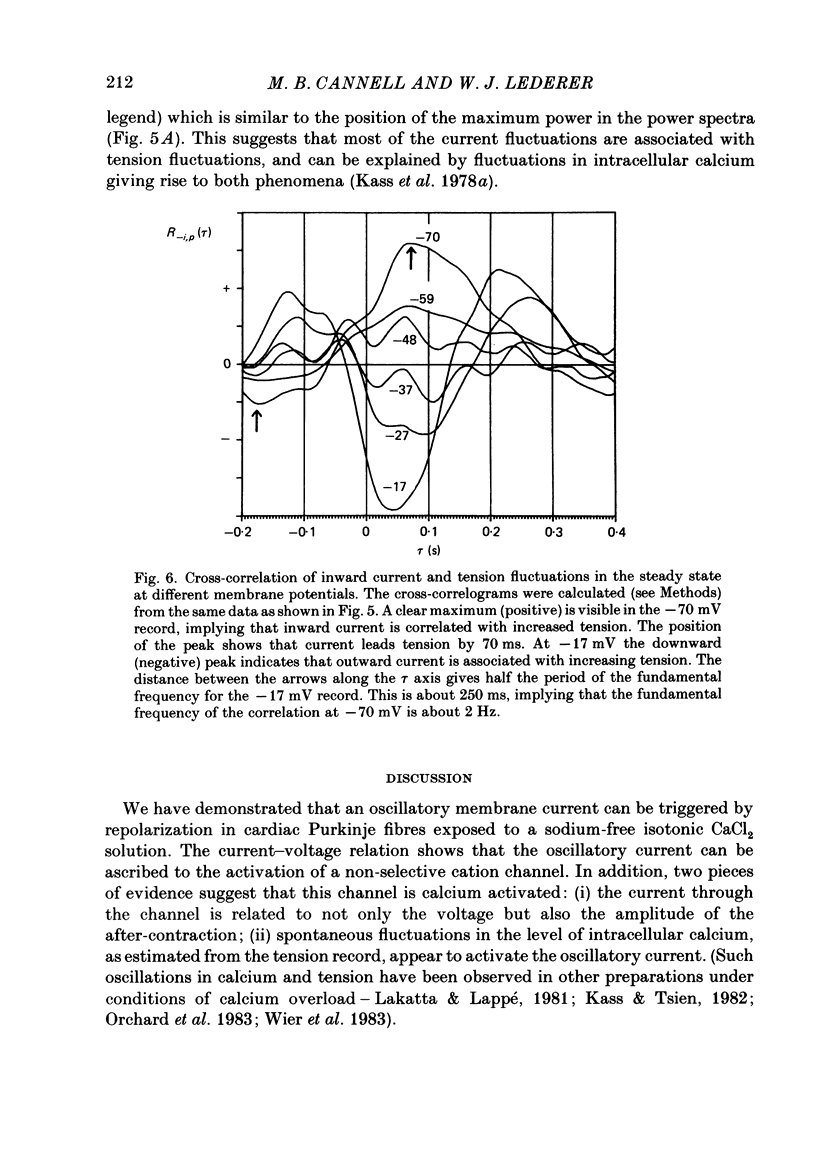
Sheep cardiac Purkinje fibres were voltage clamped with a two-microelectrode technique. Under conditions that are known to elevate intracellular calcium (0 mM-external potassium), membrane currents were examined. In the above conditions, a brief depolarizing pulse leads to an oscillatory inward current (ITI) which peaks at about 300 ms after the repolarization. An after-contraction is also observed, the peak of which occurs about 80 ms after the peak of ITI. This result is in accord with the results of Kass, Lederer, Tsien & Weingart (1978a). We replaced external sodium with an isotonic CaCl2 solution to remove the sodium-calcium exchange mechanism as a possible current carrier for ITI. In the steady state under these conditions an oscillatory membrane current and after-contraction are seen following repolarization. This current was identified as ITI on the basis of its temporal relation to both the repolarization step and the after-contraction. In isotonic CaCl2, ITI has a reversal potential of -37 mV. Because of this fact ITI cannot be explained by an electrogenic sodium-calcium exchange mechanism alone. The reversal potential suggests that ITI arises from a channel which is permeable to both potassium and calcium. Fluctuations of membrane current and of tension were recorded in the steady state at different holding potentials. Power spectral analysis showed that the current fluctuations were at a minimum at a holding potential of -37 mV. Tension fluctuations were, however, relatively constant over the range of membrane potentials examined (-17 to -70 mV). The peak power of the current fluctuations occurred at about 1.5 Hz (at a holding potential of -70 mV). This peak shifted towards higher frequencies with increasing depolarization. A similar shift in frequency was observed for the tension fluctuations. Cross-correlations between membrane current and tension were calculated for various steady membrane potentials. This analysis shows that the current fluctuations are associated with the tension fluctuations, each with a principal period of about 0.5 s. This analysis also shows that at potentials more negative than the reversal potential of ITI, increasing tension is associated with increasing inward current and that the tension fluctuations follow current fluctuations by about 70 ms. At potentials positive to the reversal potential of ITI, increasing tension was associated with increasing outward current. This analysis therefore indicates that the fluctuations in membrane current reverse at a potential similar to the reversal potential of ITI.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Orchard C. H. Characterization of oscillations of intracellular calcium concentration in ferret ventricular muscle. J Physiol. 1984 Jul;352:113–128. doi: 10.1113/jphysiol.1984.sp015281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlock P., Katzung B. G. Effects of sodium substitutes on transient inward current and tension in guinea-pig and ferret papillary muscle. J Physiol. 1985 Mar;360:105–120. doi: 10.1113/jphysiol.1985.sp015606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson R. S., Gelles J. M. The effect of ouabain, dinitrophenol, and lithium on the pacemaker current in sheep cardiac Purkinje fibers. Circ Res. 1977 May;40(5):517–524. doi: 10.1161/01.res.40.5.517. [DOI] [PubMed] [Google Scholar]
- Attwell D., Jack J. The interpretation of membrane current voltage relations: a Nernst-Planck analysis. Prog Biophys Mol Biol. 1978;34(2):81–107. doi: 10.1016/0079-6107(79)90015-4. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Ellis D. Intracellular calcium and sodium activity in sheep heart Purkinje fibres. Effect of changes of external sodium and intracellular pH. Pflugers Arch. 1982 Apr;393(2):171–178. doi: 10.1007/BF00582941. [DOI] [PubMed] [Google Scholar]
