Abstract
BACKGROUND
Ibrutinib, an inhibitor of Bruton’s tyrosine kinase, and venetoclax, an inhibitor of B-cell lymphoma 2 protein, have been approved for patients with chronic lymphocytic leukemia (CLL). Preclinical investigations have indicated potential synergistic interaction of their combination.
METHODS
We conducted an investigator-initiated phase 2 study of combined ibrutinib and venetoclax involving previously untreated high-risk and older patients with CLL. All patients had at least one of the following features: chromosome 17p deletion, mutated TP53, chromosome 11q deletion, unmutated IGHV, or an age of 65 years or older. Patients received ibrutinib monotherapy (420 mg once daily) for 3 cycles, followed by the addition of venetoclax (weekly dose escalation to 400 mg once daily). Combined therapy was administered for 24 cycles. Response assessments were performed according to International Workshop on Chronic Lymphocytic Leukemia 2008 criteria. Minimal residual disease was assessed by means of multicolor flow cytometry in bone marrow (sensitivity, 10−4).
RESULTS
A total of 80 patients were treated. The median age was 65 years (range, 26 to 83). A total of 30% of the patients were 70 years of age or older. Overall, 92% of the patients had unmutated IGHV, TP53 aberration, or chromosome 11q deletion. With combined treatment, the proportions of patients who had complete remission (with or without normal blood count recovery) and remission with undetectable minimal residual disease increased over time. After 12 cycles of combined treatment, 88% of the patients had complete remission or complete remission with incomplete count recovery, and 61% had remission with undetectable minimal residual disease. Responses were noted in older adults and across all high-risk subgroups. Three patients had laboratory evidence of tumor lysis syndrome. The adverse-event profile was similar to what has been reported with ibrutinib and venetoclax.
CONCLUSIONS
In this study, combined venetoclax and ibrutinib was an effective oral regimen for high-risk and older patients with CLL. (Funded by AbbVie and others; ClinicalTrials.gov number, NCT02756897.)
Chronic lymphocytic leukemia (cll) is the most common leukemia in the United States and was historically treated with chemoimmunotherapy. An understanding of the biologic mechanisms of CLL led to highly effective targeted therapies.1
Ibrutinib, an irreversible inhibitor of Bruton’s tyrosine kinase (BTK), is an approved treatment for CLL.2,3 In a phase 1b–2 study of ibrutinib monotherapy, with a median follow-up of 5 years, the overall response rate was 87% among patients with previously untreated CLL and 89% among those with relapsed or refractory disease.4 Most responses with continuous and indefinite treatment were partial, with persistent disease typically in the bone marrow; complete remission was uncommon. Venetoclax, an inhibitor of B-cell lymphoma 2 (BCL2) protein, was initially approved for relapsed or refractory CLL with chromosome 17p deletion [del(17p)] and recently approved in combination with rituximab.5,6 In a phase 1 study involving patients with relapsed or refractory CLL, a response rate of 79% was noted, with a 20% rate of complete remission.5 In several studies, achievement of undetectable minimal residual disease with chemoimmunotherapy-based treatment was associated with prolonged progression-free and overall survival.7,8 Remission with undetectable minimal residual disease is rare with ibrutinib; it was seen in 5 to 30% of the patients with relapsed or refractory CLL treated with venetoclax.4,5,9
Preclinical models have indicated that combined ibrutinib and venetoclax may be synergistic.10–12 Ibrutinib results in mobilization of CLL cells from protective microenvironment niches where contact provides survival signals.13 Ibrutinib-mediated inhibition of BTK results in reduced levels of myeloid-cell leukemia 1 (MCL1) protein, with an increase or no change in BCL2 levels.10 Venetoclax targets BCL2 to induce apoptosis, whereas MCL1 could protect from mitochondria-mediated cell death. A decrease in MCL1 levels by ibrutinib may lead to synergy when combined with venetoclax. Furthermore, these drugs have nonoverlapping toxic effects. This combination was reported to be safe and active in patients with mantle-cell lymphoma.14 Given the clinically complementary activity, preclinical synergism, and nonoverlapping toxic effects, we examined the safety and efficacy of combined ibrutinib and venetoclax treatment in previously untreated patients with CLL.
