Abstract
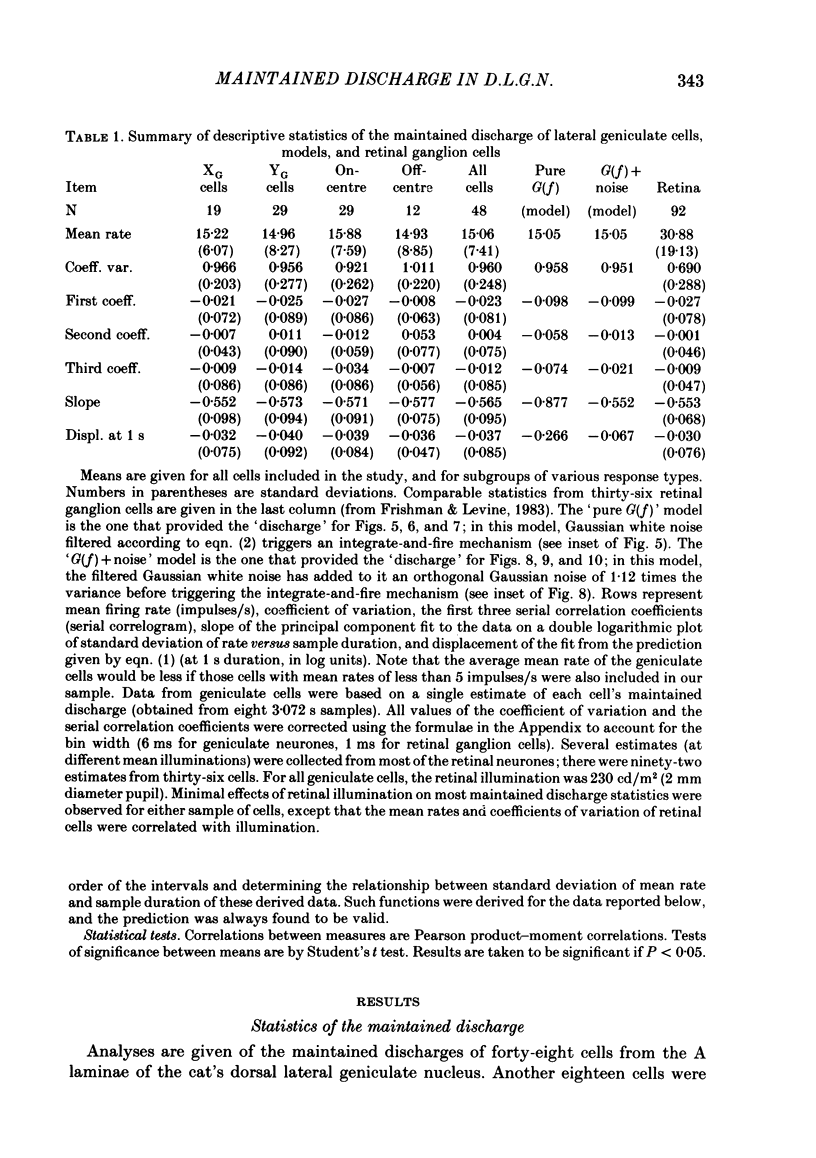
