Abstract
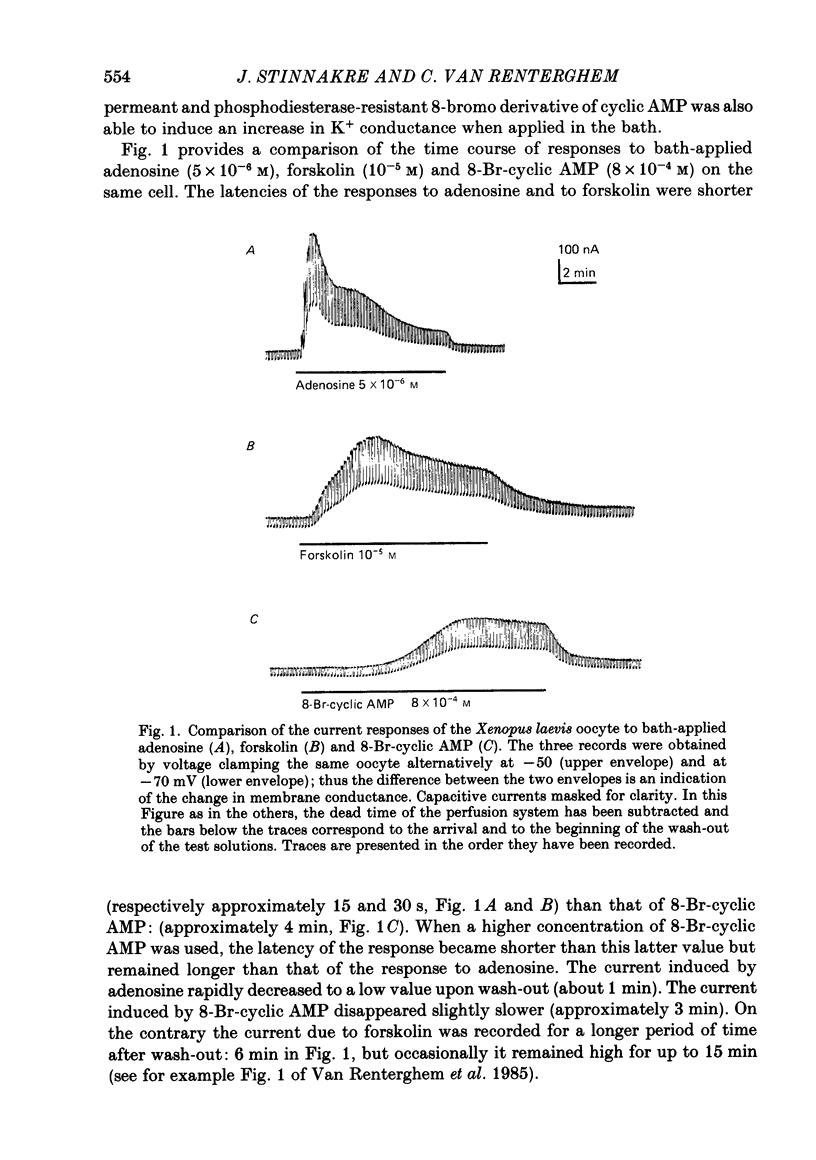
The K+ current response to bath-applied adenosine has been studied on follicle-enclosed full grown oocytes from Xenopus laevis, using the two electrodes voltage-clamp technique. The response to adenosine was mimicked by forskolin, an activator of adenylate cyclase. Forskolin applied at low concentration potentiated the response to adenosine. At low concentration, isoprenaline, a beta-adrenergic agonist known to induce a potassium current via a rise of adenosine 3',5'-phosphate (cyclic AMP) into the oocyte, potentiated the response to adenosine. Progesterone (10(-5) M) reversibly induced a slight decrease (-24%) of the response to adenosine. The calcium ionophore A23187 applied in normal external medium reduced the response to adenosine (about -70%). Intracellular injection of EGTA induced an increase (+64%) of the peak response to adenosine. Acetylcholine (0.5-10 microM) inhibited the response to 3-10 microM adenosine by 44-91%. This inhibition was suppressed by atropine and was seen even on cells which did not show any current in response to acetylcholine application. The inhibition by ACh of the sensitivity to adenosine was long lasting (more than 1 h after the wash-out of ACh). A long term inhibition (-28 to -90%) also occurred when ACh was applied alone and washed before adenosine application. It is concluded that in Xenopus oocyte: increased cyclic AMP synthesis mediates the potassium response to adenosine; intracellular calcium ion concentration modulates this response; muscarinic stimulation induces a long-lasting inhibition of the sensitivity to adenosine.
