Abstract
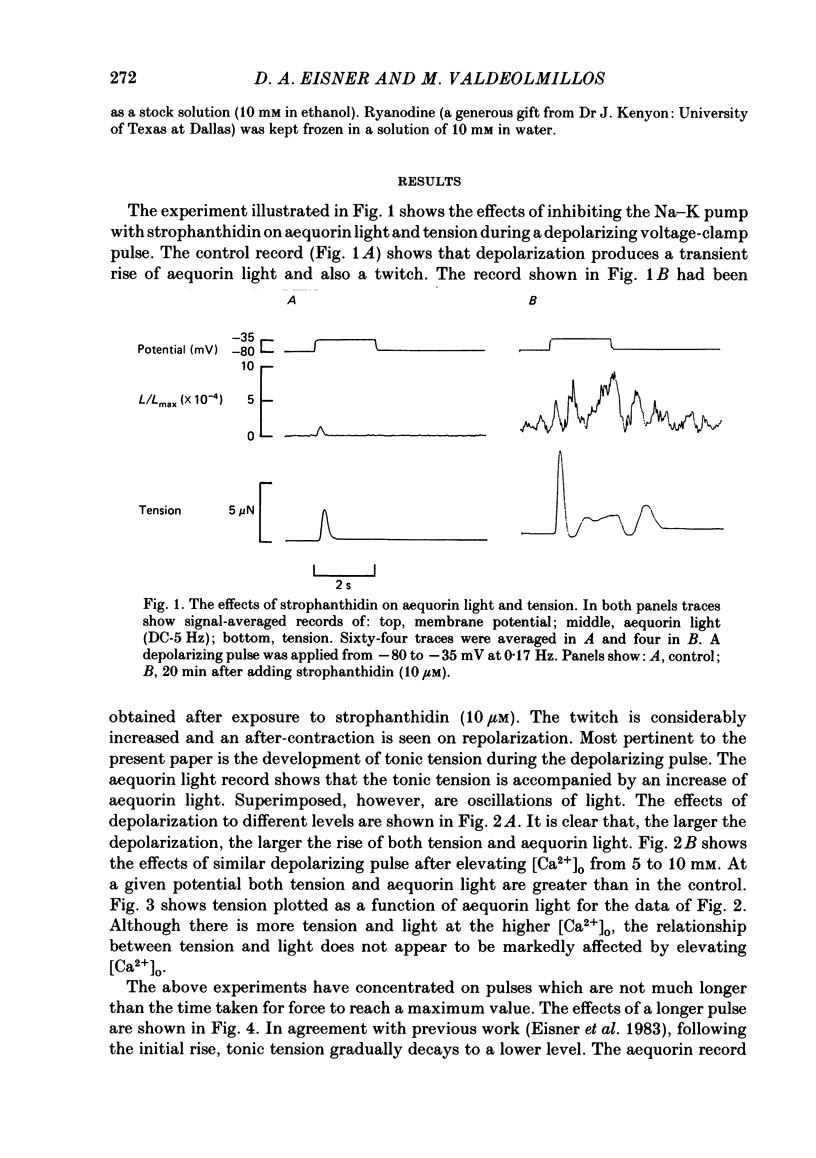
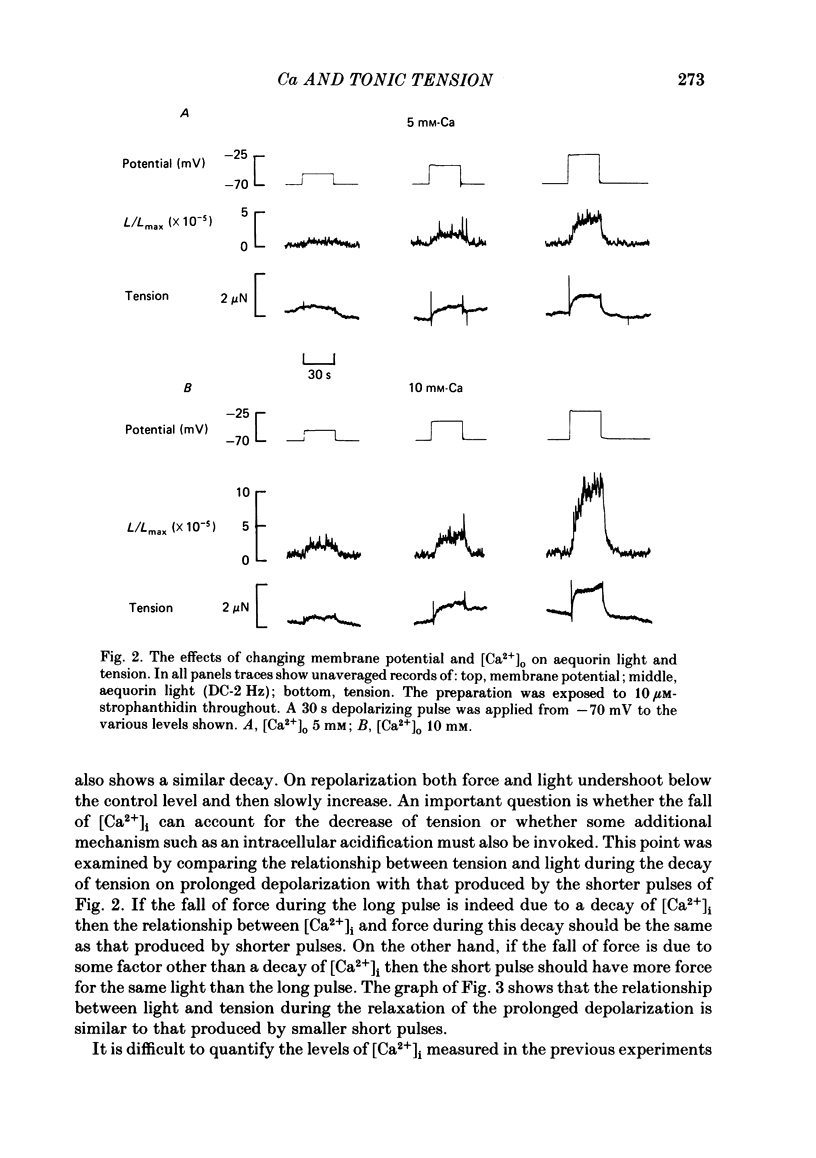
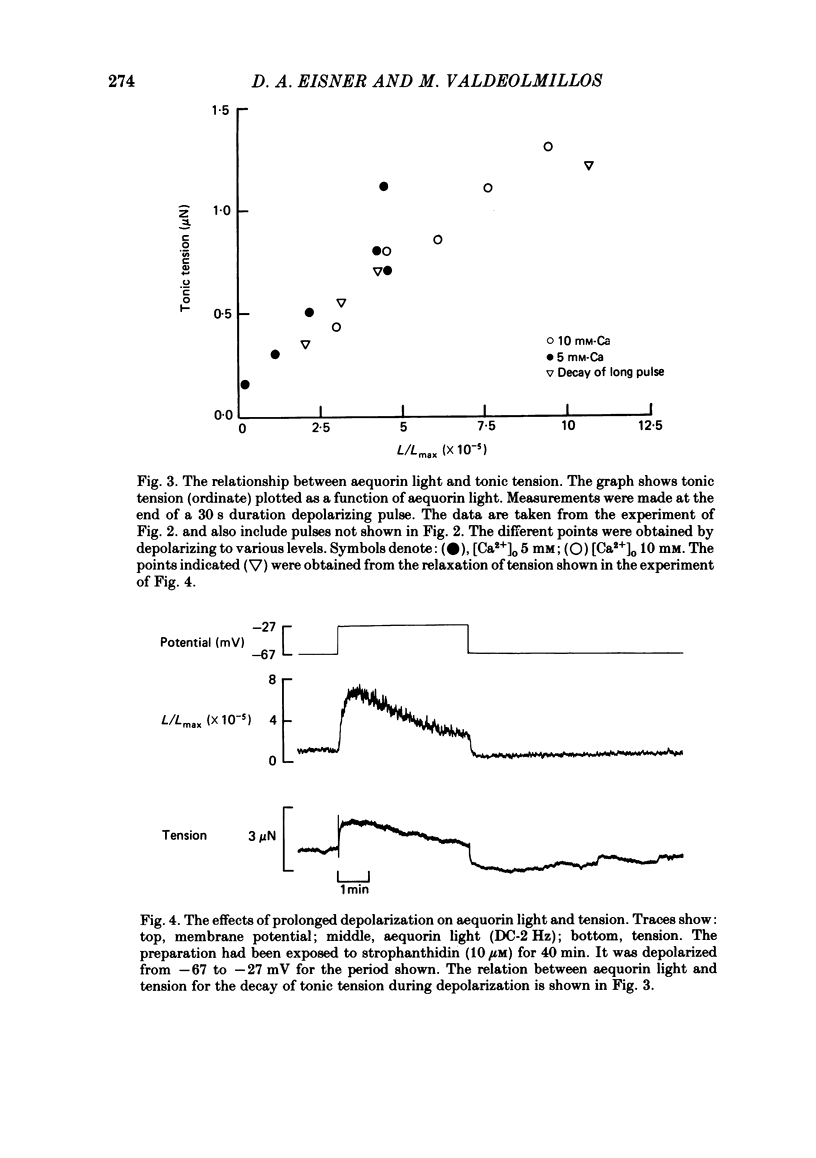
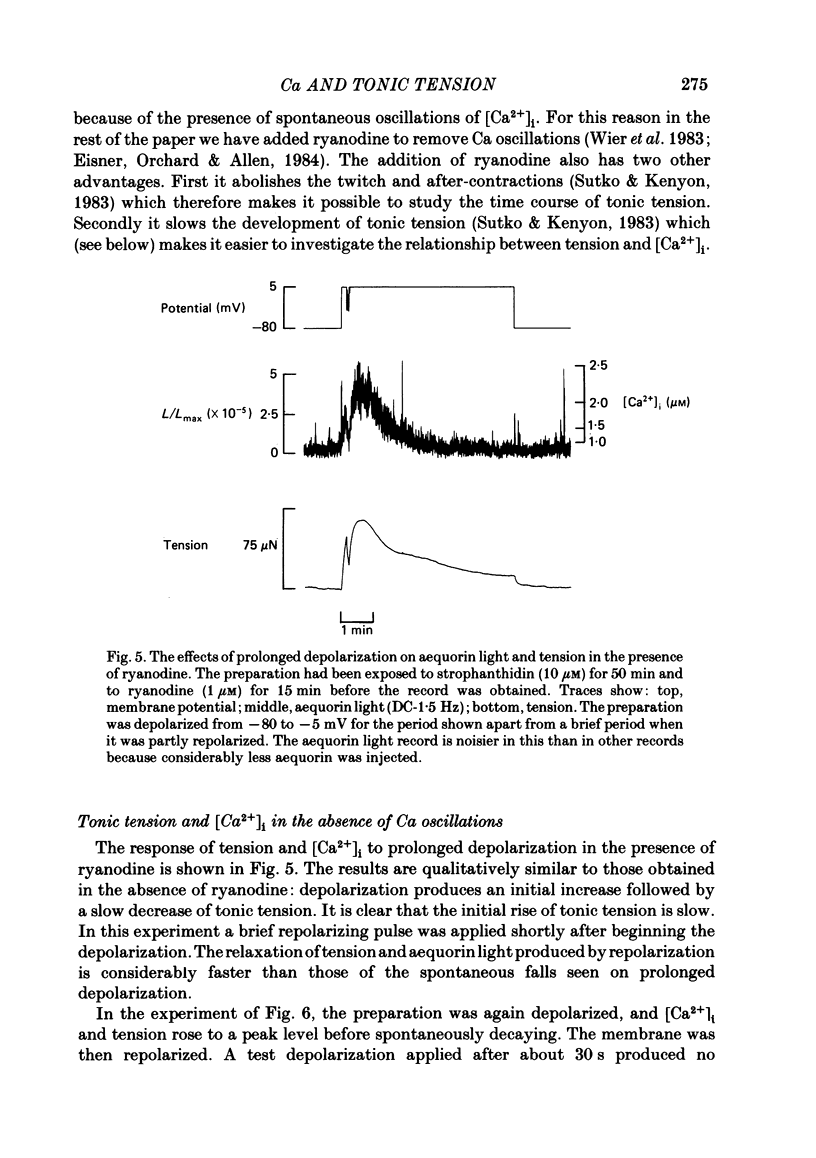
The photoprotein aequorin was micro-injected into several cells in a sheep Purkinje fibre. The intracellular Ca concentration [( Ca2+]i) was measured from the resulting light emission. Inhibition of the Na-K pump with strophanthidin resulted in the development of tonic tension which increased on depolarization. This increase was accompanied by an increase of aequorin light. Increasing external Ca concentration [( Ca2+]o) or the magnitude of the depolarization increased both light and tension. If the depolarizing pulse was maintained for several minutes then both tonic tension and aequorin light slowly decayed. The relationship between tension and light was unaffected during this decay. On repolarization the light decayed to below the level before the depolarization before slowly increasing. During this period a test depolarization produced increases of aequorin light and tension which were smaller than control. The application of ryanodine (1-10 microM) abolished all components of tension other than the tonic component. Under these conditions the time course of the increase of tonic tension and aequorin light on depolarization was sufficiently slow to be measured. In most (five out of six) experiments the relationship between light and tension during this development of tonic tension was found to be similar to that during the subsequent spontaneous decay. However, in one experiment the decay of