METHODS
PATIENT POPULATION
We designed an investigator-initiated, open-label, phase 2 study involving high-risk and older patients with previously untreated CLL. All patients had an International Workshop on Chronic Lymphocytic Leukemia (IWCLL) indication for treatment (criteria are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org).15 Patients were enrolled at the University of Texas M.D. Anderson Cancer Center (MDACC) in Houston. All patients had at least one high-risk genetic feature: del(17p), mutated TP53, chromosome 11q deletion [del(11q)], or unmutated IGHV; patients 65 years of age or older were eligible, independent of high-risk genetic features. Eligible patients had an age of 18 years or older, an Eastern Cooperative Oncology Group performance-status score of 2 or less (on a 5-point scale in which higher numbers reflect greater disability), and adequate renal function (creatinine clearance, >50 ml per minute) and hepatic function. The platelet count had to be more than 20,000 per cubic milliliter, unless a lower count was deemed to be disease-related. Additional eligibility criteria are listed in the Supplementary Appendix. All patients underwent pretreatment evaluation with computed tomographic (CT) imaging and bone marrow aspiration and biopsy and were assessed for IGHV mutation status, cytogenetic abnormalities by means of fluorescence in situ hybridization (FISH), and conventional cytogenetics (see the Supplementary Appendix). Targeted sequencing of 29 genes, including TP53, SF3B1, NOTCH1, and BIRC3 (Table S1 in the Supplementary Appendix), was performed on tumor DNA from the pretreatment samples. The study had two treatment groups: patients with previously untreated CLL (reported here) and patients with relapsed or refractory disease (not reported here).
TREATMENT PLAN
Ibrutinib monotherapy (420 mg once daily) was administered for the first 3 cycles, followed by the addition of venetoclax at the start of cycle 4 (Fig. 1A). Each cycle was 28 days. Ibrutinib monotherapy was intended to reduce the risk of venetoclax-associated tumor lysis syndrome. Weekly dose escalation of venetoclax to a target dose of 400 mg once daily and strategies for mitigation of tumor lysis syndrome were in accordance with recommendations in the prescribing information for venetoclax. Combined ibrutinib and venetoclax was administered for 24 cycles. Patients who remained positive for minimal residual disease in bone marrow at the end of combined treatment could continue ibrutinib alone until disease progression or unacceptable toxic effects.
Figure 1. Study Schema and Response to Treatment.
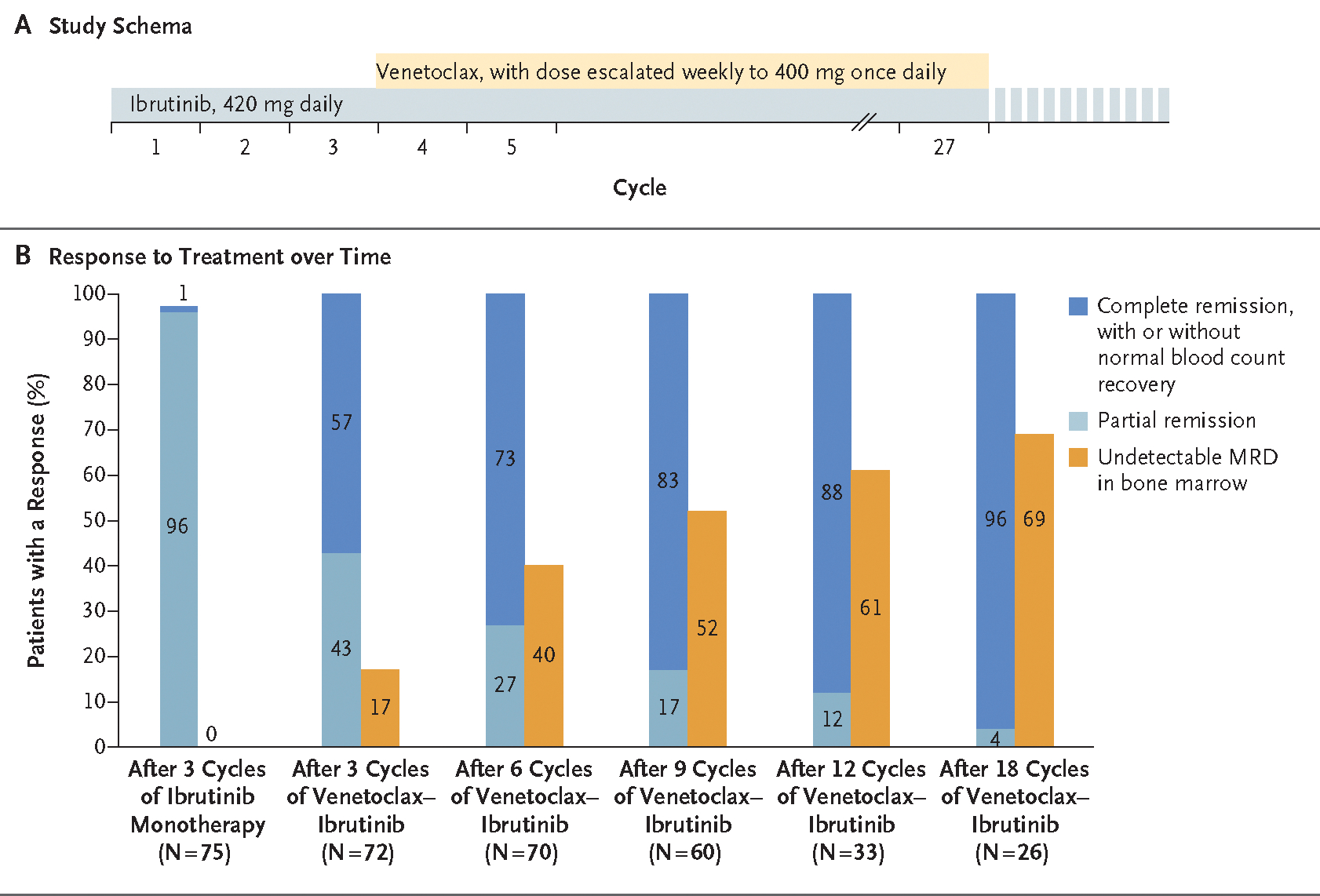
Panel A shows the study schema. Patients received ibrutinib monotherapy (420 mg once daily) for 3 cycles (each cycle was 28 days), and then venetoclax was added. The dose of venetoclax was escalated weekly to a target dose of 400 mg once daily. Combined ibrutinib and venetoclax were administered for a total of 24 cycles. Patients who remained positive for minimal residual disease (MRD) in bone marrow at the end of combined treatment could continue ibrutinib alone until disease progression or unacceptable toxic effects. Clinical responses and MRD were measured after 3 cycles of ibrutinib monotherapy and then after every 3 cycles for the first 12 cycles of the combination and every 6 cycles for cycles 13 to 24 of the combination. Panel B shows response to treatment over time. Responses (complete remission, with or without normal blood count recovery; partial remission; and undetectable MRD in bone marrow) are shown for patients after 3 cycles of ibrutinib monotherapy and at different time points for the combination therapy.