- Boyett M. R. Effect of rate-dependent changes in the transient outward current on the action potential in sheep Purkinje fibres. J Physiol. 1981;319:23–41. doi: 10.1113/jphysiol.1981.sp013889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Allen D. G. Model of calcium movements during activation in the sarcomere of frog skeletal muscle. Biophys J. 1984 May;45(5):913–925. doi: 10.1016/S0006-3495(84)84238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Vaughan-Jones R. D., Lederer W. J. Ryanodine block of calcium oscillations in heart muscle and the sodium-tension relationship. Fed Proc. 1985 Dec;44(15):2964–2969. [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- DECK K. A., KERN R., TRAUTWEIN W. VOLTAGE CLAMP TECHNIQUE IN MAMMALIAN CARDIAC FIBRES. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jun 9;280:50–62. doi: 10.1007/BF00412615. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Inotropic and arrhythmogenic effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:255–277. doi: 10.1113/jphysiol.1979.sp012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Na-Ca exchange: stoichiometry and electrogenicity. Am J Physiol. 1985 Mar;248(3 Pt 1):C189–C202. doi: 10.1152/ajpcell.1985.248.3.C189. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Sheu S. S. The role of intracellular sodium activity in the anti-arrhythmic action of local anaesthetics in sheep Purkinje fibres. J Physiol. 1983 Jul;340:239–257. doi: 10.1113/jphysiol.1983.sp014761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The control of tonic tension by membrane potential and intracellular sodium activity in the sheep cardiac Purkinje fibre. J Physiol. 1983 Feb;335:723–743. doi: 10.1113/jphysiol.1983.sp014560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The quantitative relationship between twitch tension and intracellular sodium activity in sheep cardiac Purkinje fibres. J Physiol. 1984 Oct;355:251–266. doi: 10.1113/jphysiol.1984.sp015417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Valdeolmillos M. The mechanism of the increase of tonic tension produced by caffeine in sheep cardiac Purkinje fibres. J Physiol. 1985 Jul;364:313–326. doi: 10.1113/jphysiol.1985.sp015747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrier G. R. Digitalis arrhythmias: role of oscillatory afterpotentials. Prog Cardiovasc Dis. 1977 May-Jun;19(6):459–474. doi: 10.1016/0033-0620(77)90010-x. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R., Moe G. K. Effect of calcium on acetylstrophanthidin-induced transient depolarizations in canine Purkinje tissue. Circ Res. 1973 Nov;33(5):508–515. doi: 10.1161/01.res.33.5.508. [DOI] [PubMed] [Google Scholar]
- Ferrier G. R., Saunders J. H., Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973 May;32(5):600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- Hiraoka M. Membrane current changes induced by acetylstrophanthidin in cardiac Purkinje fibers. Jpn Heart J. 1977 Nov;18(6):851–859. doi: 10.1536/ihj.18.851. [DOI] [PubMed] [Google Scholar]
- KATZUNG B. DIASTOLIC OSCILLATION IN MUSCLE TENSION AND LENGTH. J Cell Physiol. 1964 Aug;64:103–114. doi: 10.1002/jcp.1030640110. [DOI] [PubMed] [Google Scholar]
- Karagueuzian H. S., Katzung B. G. Voltage-clamp studies of transient inward current and mechanical oscillations induced by ouabain in ferret papillary muscle. J Physiol. 1982 Jun;327:255–271. doi: 10.1113/jphysiol.1982.sp014230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982 Jun;38(3):259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W., Weingart R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:209–226. doi: 10.1113/jphysiol.1978.sp012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. P., Cohen I. S. Extracellular [K+] fluctuations in voltage-clamped canine cardiac Purkinje fibers. Biophys J. 1984 Nov;46(5):663–668. doi: 10.1016/S0006-3495(84)84065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kort A. A., Lakatta E. G. Calcium-dependent mechanical oscillations occur spontaneously in unstimulated mammalian cardiac tissues. Circ Res. 1984 Apr;54(4):396–404. doi: 10.1161/01.res.54.4.396. [DOI] [PubMed] [Google Scholar]
