Abstract
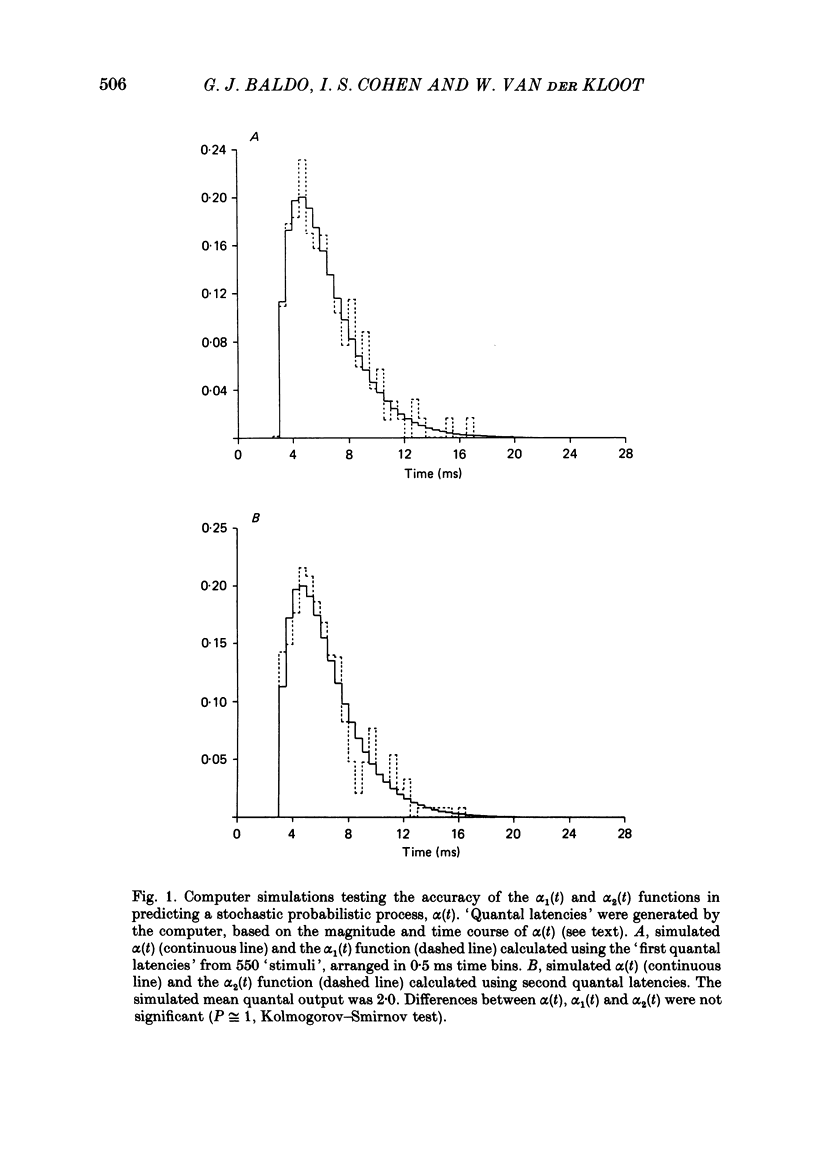
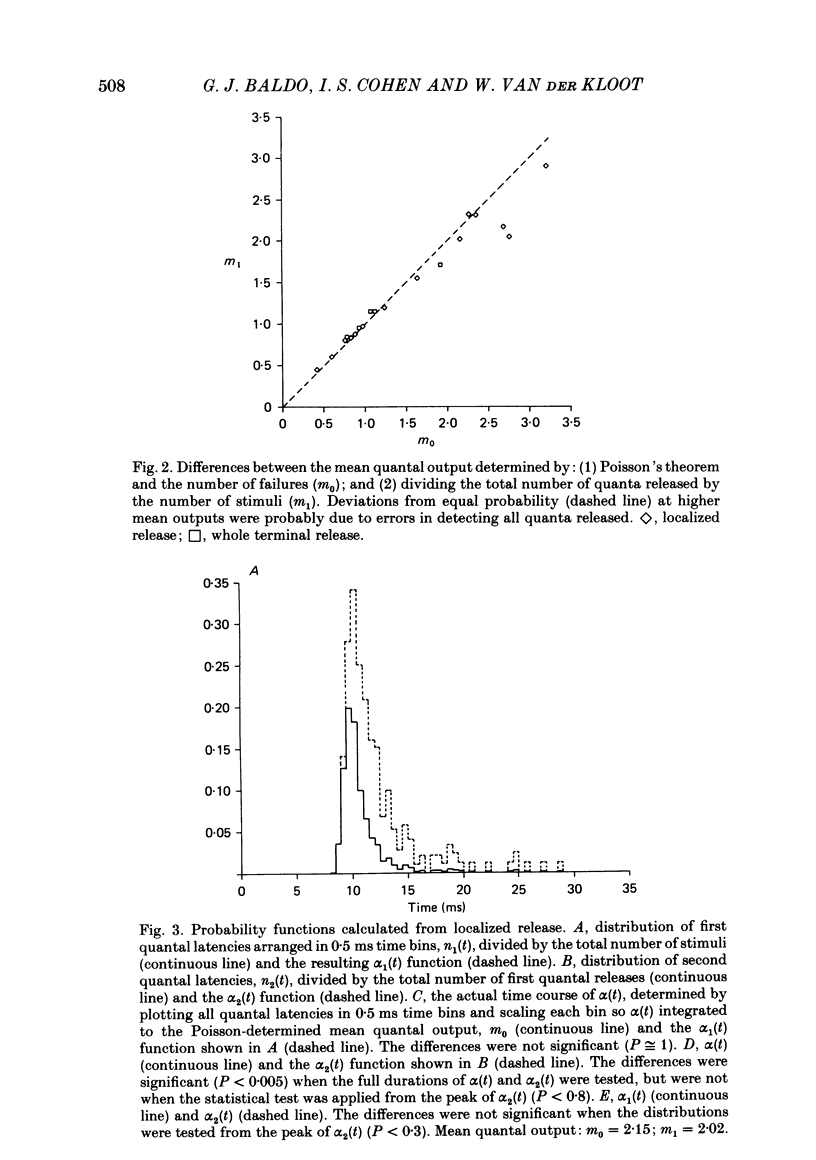
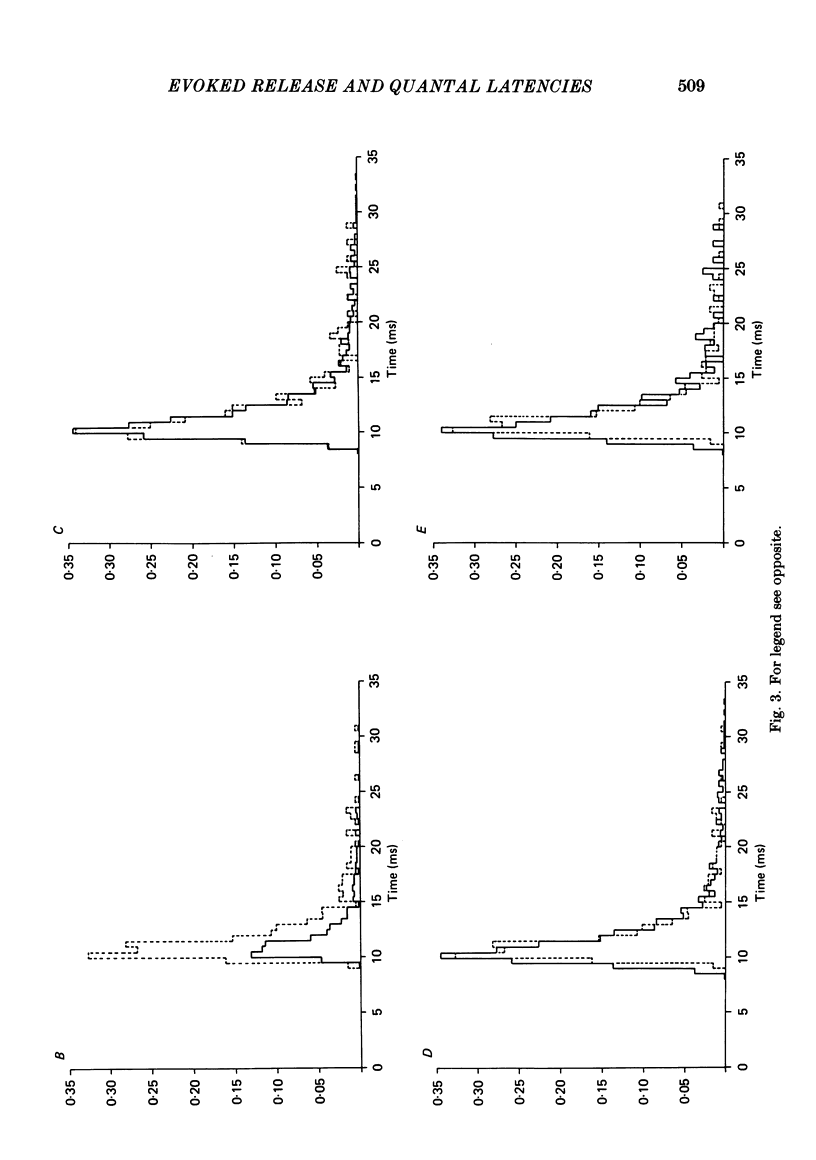
The use of end-plate current (e.p.c.) latency measurements to estimate the time course of the stochastic probabilistic process governing evoked release was investigated in the sciatic nerve-sartorius muscle preparation of the frog, Rana pipiens. We also examined the possibility that the release of a quantum depresses or enhances the subsequent release of additional quanta. Muscle end-plates were voltage clamped at 3-4 degrees C. Quantal release was restricted to a short, or localized, region of the nerve terminal using Ca2+-free, EGTA Ringer solution and a Ca2+-filled micropipette. The number of e.p.c.s containing 0, 1, 2, etc. quanta were totalled and compared to numbers predicted using Poisson's theorem. The differences between the actual and predicted numbers of events were not significant at the nineteen junctions studied (P less than 0.05). The latency of the first quantum observed in several hundred e.p.c.s was measured and used to calculate an estimate, alpha 1(t), of the time-dependent, probabilistic process, alpha (t), governing all evoked quantal release (Barrett & Stevens, 1972b). In three experiments, all quantal latencies were measured to obtain the actual alpha (t). The alpha 1(t) function gave an excellent approximation of alpha (t) (P greater than 0.2), in real and simulated latency data. The latency of the second quantum in the e.p.c.s was measured and used