All patients received antiviral prophylaxis with valacyclovir or acyclovir. Prophylaxis for pneumocystis pneumonia was not mandated.
The occurrence of tumor lysis syndrome was assessed according to the Cairo–Bishop definition (see the Supplementary Appendix).16 All patients were assessed for risk of tumor lysis syndrome (according to lymph-node tumor burden on CT imaging and the absolute lymphocyte count) before starting ibrutinib and before starting venetoclax. (For details on risk categorization, see the Supplementary Appendix.)
STUDY ASSESSMENTS
Response assessments were performed according to IWCLL 2008 criteria (see the Supplementary Appendix).15 Response assessment, including assessment of minimal residual disease in bone marrow, was done after cycle 3 (before the start of venetoclax) and then after cycles 3, 6, 9, 12, 18, and 24 of combination therapy. After completing combined therapy, patients were monitored by means of complete blood count, assessment of minimal residual disease in the blood, and physical examination every 6 cycles. Patients with residual disease on CT scan or in bone marrow after 24 cycles of the combination therapy had follow-up CT scans or bone marrow biopsy yearly until remission. Minimal residual disease was assessed by means of multicolor flow cytometry in bone marrow with a sensitivity of 10−4, with the use of standardized European Research Initiative on CLL methods (see the Supplementary Appendix).17 Responses were assessed by the first author and an MDACC radiology collaborator. Adverse events were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.3.
STUDY OVERSIGHT
The study protocol was designed by the first and last authors and is available at NEJM.org. The authors vouch for the accuracy and completeness of the data and for the adherence of the study to the protocol. The MDACC institutional review board approved this study, and the study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. The study was partially supported with funding and drug supply (venetoclax) from AbbVie. The pharmaceutical sponsor approved the study design and reviewed the manuscript but had no role in the collection or analyses of data or the writing of the manuscript.
STATISTICAL ANALYSIS
A three-stage design18 was used, with a rate of complete remission or complete remission with incomplete blood count recovery of 25% under the null hypothesis and a rate of 50% under the alternative hypothesis. With a total sample size of 80 (sample sizes were 16 in the first stage and 39 in the second stage), the study had a power of 96.6% and a one-sided type I error rate of 0.018. The futility stopping boundaries were 4 or fewer patients with a response in the first stage and 13 or fewer patients with a response in the second stage. The combination therapy would be deemed to be promising if at least 28 patients with a response were observed by the end of the study (see the Supplementary Appendix). Toxic effects were monitored in a cohort size of 3, and the trial would be stopped early if there was more than a 92% chance that the incidence of toxic effects was greater than 30%. The primary end point was the best response (complete remission or complete remission with incomplete count recovery) at any time during the treatment for up to 2 months after the completion of combined therapy. The rate of complete remission or complete remission with incomplete count recovery was estimated along with the exact 95% confidence interval. Safety data were summarized with the use of descriptive statistics. Time-to-event outcomes, including progression-free and overall survival, were estimated with the use of the Kaplan–Meier method.