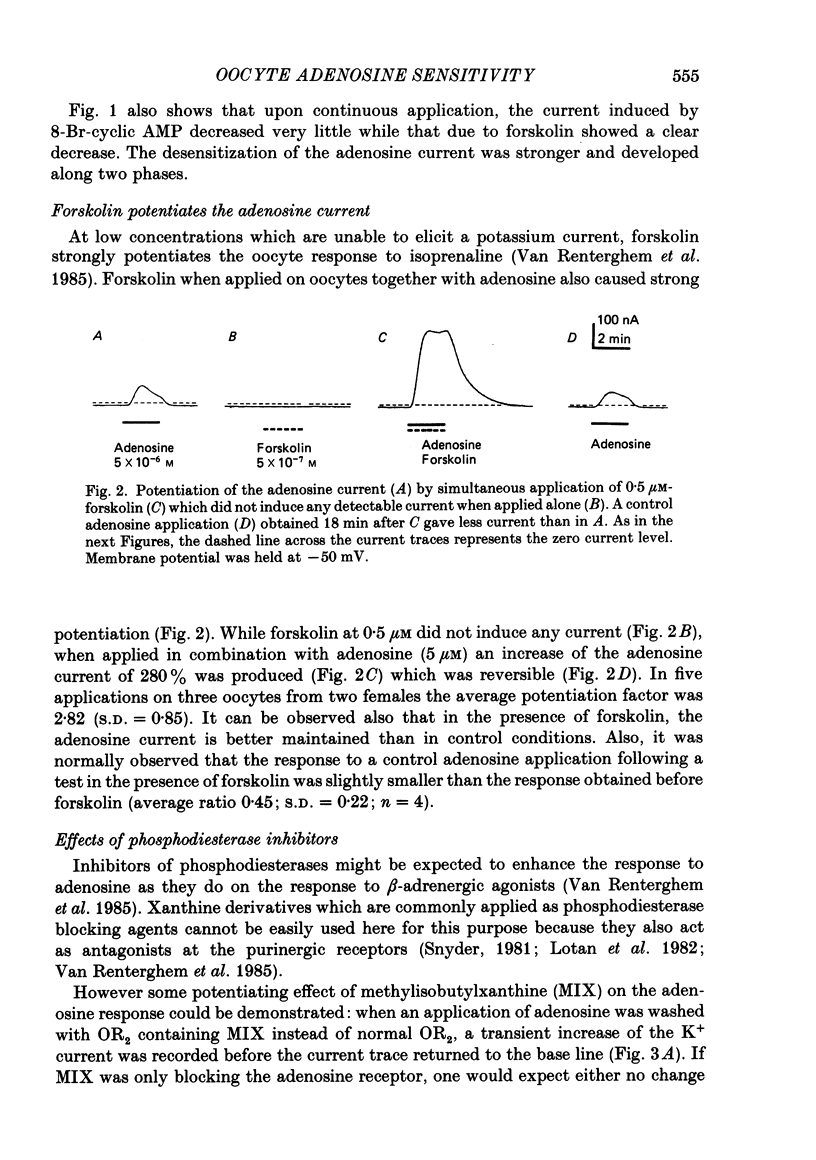
Full text
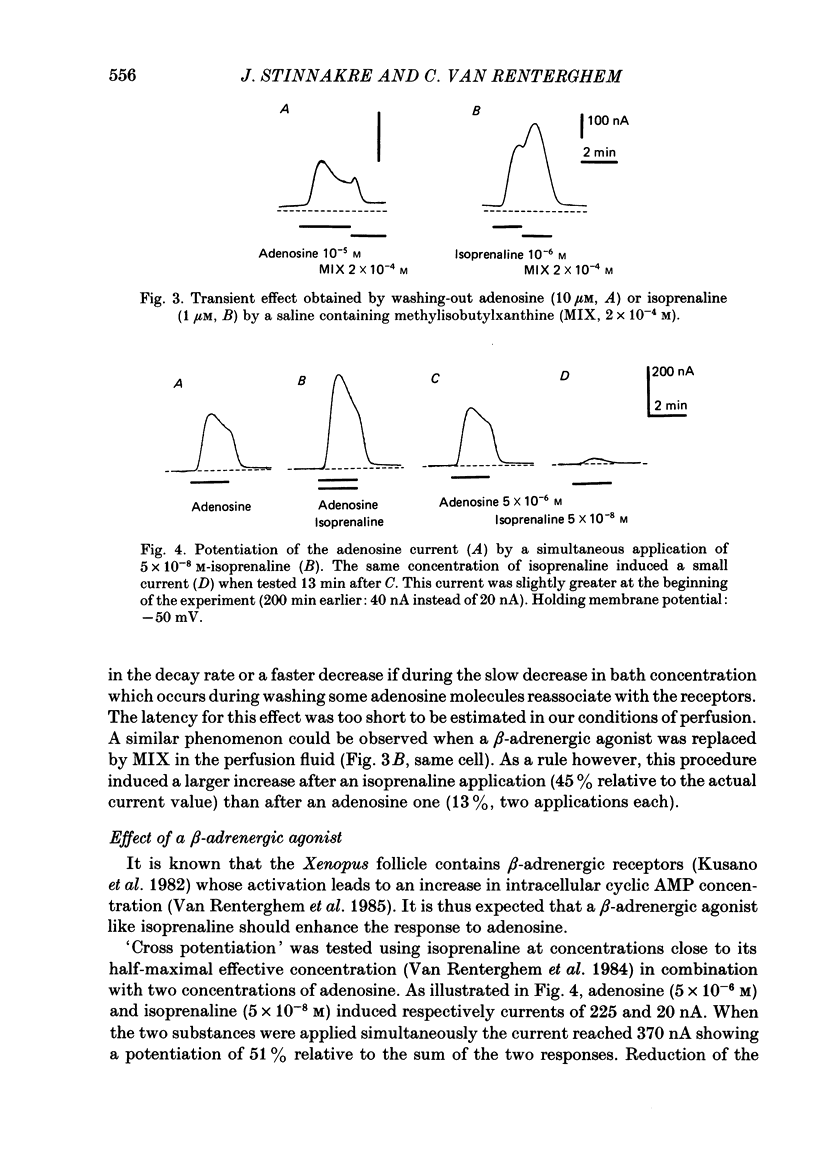
PDF


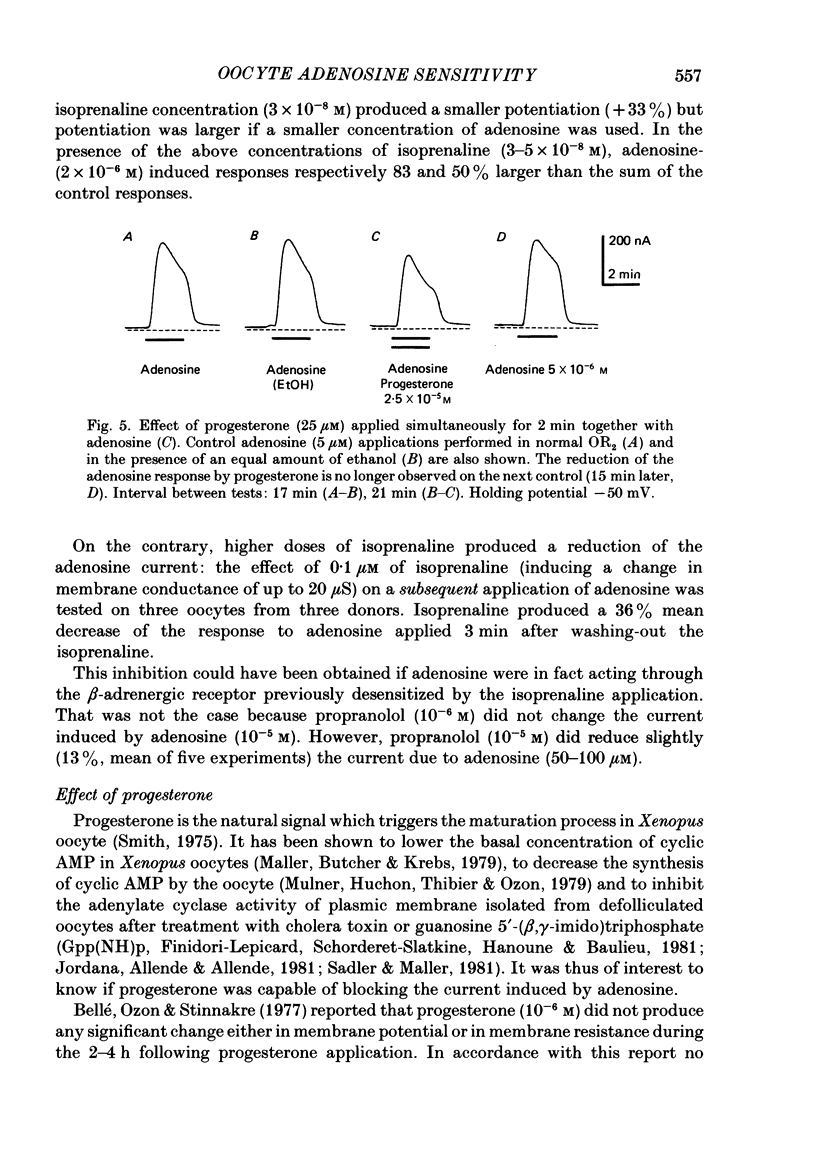
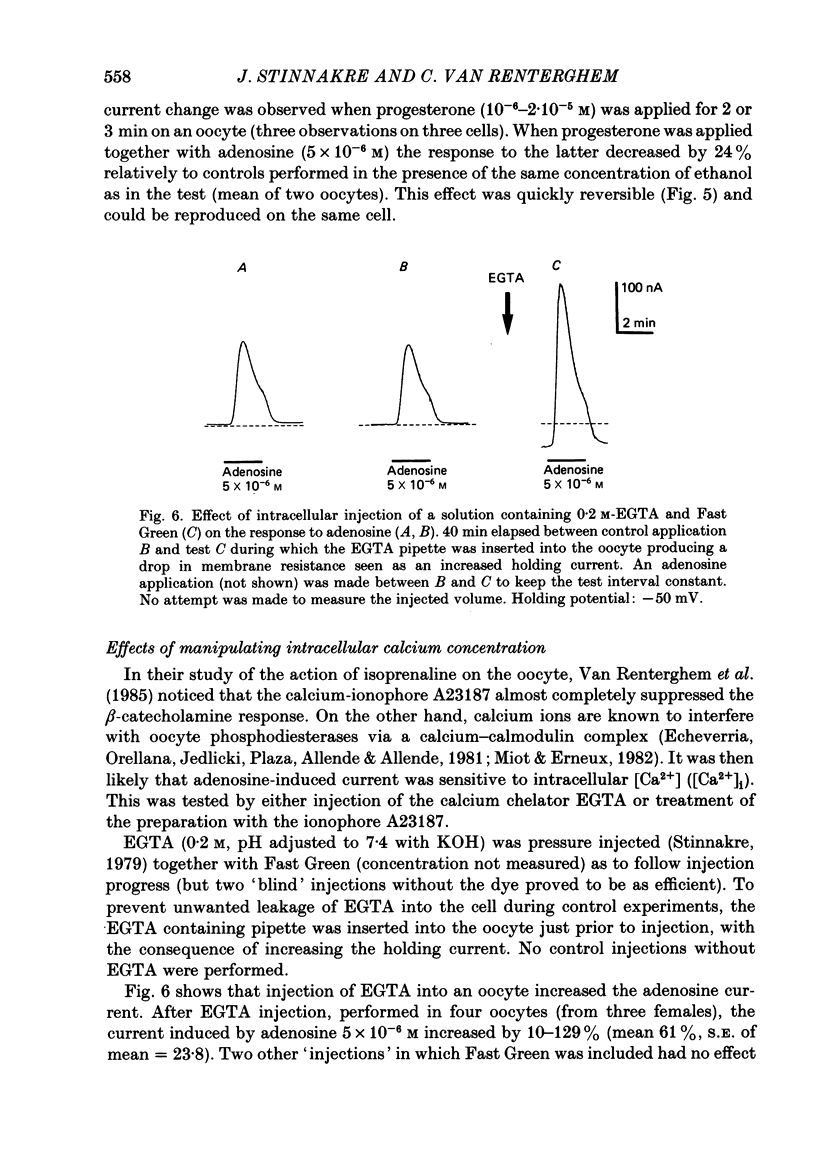
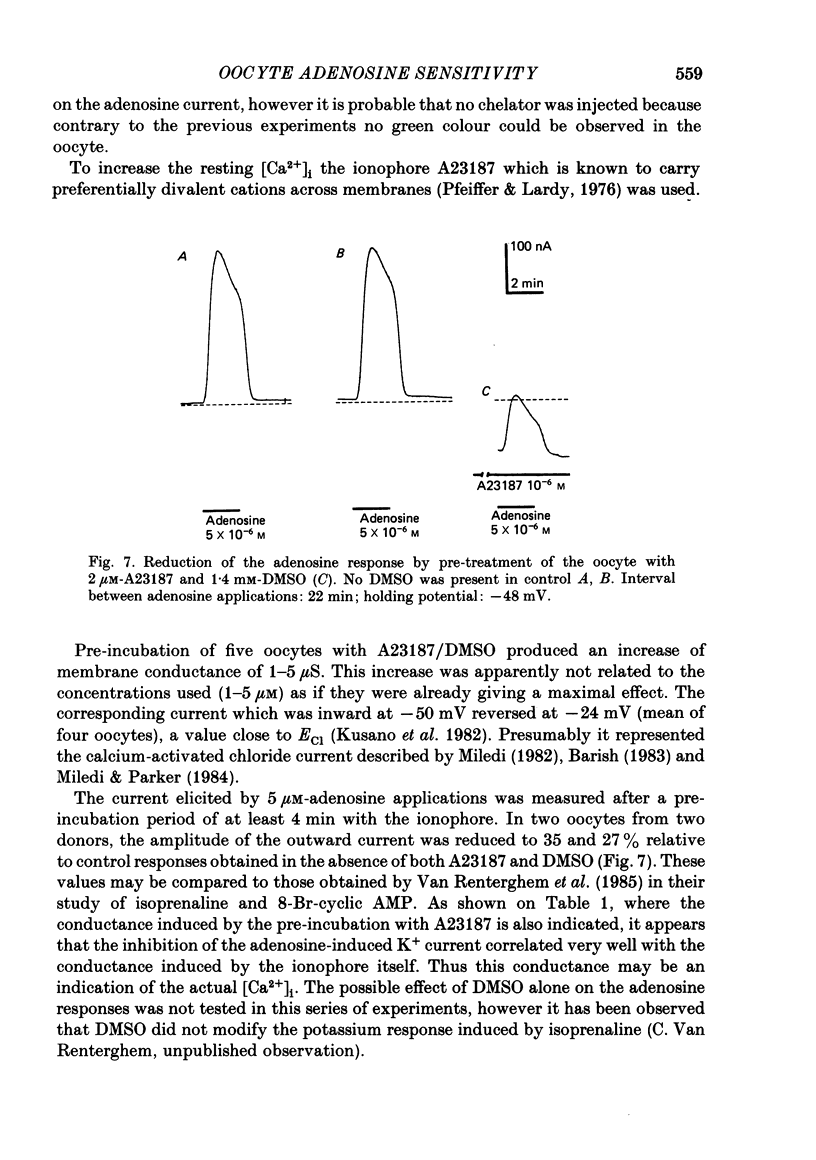

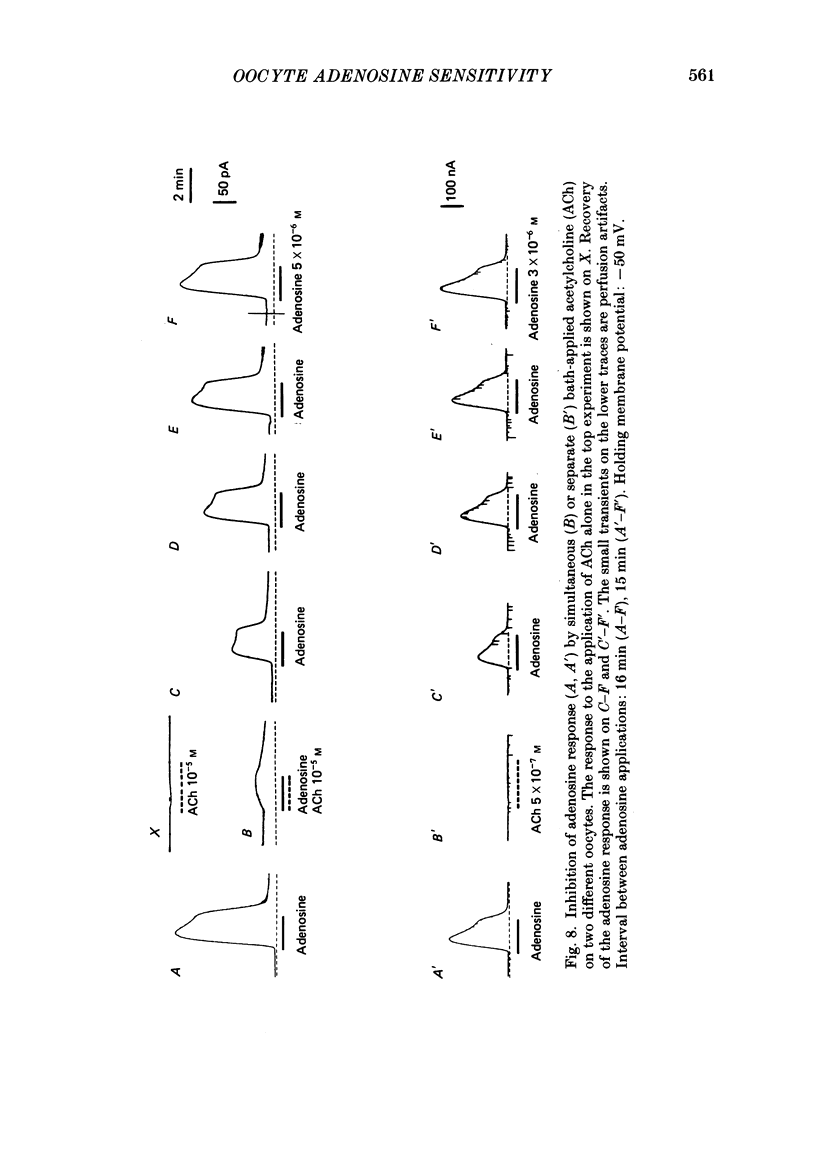
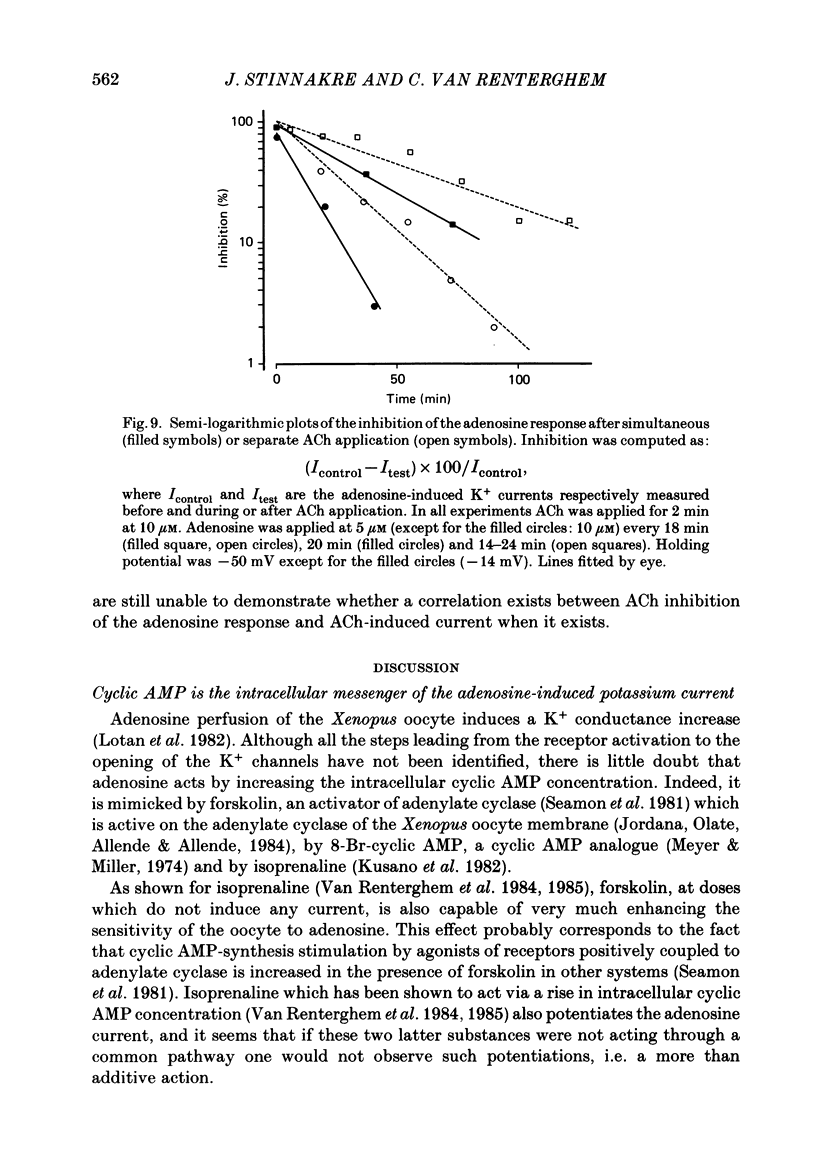







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTH L. G., BARTH L. J. Differentiation of cells of the Rana pipiens gastrula in unconditioned medium. J Embryol Exp Morphol. 1959 Jun;7:210–222. [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E. E., Godeau F., Schorderet M., Schorderet-Slatkine S. Steroid-induced meiotic division in Xenopus laevis oocytes: surface and calcium. Nature. 1978 Oct 19;275(5681):593–598. doi: 10.1038/275593a0. [DOI] [PubMed] [Google Scholar]
- Bellé R., Ozon R., Stinnakre J. Free calcium in full grown Xenopus laevis oocyte following treatment with ionophore A 23187 or progesterone. Mol Cell Endocrinol. 