to provide another estimate, alpha 2(t), of alpha (t). The alpha 2(t) function was lower (depressed) during the first few milliseconds of the evoked release period, relative to alpha 1(t). The difference was significant (P greater than 0.01) in all experiments. Our measurement procedures were tested using computer-generated 'e.p.c.s' containing randomly occurring 'quanta'. These tests showed that the early depression was due to inadequate detection of the second quantum in the e.p.c.s. The effect of Sr2+ on evoked release was examined using double-barrelled pipettes containing 1 M-SrCl2 and CaCl2 solutions. The major result was that the durations of alpha 1(t) and alpha 2(t) were equally lengthened in Sr2+, relative to Ca2+.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. F., Barrett J. N., Martin A. R., Rahamimoff R. A note on the interaction of spontaneous and evoked release at the frog neuromuscular junction. J Physiol. 1974 Mar;237(2):453–463. doi: 10.1113/jphysiol.1974.sp010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The effect of calcium ions on the secretion of quanta evoked by an impulse at nerve terminal release sites. J Gen Physiol. 1979 Oct;74(4):429–456. doi: 10.1085/jgp.74.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J. Depression of transmitter release at the neuromuscular junction of the frog. J Physiol. 1970 Mar;206(3):629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J. D., Pumplin D. W. Discrete and discontinuous action of brown widow spider venom on the presynaptic nerve terminals of frog muscle. J Physiol. 