RESULTS
PATIENT CHARACTERISTICS
From July 2016 through June 2018, a total of 80 patients (Table 1) initiated study treatment. The median age was 65 years (range, 26 to 83); 30% of the patients were 70 years of age or older. A total of 83% of the patients had unmutated IGHV; 18% had del(17p), 14% had mutated TP53, and 25% had del(11q). Overall, 92% of the patients had unmutated IGHV, TP53 aberration, or del(11q).
Table 1.
Characteristics of All 80 Patients at Study Entry.*
| Characteristic | Value |
|---|---|
| Age | |
| Median (range) — yr | 65 (26–83) |
| ≥65 yr — no. (%) | 43 (54) |
| ≥70 yr — no. (%) | 24 (30) |
| Male sex — no. (%) | 57 (71) |
| Baseline laboratory tests | |
| Median absolute lymphocyte count (range) — ×10−9/liter | 75.6 (1.1–338.0) |
| Median hemoglobin (range) — g/dl | 11.6 (7.7–15.8) |
| Median platelet count (range) — ×10−9/liter | 130 (28–334) |
| Median serum β2-microglobulin (range) — mg/liter | 3.5 (1.7–13.7) |
| Hierarchical FISH — no. (%) | |
| Chromosome 17p deletion | 14 (18) |
| Chromosome 11q deletion | 20 (25) |
| Trisomy 12 | 17 (21) |
| Negative | 10 (12) |
| Chromosome 13q deletion | 19 (24) |
| IGHV status — no./total no. (%) | |
| Unmutated | 63/76 (83) |
| Mutated | 13/76 (17) |
| Cytogenetics — no./total no. (%) | |
| Complex | 12/78 (15) |
| Diploid | 32/78 (41) |
| Gene mutation — no./total no. (%) | |
| TP53 | 11/79 (14) |
| NOTCH1 | 22/79 (28) |
| SF3B1 | 18/79 (23) |
| BIRC3 | 5/79 (6) |
| Positivity for ZAP70 — no./total no. (%)† | 60/79 (76) |
| Positivity for CD38 — no. (%)‡ | 42 (52) |
FISH denotes fluorescence in situ hybridization.
ZAP70 was assessed by means of immunohistochemical analysis or flow cytometry; expression of 20% or more was considered positive.
CD38 was assessed by means of flow cytometry; expression of 30% or more was considered positive.
Of the 80 patients, 5 withdrew from the study during the ibrutinib-monotherapy phase (reasons for withdrawal are described below) and did not initiate venetoclax. The median follow-up was 14.8 months.
EFFICACY
Responses are shown in Figure 1B, and in Table S2 in the Supplementary Appendix. After the initial 3 cycles of ibrutinib monotherapy, most responses were partial. After the addition of venetoclax, the proportions of patients who had complete remission or complete remission with incomplete count recovery and remission with undetectable minimal residual disease in bone marrow increased over time (Fig. 1B, and Fig. S1 in the Supplementary Appendix). Of the total 80 patients enrolled, 59 (74%; 95% confidence interval [CI], 63 to 83) had complete remission or complete remission with incomplete count recovery as their best response. After 6 cycles of the combination, 51 of 70 patients (73%; 95% CI, 61 to 83) had complete remission or complete remission with incomplete count recovery, and 28 of 70 patients (40%; 95% CI, 28 to 52) had remission with undetectable minimal residual disease in bone marrow. After 12 cycles of the combination, 29 of 33 patients (88%; 95% CI, 72 to 97) were in complete remission or complete remission with incomplete count recovery, and 20 of 33 patients (61%; 95% CI, 42 to 77) had remission with undetectable minimal residual disease in bone marrow. After 18 cycles of the combination, 25 of 26 patients (96%; 95% CI, 80 to 100) were in complete remission or complete remission with incomplete count recovery, with 18 of 26 (69%; 95% CI, 48 to 86) having remission with undetectable minimal residual disease in bone marrow. Three patients completed 24 cycles of combined therapy; all had complete remission or complete remission with incomplete count recovery, with undetectable minimal residual disease in bone marrow.
Patients 65 years of age or older had a high rate of response. A total of 74% had complete remission or complete remission with incomplete count recovery, and 44% had undetectable minimal residual disease in bone marrow after 6 cycles of the combination; these rates increased to 94% and 76%, respectively, after 12 cycles of the combination (Table 2). Responses were seen across all high-risk subgroups, independent of IGHV mutation status, FISH category, TP53 mutation, NOTCH1 mutation, and SF3B1 mutation (Table 2).
Table 2.