1977 Jul;8(1):65–72. doi: 10.1016/0303-7207(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biegon R. L., Pappano A. J. Dual mechanism for inhibition of calcium-dependent action potentials by acetylcholine in avian ventricular muscle. Relationship to cyclic AMP. Circ Res. 1980 Mar;46(3):353–362. doi: 10.1161/01.res.46.3.353. [DOI] [PubMed] [Google Scholar]
- Bravo R., Otero C., Allende C. C., Allende J. E. Amphibian oocyte maturation and protein synthesis: related inhibition by cyclic AMP, theophylline, and papaverine. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1242–1246. doi: 10.1073/pnas.75.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Burnstock G. Evidence in support of the P1/P2 purinoceptor hypothesis in the guinea-pig taenia coli. Br J Pharmacol. 1981 Jul;73(3):617–624. doi: 10.1111/j.1476-5381.1981.tb16796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J., Iyengar R., Birnbaumer L., Sekura R. D., Manclark C. R. Pertussis toxin substrate, the putative Ni component of adenylyl cyclases, is an alpha beta heterodimer regulated by guanine nucleotide and magnesium. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4276–4280. doi: 10.1073/pnas.80.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Landau E. M., Lass Y. Xenopus oocyte resting potential, muscarinic responses and the role of calcium and guanosine 3',5'-cyclic monophosphate. J Physiol. 1984 Jul;352:551–574. doi: 10.1113/jphysiol.1984.sp015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Landau E. M. Types of muscarinic response in Xenopus oocytes. Life Sci. 1980 Oct 13;27(15):1423–1428. doi: 10.1016/0024-3205(80)90407-5. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Finidori-Lepicard J., Schorderet-Slatkine S., Hanoune J., Baulieu E. E. Progesterone inhibits membrane-bound adenylate cyclase in Xenopus laevis oocytes. Nature. 1981 Jul 16;292(5820):255–257. doi: 10.1038/292255a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Harden T. K., Scheer A. G., Smith M. M. Differential modification of the interaction of cardiac muscarinic cholinergic and beta-adrenergic receptors with a guanine nucleotide binding component(s). Mol Pharmacol. 1982 May;21(3):570–580. [PubMed] [Google Scholar]