force was greater than could be accounted for by the fall of [Ca2+]i. It is concluded that most of the spontaneous relaxation of tonic tension can be attributed to a fall of [Ca2+]i rather than to other explanations such as an intracellular acidification or increase of inorganic phosphate concentration.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Eisner D. A., Orchard C. H. Factors influencing free intracellular calcium concentration in quiescent ferret ventricular muscle. J Physiol. 1984 May;350:615–630. doi: 10.1113/jphysiol.1984.sp015221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Orchard C. H. The effects of changes of pH on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1983 Feb;335:555–567. doi: 10.1113/jphysiol.1983.sp014550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Vaughan-Jones R. D., Lederer W. J. Ryanodine block of calcium oscillations in heart muscle and the sodium-tension relationship. Fed Proc. 1985 Dec;44(15):2964–2969. [PubMed] [Google Scholar]
- Chapman R. A. The effects of temperature and metabolic inhibitors on the spontaneous relaxation of the potassium contracture of the heart of the frog Rana pipiens. J Physiol. 1973 Jun;231(2):233–249. doi: 10.1113/jphysiol.1973.sp010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Tunstall J. The interaction of sodium and calcium ions at the cell membrane and the control of contractile strength in frog atrial muscle. J Physiol. 1980 Aug;305:109–123. doi: 10.1113/jphysiol.1980.sp013353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Inotropic and arrhythmogenic effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:255–277. doi: 10.1113/jphysiol.1979.sp012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The control of tonic tension by membrane potential and intracellular sodium activity in the sheep cardiac Purkinje fibre. J Physiol. 1983 Feb;335:723–743. doi: 10.1113/jphysiol.1983.sp014560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The effects of rubidium ions and membrane potentials on the intracellular sodium activity of sheep Purkinje fibres. J Physiol. 1981 Aug;317:189–205. doi: 10.1113/jphysiol.1981.sp013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Orchard C. H., Allen D. G. Control of intracellular ionized calcium concentration by sarcolemmal and intracellular mechanisms. J Mol Cell Cardiol. 1984 Feb;16(2):137–146. doi: 10.1016/s0022-2828(84)80702-6. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Valdeolmillos M. The mechanism of the increase of tonic tension produced by caffeine in sheep cardiac Purkinje fibres. J Physiol. 1985 Jul;364:313–326. doi: 10.1113/jphysiol.1985.sp015747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. High potassium and low sodium contractures in sheep cardiac muscle. J Gen Physiol. 1971 Nov;58(5):483–510. doi: 10.1085/jgp.58.5.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Metzger P., Weingart R. Free magnesium in sheep, ferret and frog striated muscle at rest measured with ion-selective micro-electrodes. J Physiol. 1982 Dec;333:173–188. doi: 10.1113/jphysiol.1982.sp014447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- January C. T., Fozzard H. A. The effects of membrane potential, extracellular potassium, and tetrodotoxin on the intracellular sodium ion activity of sheep cardiac muscle. Circ Res. 1984 Jun;54(6):652–665. doi: 10.1161/01.res.54.6.652. [DOI] [PubMed] [Google Scholar]
- Kentish J. C. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard C. H., Eisner D. A., Allen D. G. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983 Aug 25;304(5928):735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- Powell T., Tatham P. E., Twist V. W. Cytoplasmic free calcium measured by quin2 fluorescence in isolated ventricular myocytes at rest and during potassium-depolarization. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1012–1020. doi: 10.1016/0006-291x(84)91192-6. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin R. A., Abercrombie R. F. The influence of external cations and membrane potential on Ca-activated Na efflux in Myxicola giant axons. J Gen Physiol. 1978 Apr;71(4):453–466. doi: 10.1085/jgp.71.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J. L., Kenyon J. L. Ryanodine modification of cardiac muscle responses to potassium-free solutions. Evidence for inhibition of sarcoplasmic reticulum calcium release. J Gen Physiol. 1983 Sep;82(3):385–404. doi: 10.1085/jgp.82.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones R. D., Lederer W. J., Eisner D. A. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983 Feb 10;301(5900):522–524. doi: 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Kort A. A., Stern M. D., Lakatta E. G., Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]