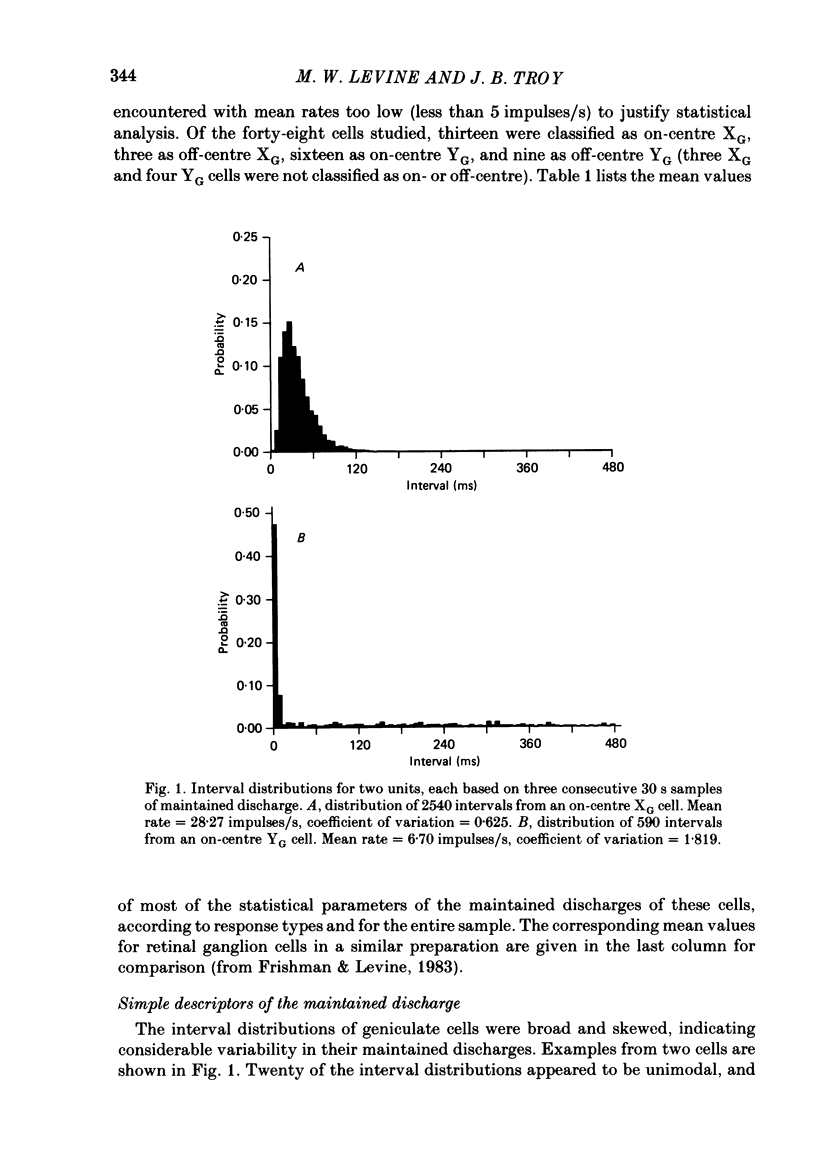
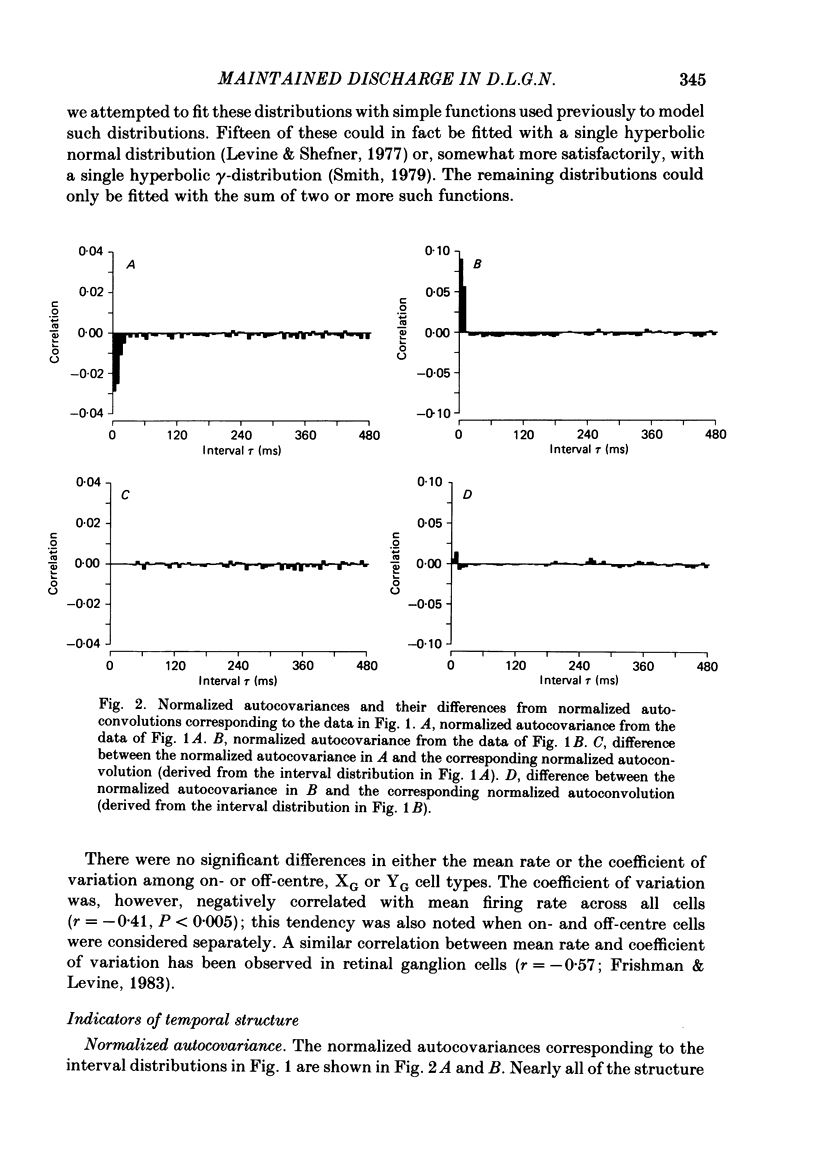