Remission with Undetectable Minimal Residual Disease (MRD) in Bone Marrow, According to Pretreatment Characteristics.
| Characteristic | After 3 Cycles of Venetoclax-Ibrutinib | After 6 Cycles of Venetoclax-Ibrutinib | After 9 Cycles of Venetoclax-Ibrutinib | After 12 Cycles of Venetoclax-Ibrutinib |
|---|---|---|---|---|
| no. of patients with undetectable MRD/total no. (%) | ||||
| All patients | 12/72 (17) | 28/70 (40) | 31/60 (52) | 20/33 (61) |
| Age | ||||
| <65 yr | 4/32 (12) | 11/31 (35) | 14/27 (52) | 7/16 (44) |
| ≥65 yr | 8/40 (20) | 17/39 (44) | 17/33 (52) | 13/17 (76) |
| ≥70 yr | 4/23 (17) | 10/23 (43) | 11/18 (61) | 6/7 (86) |
| Sex | ||||
| Female | 2/21 (10) | 7/19 (37) | 8/17 (47) | 6/9 (67) |
| Male | 10/51 (20) | 21/51 (41) | 23/43 (53) | 14/24 (58) |
| FISH | ||||
| Chromosome 17p deletion | 1/12 (8) | 3/12 (25) | 6/12 (50) | 5/6 (83) |
| Chromosome 11q deletion | 4/17 (24) | 10/17 (59) | 10/13 (77) | 7/9 (78) |
| Trisomy 12 | 2/16 (12) | 5/16 (31) | 6/13 (46) | 2/5 (40) |
| Negative | 1/10 (10) | 5/9 (56) | 4/8 (50) | 2/4 (50) |
| Chromosome 13q deletion | 4/17 (24) | 5/16 (31) | 5/14 (36) | 4/9 (44) |
| Complex cytogenetics | 2/11 (18) | 3/10 (30) | 6/10 (60) | 4/5 (80) |
| IGHV status | ||||
| Unmutated | 11/57 (19) | 25/55 (45) | 27/49 (55) | 15/24 (62) |
| Mutated | 1/11 (9) | 3/11 (27) | 2/8 (25) | 3/6 (50) |
| Gene mutation | ||||
| TP53 | 0/9 | 1/8 (12) | 2/8 (25) | 3/4 (75) |
| NOTCH1 | 2/18 (11) | 6/17 (35) | 6/15 (40) | 6/10 (60) |
| SF3B1 | 4/14 (29) | 8/15 (53) | 7/12 (58) | 3/7 (43) |
| Absolute lymphocyte count | ||||
| ≥100×109/liter | 6/28 (21) | 12/27 (44) | 12/23 (52) | 9/13 (69) |
| <100×109/liter | 6/44 (14) | 16/43 (37) | 19/37 (51) | 11/20 (55) |
| Platelet count | ||||
| <100×109/liter | 3/18 (17) | 5/18 (28) | 5/16 (31) | 6/11 (55) |
| ≥100×109/liter | 9/54 (17) | 23/52 (44) | 26/44 (59) | 14/22 (64) |
| Serum β2-microglobulin | ||||
| ≥3.5 mg/liter | 8/39 (21) | 18/37 (49) | 17/31 (55) | 8/11 (73) |
| <3.5 mg/liter | 4/33 (12) | 10/33 (30) | 14/29 (48) | 12/22 (55) |
The estimated 1-year progression-free and overall survival were 98% (95% CI, 94 to 100) and 99% (95% CI, 96 to 100), respectively (Fig. S2A and S2B in the Supplementary Appendix). No patient has had CLL progression. Richter’s transformation developed in one patient; this was a 63-year-old man with CLL with high-risk genomics (unmutated IGHV and NOTCH1 mutation) in whom back pain developed during dose escalation of venetoclax and who was noted to have transformation to diffuse large B-cell lymphoma (DLBCL).
One patient died. This was a 60-year-old man who was having headache and numbness on the right side for 1 week before starting ibrutinib. The patient received 1 day of ibrutinib monotherapy, had progressive neurologic symptoms, and was found to have cryptococcal infection of the central nervous system; ibrutinib treatment was discontinued. The patient died 6 months later from complications of disseminated cryptococcal infection. Given that the patient had symptoms before starting ibrutinib and received only 1 day of ibrutinib, the death was deemed by the treating physician and the first author to be unrelated to ibrutinib.
STRATIFICATION ACCORDING TO RISK OF TUMOR LYSIS SYNDROME
At the time of ibrutinib initiation, 13% of the patients were at high risk, 72% at medium risk, and 15% at low risk for venetoclax-associated tumor lysis syndrome. At the time of venetoclax initiation, 3% of the patients were at high risk, 43% at medium risk, and 54% at low risk for tumor lysis syndrome. Ibrutinib monotherapy led to downgrading of the risk category in 80% of the high-risk patients and 48% of the medium-risk patients. (For changes in lymph-node tumor burden and absolute lymphocyte count with ibrutinib monotherapy, see Figs. S3 and S4, respectively, in the Supplementary Appendix.) Three patients (two at medium risk and one at low risk for tumor lysis syndrome) had laboratory evidence of tumor lysis syndrome. No patient had clinical evidence of tumor lysis syndrome.
