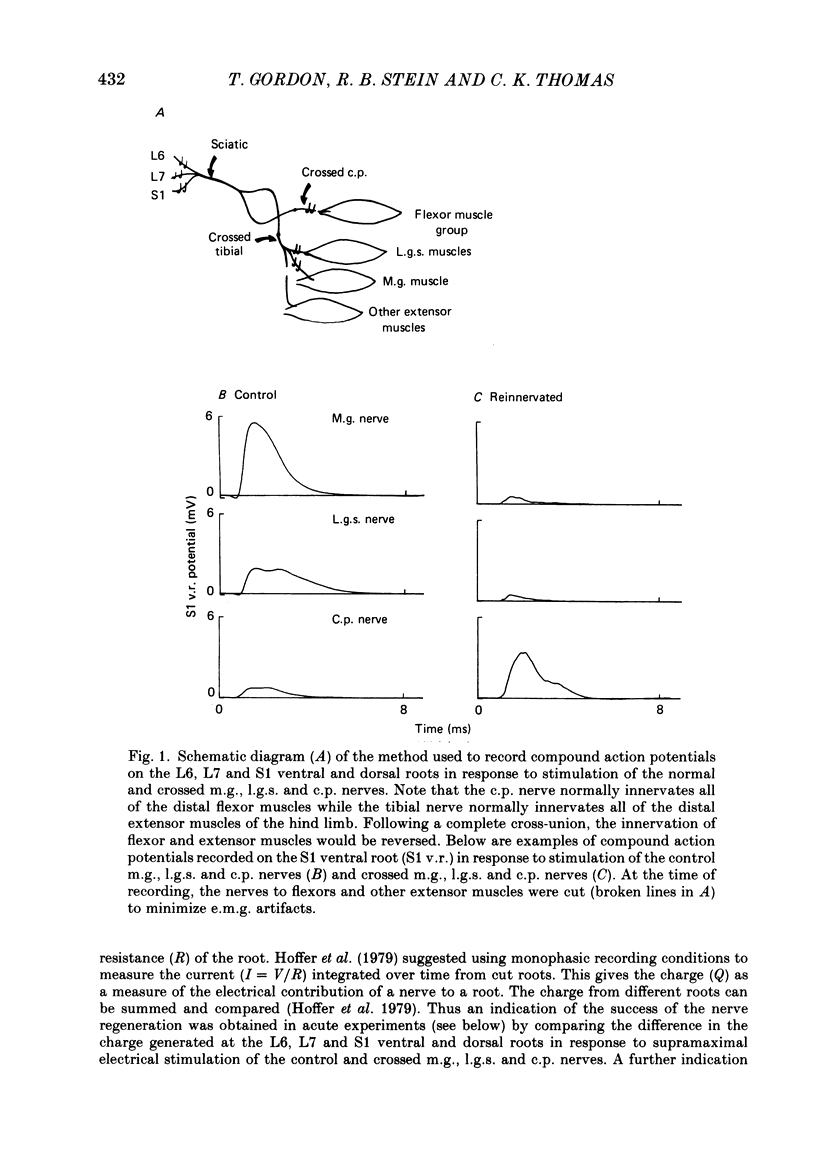
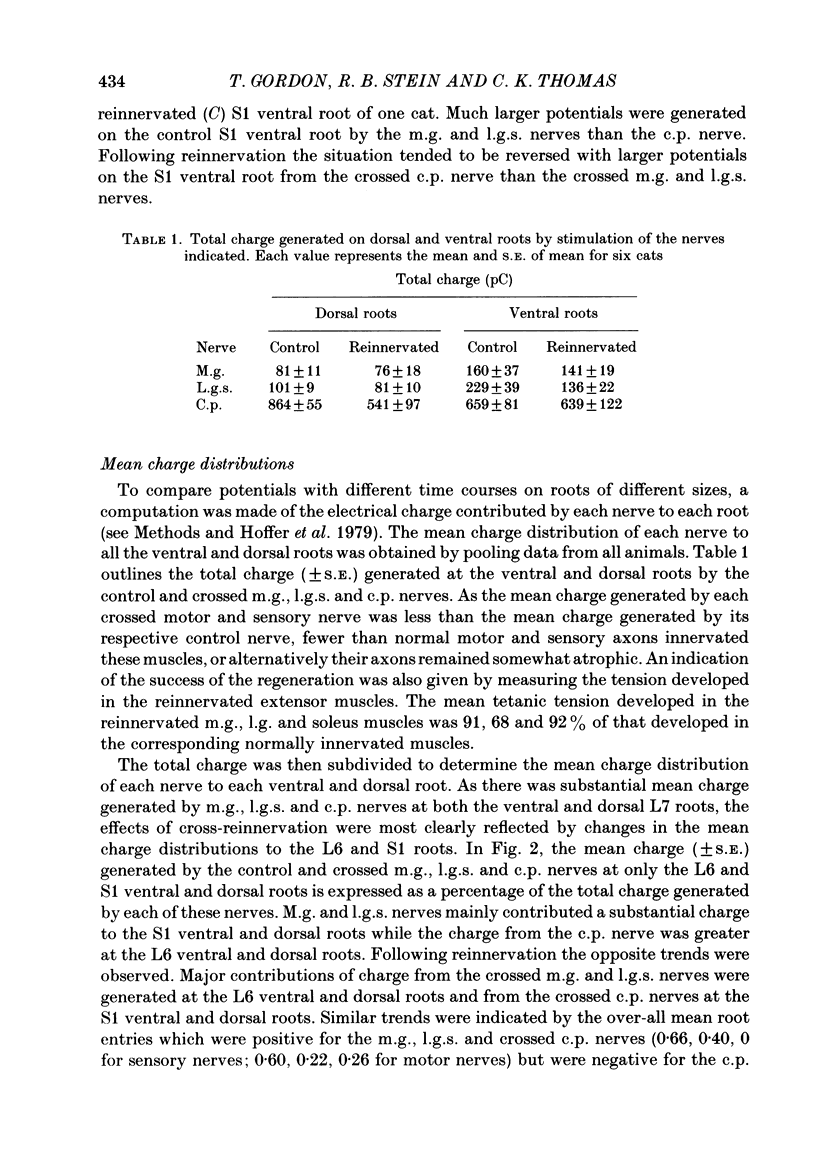
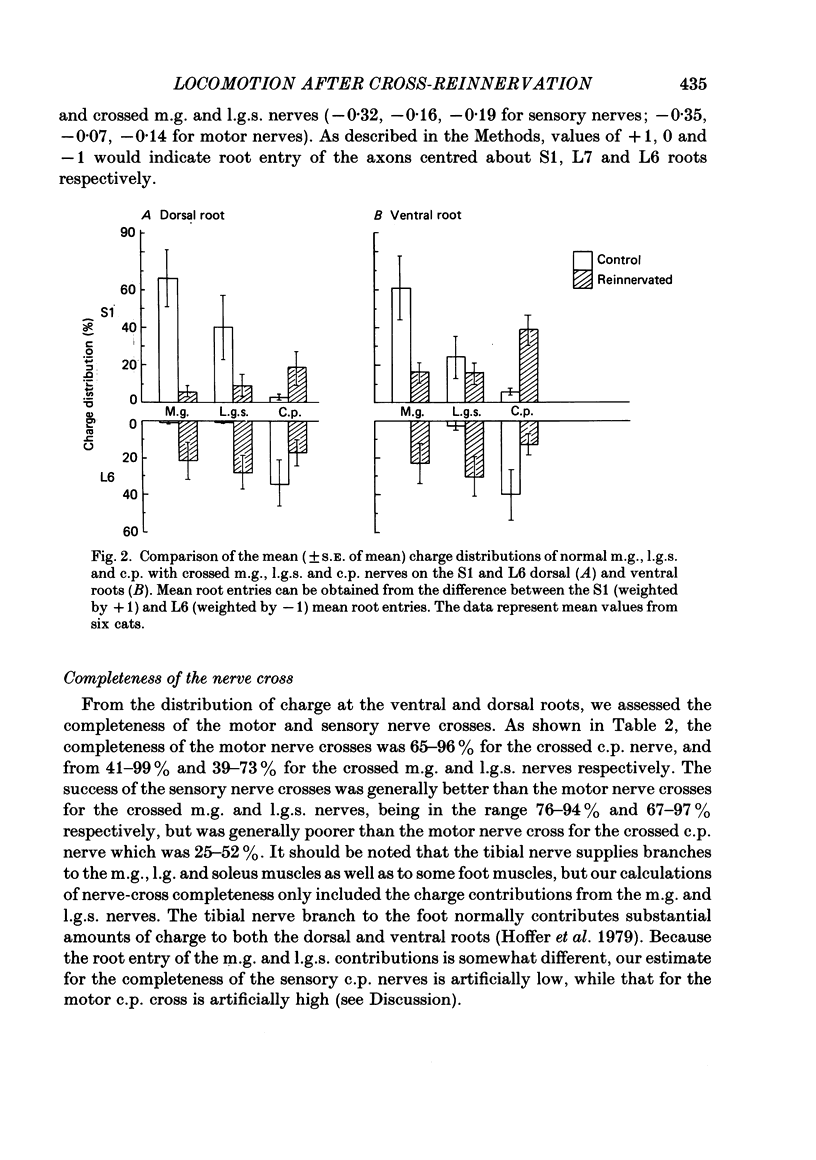
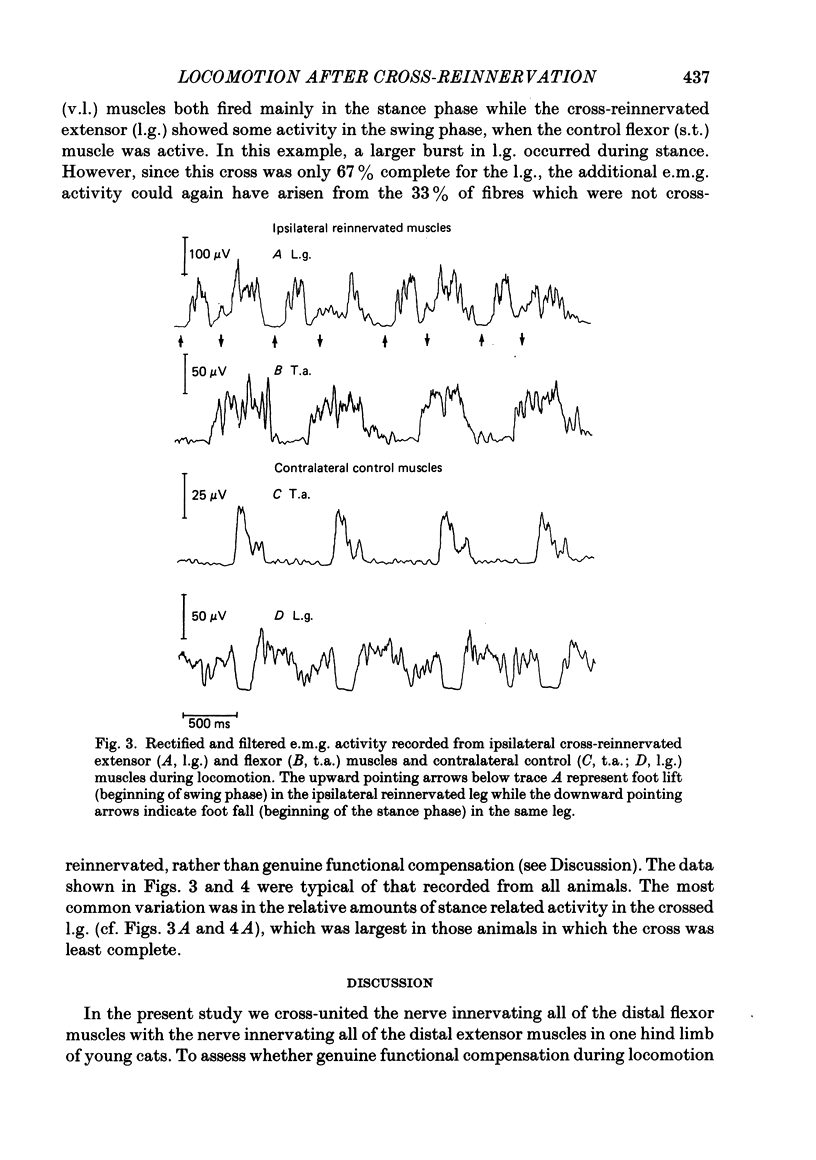
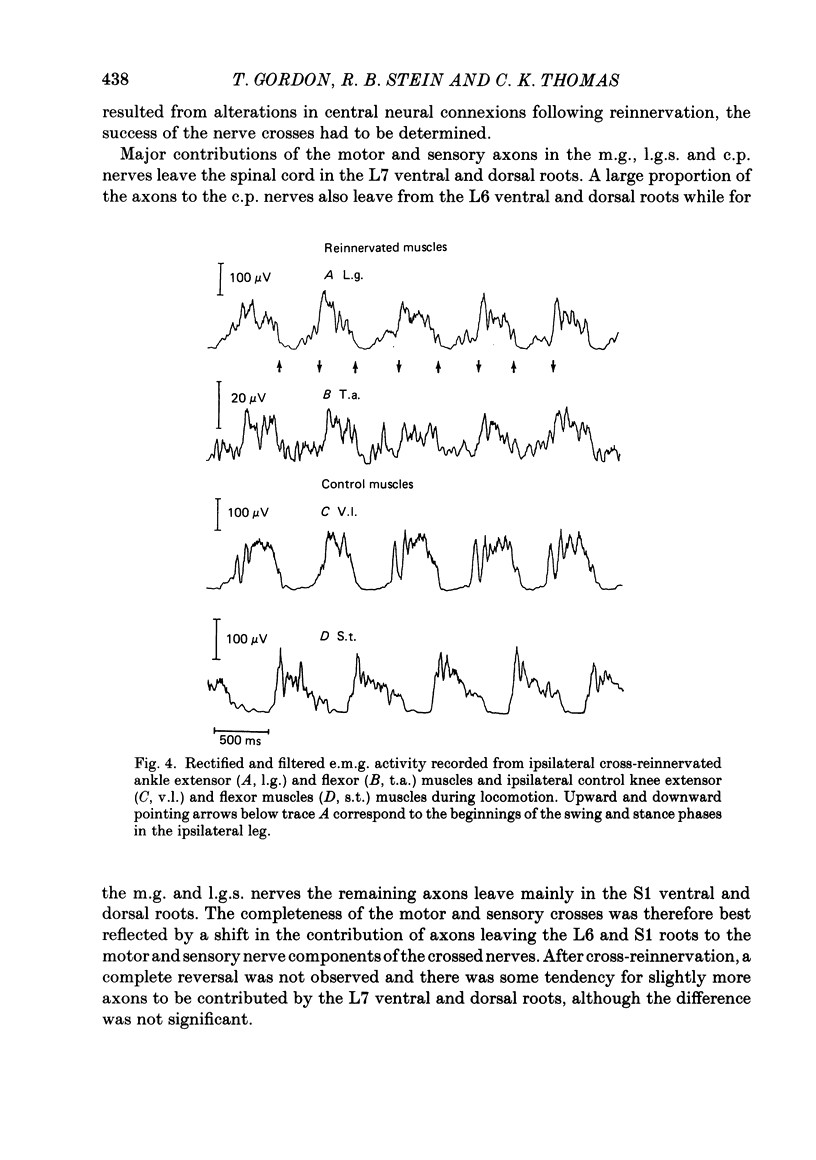
Abstract
Peripheral nerves to flexor (common peroneal) and extensor (tibial) nerves in a hind limb of seven 2-6 month old cats were cut and cross-united to study the plasticity in the spinal cord. The extent to which motoneurones from extensor and flexor motor pools were misdirected to their antagonistic muscles was determined by measuring the potentials generated at the spinal roots from the crossed nerves. The axons contributing to the extensor nerves normally leave the cord in the L7 and S1 ventral and dorsal roots while the axons contributing to the flexor nerves normally leave the cord in the L6 and L7 ventral and dorsal roots. Following cross-union, medial gastrocnemius (m.g.) and lateral gastrocnemius-soleus (l.g.s.) nerves were primarily supplied by L6 and L7 ventral and dorsal roots, and common peroneal (c.p.) nerves were primarily supplied by L7 and S1 ventral and dorsal roots. A method for quantifying the completeness of cross-reinnervation was developed. The pattern of e.m.g. activity in cross-reinnervated muscles during locomotion was primarily determined by the innervating nerve with the reinnervated flexor muscles being activated during the extensor phase. However, the cross-reinnervated extensor muscles showed evidence of extensor activity in addition to the double-burst pattern typical of flexor nerves. This extensor activity was more prominent when the nerve cross was less complete. We conclude that during locomotion the activity of spinal motoneurones was not substantially modified by inappropriate peripheral connexions, even when the nerve cross was carried out in young animals. This conclusion is discussed in relation to previous studies which suggested some degree of functional modification.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkman C., Porter R., Norman J. Plasticity of motor behavior in monkeys with crossed forelimb nerves. Science. 1983 Apr 22;220(4595):438–440. doi: 10.1126/science.6836289. [DOI] [PubMed] [Google Scholar]
- Brushart T. M., Mesulam M. M. Alteration in connections between muscle and anterior horn motoneurons after peripheral nerve repair. Science. 1980 May 9;208(4444):603–605. doi: 10.1126/science.7367884. [DOI] [PubMed] [Google Scholar]
- Cohen A. H. Functional recovery following cross-reinnervation of antagonistic forelimb muscles in rats. Acta Physiol Scand. 1978 Jul;103(3):331–333. doi: 10.1111/j.1748-1716.1978.tb06220.x. [DOI] [PubMed] [Google Scholar]