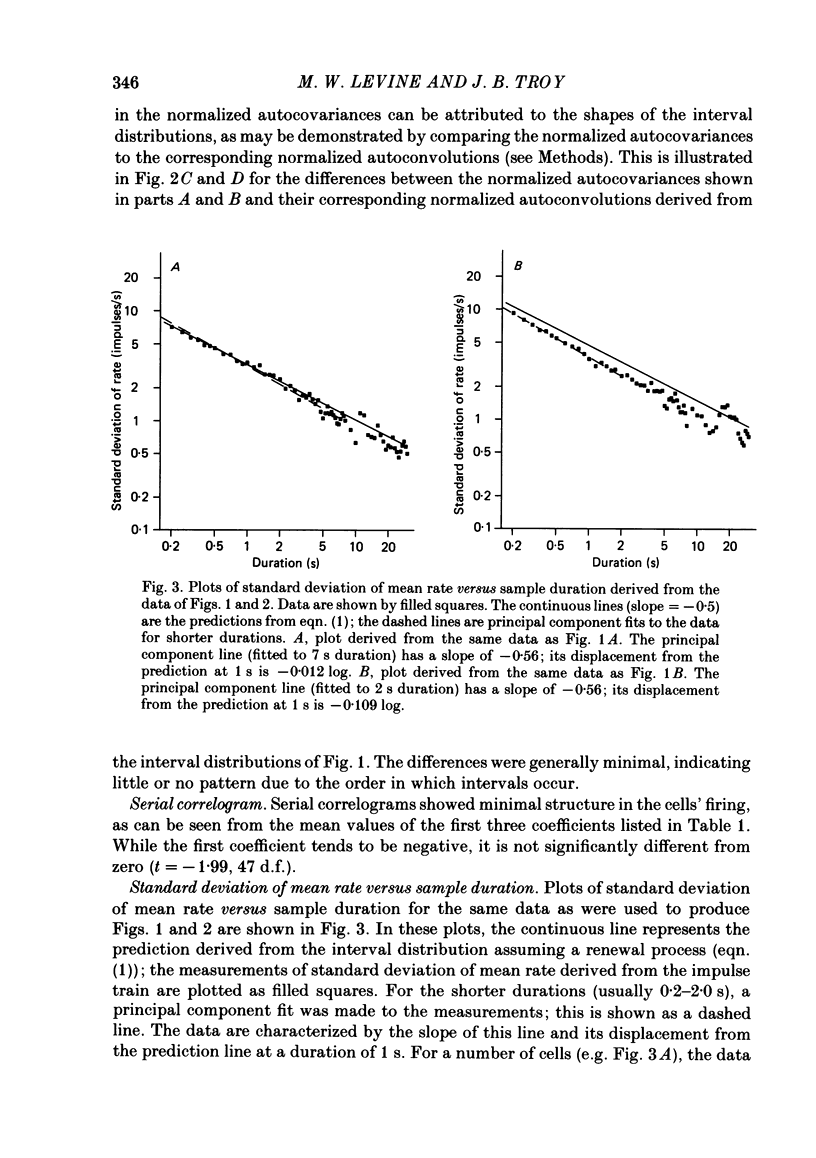