- Hazeki O., Ui M. Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem. 1981 Mar 25;256(6):2856–2862. [PubMed] [Google Scholar]
- Jordana X., Olate J., Allende C. C., Allende J. E. Studies on the mechanism of inhibition of amphibian oocyte adenylate cyclase by progesterone. Arch Biochem Biophys. 1984 Feb 1;228(2):379–387. doi: 10.1016/0003-9861(84)90001-8. [DOI] [PubMed] [Google Scholar]
- Katada T., Ui M. Islet-activating protein. A modifier of receptor-mediated regulation of rat islet adenylate cyclase. J Biol Chem. 1981 Aug 25;256(16):8310–8317. [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Dascal N., Cohen S., Lass Y. Adenosine-induced slow ionic currents in the Xenopus oocyte. Nature. 1982 Aug 5;298(5874):572–574. doi: 10.1038/298572a0. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Butcher F. R., Krebs E. G. Early effect of progesterone on levels of cyclic adenosine 3':5'-monophosphate in Xenopus oocytes. J Biol Chem. 1979 Feb 10;254(3):579–582. [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Meyer R. B., Jr, Miller J. P. Analogs of cyclic AMP and cyclic GMP: general methods of synthesis and the relationship of structure to enzymic activity. Life Sci. 1974 Mar 16;14(6):1019–1040. doi: 10.1016/0024-3205(74)90228-8. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miot F., Erneux C. Characterization of the soluble cyclic nucleotide phosphodiesterases in Xenopus laevis oocytes. Evidence for a calmodulin-dependent enzyme. Biochim Biophys Acta. 1982 Feb 18;701(2):253–259. doi: 10.1016/0167-4838(82)90121-2. [DOI] [PubMed] [Google Scholar]
- Mulner O., Huchon D., Thibier C., Ozon R. Cyclic AMP synthesis in Xenopus laevis oocytes: inhibition by progesterone. Biochim Biophys Acta. 1979 Jan 4;582(1):179–184. doi: 10.1016/0304-4165(79)90301-5. [DOI] [PubMed] [Google Scholar]
- Mulner O., Tso J., Huchon D., Ozon R. Calmodulin modulates the cyclic AMP level in Xenopus oocyte. Cell Differ. 1983 Apr;12(4):211–218. doi: 10.1016/0045-6039(83)90030-1. [DOI] [PubMed] [Google Scholar]