1975 Nov;252(2):491–508. doi: 10.1113/jphysiol.1975.sp011154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Kita H., Van Der Kloot W. The intervals between miniature end-plate potentials in the frog are unlikely to be independently or exponentially distributed. J Physiol. 1974 Jan;236(2):327–339. doi: 10.1113/jphysiol.1974.sp010437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Kita H., Van Der Kloot W. The stochastic properties of spontaneous quantal release of transmitter at the frog neuromuscular junction. J Physiol. 1974 Jan;236(2):341–361. doi: 10.1113/jphysiol.1974.sp010438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., ENGBAEK L. The nature of the neuromuscular block produced by magnesium. J Physiol. 1954 May 28;124(2):370–384. doi: 10.1113/jphysiol.1954.sp005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Miledi R., Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969 Jan;200(1):267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE MEASUREMENT OF SYNAPTIC DELAY, AND THE TIME COURSE OF ACETYLCHOLINE RELEASE AT THE NEUROMUSCULAR JUNCTION. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:483–495. doi: 10.1098/rspb.1965.0016. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri H., Rahamimoff R. Clumping and oscillations in evoked transmitter release at the frog neuromuscular junction. J Physiol. 1978 May;278:513–523. doi: 10.1113/jphysiol.1978.sp012321. [DOI] [PMC free article] [PubMed] [Google Scholar]