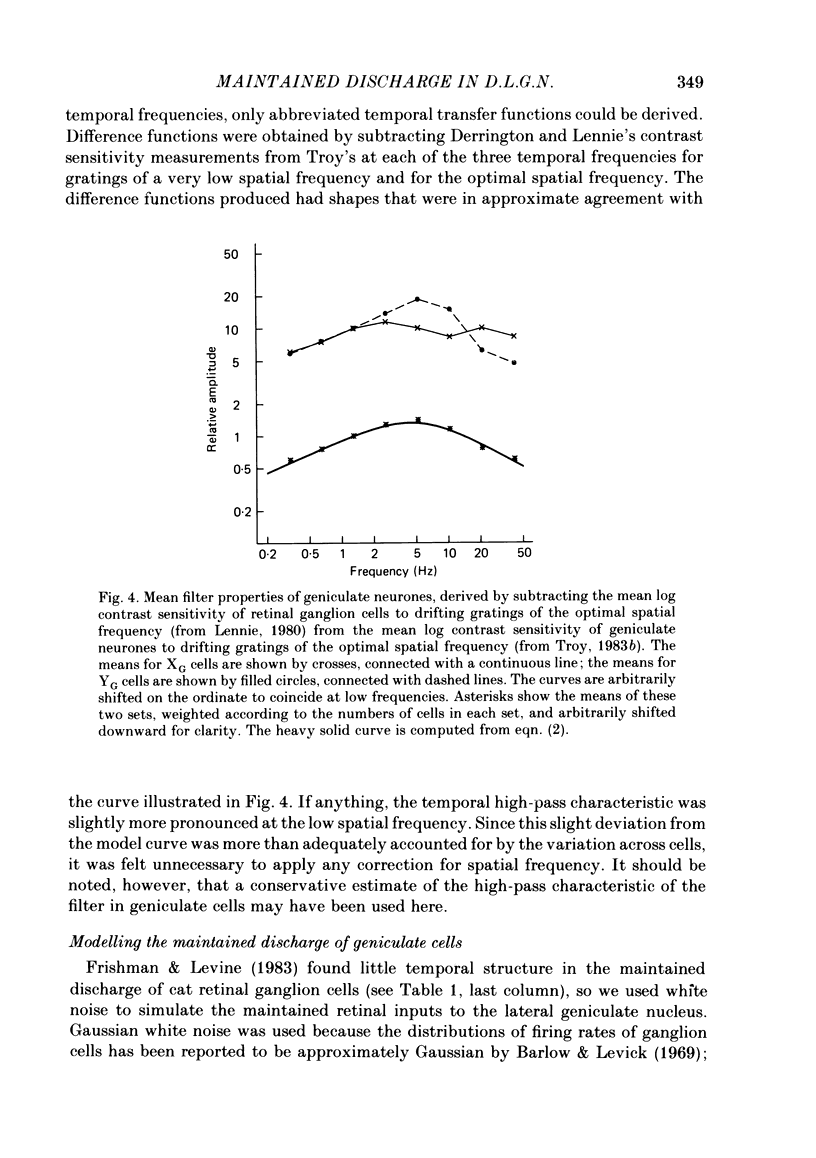