- Olate J., Allende C. C., Allende J. E., Sekura R. D., Birnbaumer L. Oocyte adenylyl cyclase contains Ni, yet the guanine nucleotide-dependent inhibition by progesterone is not sensitive to pertussis toxin. FEBS Lett. 1984 Sep 17;175(1):25–30. doi: 10.1016/0014-5793(84)80562-1. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer D. R., Lardy H. A. Ionophore A23187: the effect of H+ concentration on complex formation with divalent and monovalent cations and the demonstration of K+ transport in mitochondria mediated by A23187. Biochemistry. 1976 Mar 9;15(5):935–943. doi: 10.1021/bi00650a001. [DOI] [PubMed] [Google Scholar]
- Phillis J. W., Barraco R. A. Adenosine, adenylate cyclase, and transmitter release. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1985;19:243–257. [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Sadler S. E., Maller J. L. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J Biol Chem. 1981 Jun 25;256(12):6368–6373. [PubMed] [Google Scholar]
- Schorderet-Slatkine S., Schorderet M., Baulieu E. E. Cyclic AMP-mediated control of meiosis: effects of progesterone, cholera toxin, and membrane-active drugs in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):850–854. doi: 10.1073/pnas.79.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Physiological roles for adenosine and adenosine 5'-triphosphate in the nervous system. Neuroscience. 1981;6(4):523–555. doi: 10.1016/0306-4522(81)90145-7. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Penit-Soria J., Stinnakre J. Beta-adrenergic induced potassium current in Xenopus oocyte: involvement of cyclic AMP. Biochimie. 1984 Feb;66(2):135–138. doi: 10.1016/0300-9084(84)90202-5. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Renit-Soria J., Stinnakre J. beta-Adrenergic induced K+ current in Xenopus oocytes: role of cAMP, inhibition by muscarinic agents. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):389–402. doi: 10.1098/rspb.1985.0008. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wasserman W. J., Pinto L. H., O'Connor C. M., Smith L. D. Progesterone induces a rapid increase in [Ca2+]in of Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1534–1536. doi: 10.1073/pnas.77.3.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]