DISCONTINUATION OF STUDY TREATMENTS
A total of 11 patients (14%) discontinued study treatments. Five patients withdrew from the study during ibrutinib monotherapy: 1 each had rash, hypertension, use of prohibited medication, and unrelated infection (cryptococcus), and 1 withdrew consent in order to receive treatment with a local physician. Six patients withdrew from the study during the combination phase: 2 had recurrent neutropenia, and 1 each had DLBCL transformation, fallopian-tube cancer, allogeneic stem-cell transplantation, and hemolytic anemia. The patient with hemolytic anemia later received a diagnosis of myelodysplastic syndrome.
SAFETY
Toxic effects of grade 3 or higher were noted in 60% of the patients. Nonhematologic adverse events that occurred in at least 5% of the patients are listed in Table 3. Easy bruising, arthralgia, and diarrhea were the most common nonhematologic adverse events. An increase in the aminotransferase level was noted in 5 patients (grade 1 or 2 in 4 patients and grade 3 in 1 patient). Grade 3 or 4 neutropenia occurred in 38 of 80 patients (48%) (grade 3 in 21 patients and grade 4 in 17). The incidence of grade 3 or 4 neutropenia was similar among patients younger than 65 years of age (18 of 37 patients [49%]) and among those 65 years of age or older (20 of 43 patients [47%]). A total of 19 of 80 patients (24%) had grade 3 or 4 neutropenia during ibrutinib monotherapy; 29 of 75 patients (39%) had grade 3 or 4 neutropenia during the combination phase (10 patients had grade 3 or 4 neutropenia during both ibrutinib monotherapy and the combination phase). A total of 19 patients (24%) received granulocyte colony-stimulating factor (G-CSF) support for neutropenia. Grade 3 thrombocytopenia occurred in 2 patients (2%) during the combination phase. No patient had grade 4 thrombocytopenia.
Table 3.
Nonhematologic Adverse Events in All 80 Patients.*
| Event | Any Grade | Grade 3 or Higher |
|---|---|---|
| no. of patients (%) | ||
| Easy bruising | 48 (60) | 0 |
| Arthralgia | 38 (48) | 1 (1) |
| Diarrhea | 33 (41) | 1 (1) |
| Nausea or vomiting | 29 (36) | 0 |
| Myalgia | 22 (28) | 1 (1) |
| Rash | 19 (24) | 0 |
| Nail changes | 13 (16) | 0 |
| Atrial fibrillation or flutter | 12 (15) | 8 (10) |
| Oral mucositis | 12 (15) | 0 |
| Hypertension | 11 (14) | 8 (10) |
| Fatigue | 11 (14) | 1 (1) |
| Dry skin | 9 (11) | 0 |
| Gastroesophageal reflux disease | 8 (10) | 0 |
| Constipation | 7 (9) | 0 |
| Headache | 6 (8) | 0 |
| Increased creatinine level | 6 (8) | 0 |
| Epistaxis | 5 (6) | 0 |
| Increased aminotransferase level | 5 (6) | 1 (1) |
Shown are adverse events (irrespective of the relationship to the study drugs) that were reported in at least 5% of the patients.
Neutropenic fever occurred in four patients (in one during ibrutinib monotherapy and in three during the combination phase); cultures remained negative in three patients, and one patient had pneumocystis pneumonia. Aspergillus and cryptococcus pneumonia developed in one patient during cycle 1 of ibrutinib monotherapy. Additional infectious complications leading to hospitalization included pneumonia (nonneutropenic, culture negative) in three patients, cellulitis in two patients, and nonneutropenic fever, anaplasmosis infection, septic arthritis, and appendicitis, each in one patient.
The dose of ibrutinib was reduced in 35 of 80 patients (44%). The most common reasons for dose reduction were atrial fibrillation (in 9 patients), neutropenia (in 5), rash (in 5), hypertension (in 3), and myalgia (in 3). The dose of venetoclax was reduced in 18 of 75 patients (24%). The most common reason for dose reduction was neutropenia (in 12 patients). Additional reasons for dose reductions are listed in the Supplementary Appendix.
Atrial fibrillation occurred in 12 patients (15%) (grade 1 or 2 in 4 patients, grade 3 in 7, and grade 4 in 1). Atrial fibrillation developed in 7 patients during ibrutinib monotherapy and in 5 patients during the combination phase (Table S3 in the Supplementary Appendix).