- Davis L. A., Gordon T., Hoffer J. A., Jhamandas J., Stein R. B. Compound action potentials recorded from mammalian peripheral nerves following ligation or resuturing. J Physiol. 1978 Dec;285:543–559. doi: 10.1113/jphysiol.1978.sp012588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Stein R. B. Time course and extent of recovery in reinnervated motor units of cat triceps surae muscles. J Physiol. 1982 Feb;323:307–323. doi: 10.1113/jphysiol.1982.sp014074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev. 1975 Apr;55(2):247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., Stein R. B., Gordon T. Differential atrophy of sensory and motor fibers following section of cat peripheral nerves. Brain Res. 1979 Dec 14;178(2-3):347–361. doi: 10.1016/0006-8993(79)90698-x. [DOI] [PubMed] [Google Scholar]
- Jolesz F., Sreter F. A. Development, innervation, and activity-pattern induced changes in skeletal muscle. Annu Rev Physiol. 1981;43:531–552. doi: 10.1146/annurev.ph.43.030181.002531. [DOI] [PubMed] [Google Scholar]
- Mackel R., Kunesch E., Waldhör F., Struppler A. Reinnervation of mechanoreceptors in the human glabrous skin following peripheral nerve repair. Brain Res. 1983 May 23;268(1):49–65. doi: 10.1016/0006-8993(83)90389-x. [DOI] [PubMed] [Google Scholar]
- Miledi R., Stefani E. Non-selective re-innervation of slow and fast muscle fibres in the rat. Nature. 1969 May 10;222(5193):569–571. doi: 10.1038/222569a0. [DOI] [PubMed] [Google Scholar]
- Shik M. L., Orlovsky G. N. Neurophysiology of locomotor automatism. Physiol Rev. 1976 Jul;56(3):465–501. doi: 10.1152/physrev.1976.56.3.465. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Fujito Y., Oda Y., Maeda J. Formation of functional synapses in the adult cat red nucleus from the cerebrum following cross-innervating of forelimb flexor and extensor nerves. I. Appearance of new synaptic potentials. Exp Brain Res. 1982;45(1-2):1–12. doi: 10.1007/BF00235757. [DOI] [PubMed] [Google Scholar]