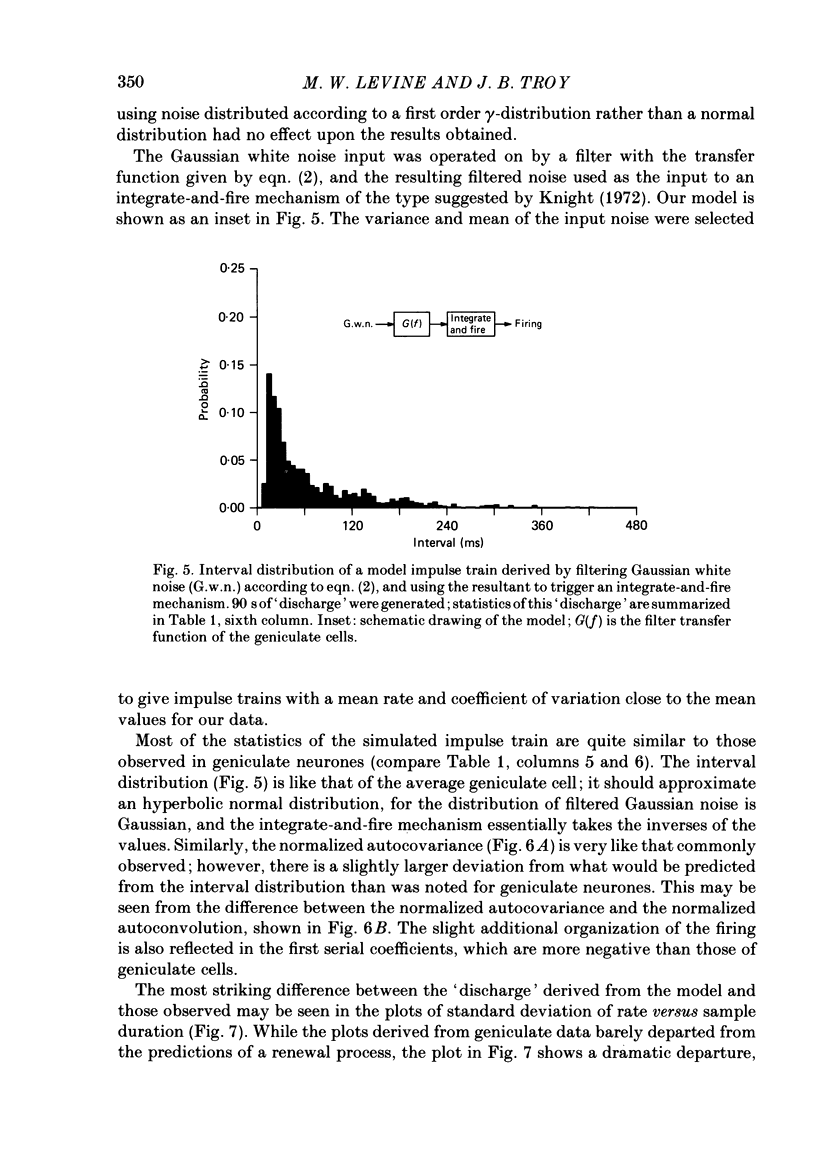
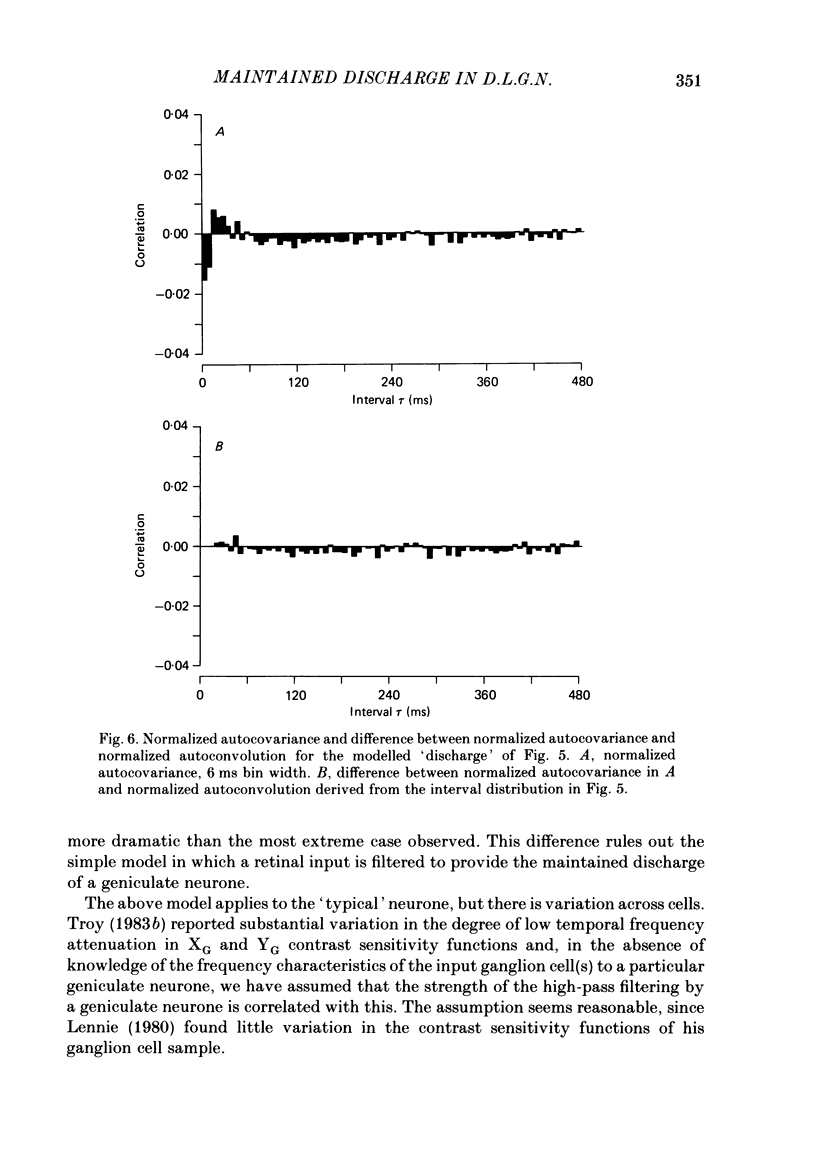