DISCUSSION
Our data showed that combination therapy with ibrutinib and venetoclax was effective in patients with CLL, with no new toxic effects from the combination that were not reported previously for the individual agents. Toxic effects of grade 3 or higher were noted in 60% of the patients (neutropenia accounted for most of these effects). As expected, the majority of patients had a partial response after 3 cycles of ibrutinib monotherapy. With the addition of venetoclax, we noted a rapid conversion of partial responses into complete responses (with or without normal count recovery) and a steady increase in the proportion of patients with undetectable minimal residual disease in bone marrow. After 12 cycles of the combination, 88% of the patients were in complete remission or complete remission with incomplete count recovery, with 61% in remission with undetectable minimal residual disease in bone marrow. These efficacy results are substantially better than what has been reported with ibrutinib or venetoclax monotherapy for patients with CLL; with monotherapy, the majority of responses have been partial, and remissions with undetectable minimal residual disease in bone marrow have been rare, especially with ibrutinib.2,4,5,19,20 These results also appear favorable as compared with chemoimmunotherapy (in the CLL10 trial, the rates of complete remission and undetectable minimal residual disease in peripheral blood were 40% and 74%, respectively, in the fludarabine–cyclophosphamide–rituximab group and 31% and 63%, respectively, in the bendamustine–rituximab group).21
Because CLL typically occurs in older adults, the majority of patients who need treatment are older than 65 years of age. This group of patients often has unacceptable side effects and has a lower rate of complete remission and undetectable minimal residual disease with chemoimmunotherapy than younger patients.21–23 We found significant efficacy, with a rate of complete remission or complete remission with incomplete count recovery of 94% and a rate of undetectable minimal residual disease in bone marrow of 76% after 12 cycles of combined treatment among patients 65 years of age or older. It is notable that the incidence of grade 3 or 4 neutropenia was similar among patients 65 years of age or older (47%) and those younger than 65 years of age (49%). Responses with combined ibrutinib and venetoclax were seen across all genetic subgroups, including high-risk subgroups such as patients with del(17p), del(11q), unmutated IGHV, or mutated TP53.
No new safety concerns were noted with combination therapy. The toxicity profile was similar to what has been noted for ibrutinib or venetoclax monotherapy. Atrial fibrillation, probably related to ibrutinib, occurred in 15% of the patients — an incidence similar to that in published data.24 Grade 3 or 4 neutropenia was noted in 48% of the patients and was managed by G-CSF support and dose interruptions or dose reductions of study drugs. The incidence of grade 3 or 4 neutropenia noted in this trial (48%) is similar to the rate reported in other venetoclax trials (venetoclax monotherapy in relapsed or refractory CLL5 [41%]; venetoclax plus rituximab in relapsed or refractory CLL [MURANO trial]6 [58%]). The risk of neutropenic fever was 5%, a risk similar to the 3.6% risk of neutropenic fever reported in the MURANO trial.6 The current median follow-up of the trial is 14.8 months; a longer follow-up is needed to adequately assess the long-term safety of this combination.
Treatment with ibrutinib or venetoclax monotherapy is until disease progression; venetoclax administered for 2 years when combined with 6 cycles of rituximab was recently approved for relapsed or refractory CLL. In the present trial, the combined treatment will stop after 24 cycles. A time-limited treatment strategy leading to deep remission and a treatment-free interval potentially has advantages over continuous indefinite treatment: increased depth of remission may lead to prolonged progression-free and overall survival, as has been noted with chemoimmunotherapy; deep remissions may avoid resistance and eliminate aggressive clones; time-limited treatment reduces the potential for ongoing long-term toxic effects; and time-limited treatment may have lower cost. Longer follow-up of this trial is needed to assess outcomes beyond the 24 cycles of combined therapy. The appropriate duration of treatment with targeted agents such as combined ibrutinib and venetoclax in patients with CLL remains uncertain, and this study and several other ongoing trials (ClinicalTrials.gov numbers, NCT02910583 and NCT03462719; and EudraCT number, 2013-001944-76) are exploring this question.25 Whether treatment with targeted agents can be discontinued safely in patients with remission with undetectable minimal residual disease remains an unanswered question at this time, although some early data suggest that this might be the case.26,27
In conclusion, we found combined ibrutinib and venetoclax to be an active regimen for high-risk and older patients with previously untreated CLL. High rates of complete response and remission with undetectable minimal residual disease in bone marrow were noted with this regimen without unanticipated toxic effects at early time points of follow-up.
Supplementary Material
Acknowledgments
Supported by AbbVie, a grant from the University of Texas M.D. Anderson Cancer Center (MDACC) Chronic Lymphocytic Leukemia Moon Shot program (to Drs. Jain and Gandhi), a grant from the Andrew Sabin Family Foundation (to Drs. Jain and Gandhi), a grant from the CLL Global Research Foundation (to Dr. Gandhi), and MDACC Support Grant P30 CA016672.
We thank the patients who participated in this trial and their families, the MDACC Investigational New Drug office for their oversight of the study, and the referring physicians, as well as the entire clinical and research staff at the Department of Leukemia, MDACC.