- Kort A. A., Lakatta E. G., Marban E., Stern M. D., Wier W. G. Fluctuations in intracellular calcium concentration and their effect on tonic tension in canine cardiac Purkinje fibres. J Physiol. 1985 Oct;367:291–308. doi: 10.1113/jphysiol.1985.sp015825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta E. G., Lappé D. L. Diastolic scattered light fluctuation, resting force and twitch force in mammalian cardiac muscle. J Physiol. 1981 Jun;315:369–394. doi: 10.1113/jphysiol.1981.sp013753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Spindler A. J., Eisner D. A. Thick slurry bevelling: a new technique for bevelling extremely fine microelectrodes and micropipettes. Pflugers Arch. 1979 Sep;381(3):287–288. doi: 10.1007/BF00583261. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Tsien R. W. Transient inward current underlying arrhythmogenic effects of cardiotonic steroids in Purkinje fibres. J Physiol. 1976 Dec;263(2):73–100. doi: 10.1113/jphysiol.1976.sp011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H., Noma A., Kurachi Y., Irisawa H. Transient depolarization and spontaneous voltage fluctuations in isolated single cells from guinea pig ventricles. Calcium-mediated membrane potential fluctuations. Circ Res. 1982 Aug;51(2):142–151. doi: 10.1161/01.res.51.2.142. [DOI] [PubMed] [Google Scholar]
- Mullins L. J. A mechanism for Na/Ca transport. J Gen Physiol. 1977 Dec;70(6):681–695. doi: 10.1085/jgp.70.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Noble D. The surprising heart: a review of recent progress in cardiac electrophysiology. J Physiol. 1984 Aug;353:1–50. doi: 10.1113/jphysiol.1984.sp015320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H., Eisner D. A., Allen D. G. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983 Aug 25;304(5928):735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Posner C. J., Berman D. A. Mathematical analysis of oscillatory and non-oscillatory recovery of contractility after a rested-state contraction and its modification by calcium. Circ Res. 1969 Dec;25(6):725–733. doi: 10.1161/01.res.25.6.725. [DOI] [PubMed] [Google Scholar]
- Rosen M. R., Gelband H., Hoffman B. F. Correlation between effects of ouabain on the canine electrocardiogram and transmembrane potentials of isolated Purkinje fibers. Circulation. 1973 Jan;47(1):65–72. doi: 10.1161/01.cir.47.1.65. [DOI] [PubMed] [Google Scholar]
- Rosen M. R., Gelband H., Merker C., Hoffman B. F. Mechanisms of digitalis toxicity. Effects of ouabain on phase four of canine Purkinje fiber transmembrane potentials. Circulation. 1973 Apr;47(4):681–689. doi: 10.1161/01.cir.47.4.681. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M. D., Kort A. A., Bhatnagar G. M., Lakatta E. G. Scattered-light intensity fluctuations in diastolic rat cardiac muscle caused by spontaneous Ca++-dependent cellular mechanical oscillations. J Gen Physiol. 1983 Jul;82(1):119–153. doi: 10.1085/jgp.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Kenyon J. L. Ryanodine modification of cardiac muscle responses to potassium-free solutions. Evidence for inhibition of sarcoplasmic reticulum calcium release. J Gen Physiol. 1983 Sep;82(3):385–404. doi: 10.1085/jgp.82.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalle M., Mugelli A. An oscillatory current in sheep cardiac Purkinje fibers. Circ Res. 1981 May;48(5):618–631. doi: 10.1161/01.res.48.5.618. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Hess P. Excitation-contraction coupling in cardiac Purkinje fibers. Effects of cardiotonic steroids on the intracellular [Ca2+] transient, membrane potential, and contraction. J Gen Physiol. 1984 Mar;83(3):395–415. doi: 10.1085/jgp.83.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Kort A. A., Stern M. D., Lakatta E. G., Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]