Footnotes
Contributor Information
Nitin Jain, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Michael Keating, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Philip Thompson, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Alessandra Ferrajoli, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Jan Burger, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Gautam Borthakur, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Koichi Takahashi, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Zeev Estrov, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Nathan Fowler, Department of Lymphoma and Myeloma, University of Texas M.D. Anderson Cancer Center, Houston.
Tapan Kadia, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Marina Konopleva, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Yesid Alvarado, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Musa Yilmaz, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Courtney DiNardo, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Prithviraj Bose, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Maro Ohanian, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Naveen Pemmaraju, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Elias Jabbour, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Koji Sasaki, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Rashmi Kanagal-Shamanna, Department of Hematopathology, University of Texas M.D. Anderson Cancer Center, Houston.
Keyur Patel, Department of Hematopathology, University of Texas M.D. Anderson Cancer Center, Houston.
Jeffrey Jorgensen, Department of Hematopathology, University of Texas M.D. Anderson Cancer Center, Houston.
Naveen Garg, Department of Diagnostic Radiology, University of Texas M.D. Anderson Cancer Center, Houston.
Xuemei Wang, Department of Biostatistics, University of Texas M.D. Anderson Cancer Center, Houston.
Katrina Sondermann, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Nichole Cruz, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Chongjuan Wei, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Ana Ayala, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
William Plunkett, Department of Experimental Therapeutics, University of Texas M.D. Anderson Cancer Center, Houston.
Hagop Kantarjian, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
Varsha Gandhi, Department of Experimental Therapeutics, University of Texas M.D. Anderson Cancer Center, Houston.
William Wierda, Department of Leukemia, University of Texas M.D. Anderson Cancer Center, Houston.
REFERENCES
- 1.Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet 2018;391:1524–37. [DOI] [PubMed] [Google Scholar]
- 2.Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015;373:2425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014;371:213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien S, Furman RR, Coutre S, et al. Single-agent ibrutinib in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia: a 5-year experience. Blood 2018;131:1910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax–rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med 2018;378:1107–20. [DOI] [PubMed] [Google Scholar]
- 7.Böttcher S, Ritgen M, Fischer K, et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J Clin Oncol 2012;30:980–8. [DOI] [PubMed] [Google Scholar]
- 8.Dimier N, Delmar P, Ward C, et al. A model for predicting effect of treatment on progression-free survival using MRD as a surrogate end point in CLL. Blood 2018;131:9 55–62. [DOI] [PubMed] [Google Scholar]
- 9.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax for patients with chronic lymphocytic leukemia with 17p deletion: results from the full population of a phase II pivotal trial. J Clin Oncol 2018;36:1 973–80. [DOI] [PubMed] [Google Scholar]
- 10.Cervantes-Gomez F, Lamothe B, Woyach JA, et al. Pharmacological and protein profiling suggests venetoclax (ABT-199) as optimal partner with ibrutinib in chronic lymphocytic leukemia. Clin Cancer Res 2015;21:3705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng J, Isik E, Fernandes SM, Brown JR, Letai A, Davids MS. Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lymphocytic leukemia. Leukemia 2017;31:2075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slinger E, Balasubramanian S, Leverson JD, Eldering E, Kater AP. Combinatorial treatment of chronic lymphocytic leukemia with ibrutinib and venetoclax is superior to treatment with single agents in the TCL1 mouse model. Blood 2017;130:Suppl 1:3018. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011;117:6287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med 2018;378:1211–23. [DOI] [PubMed] [Google Scholar]
- 15.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol 2004;127:3–11. [DOI] [PubMed] [Google Scholar]
- 17.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia 2007;21:956–64. [DOI] [PubMed] [Google Scholar]
- 18.Chen TT. Optimal three-stage designs for phase II cancer clinical trials. Stat Med 1997;16:2701–11. [DOI] [PubMed] [Google Scholar]
- 19.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn IE, Farooqui MZH, Tian X, et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood 2018;131:2357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichhorst B, Fink AM, Bahlo J, et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol 2016;17:928–42. [DOI] [PubMed] [Google Scholar]
- 22.Keating MJ, O’Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005;23:4079–88. [DOI] [PubMed] [Google Scholar]
- 23.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014;370:1101–10. [DOI] [PubMed] [Google Scholar]
- 24.Brown JR, Moslehi J, O’Brien S, et al. Characterization of atrial fibrillation adverse events reported in ibrutinib randomized controlled registration trials. Haematologica 2017;102:1796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain N Selecting frontline therapy for CLL in 2018. Hematology Am Soc Hematol Educ Program 2018;2018:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seymour JF, Ma S, Brander DM, et al. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol 2017;18:230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain N, Thompson PA, Burger JA, et al. Ibrutinib, fludarabine, cyclophosphamide, and obinutuzumab (iFCG) for firstline treatment of patients with CLL with mutated IGHV and without TP53 aberrations. Blood 2018;132:Suppl 1:695. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