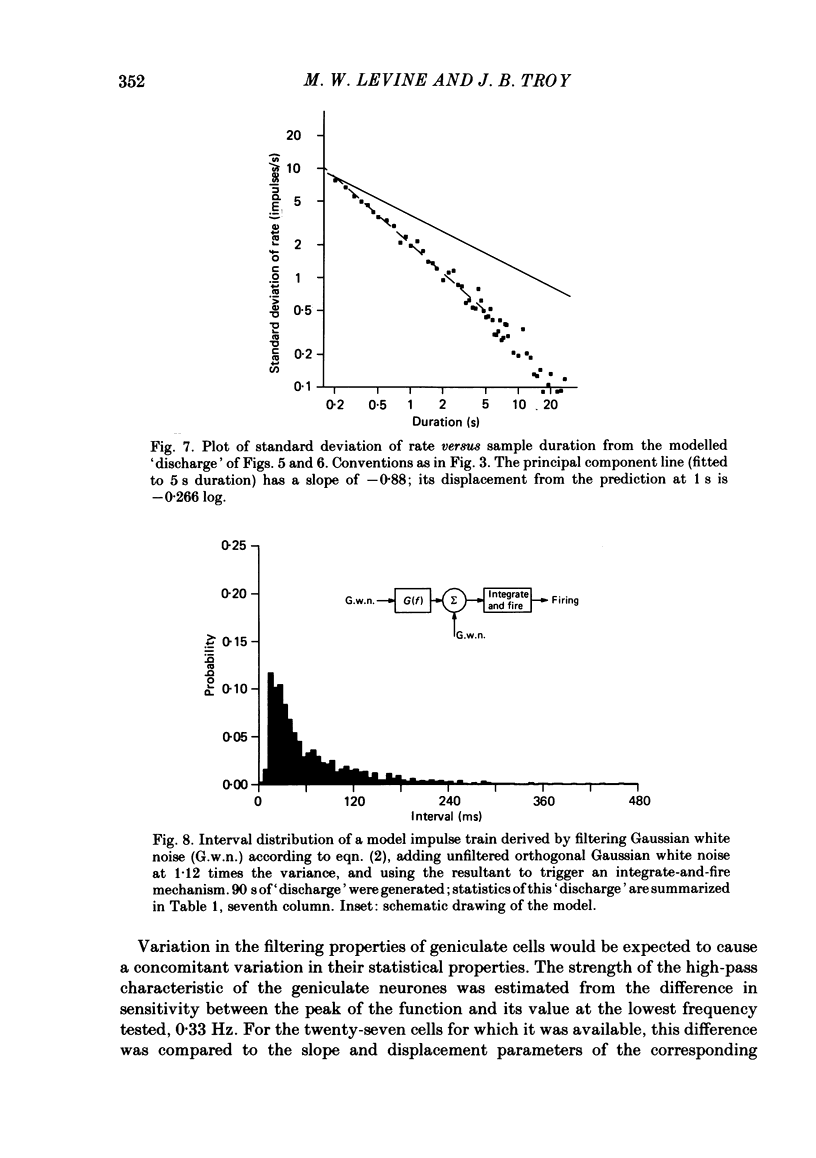
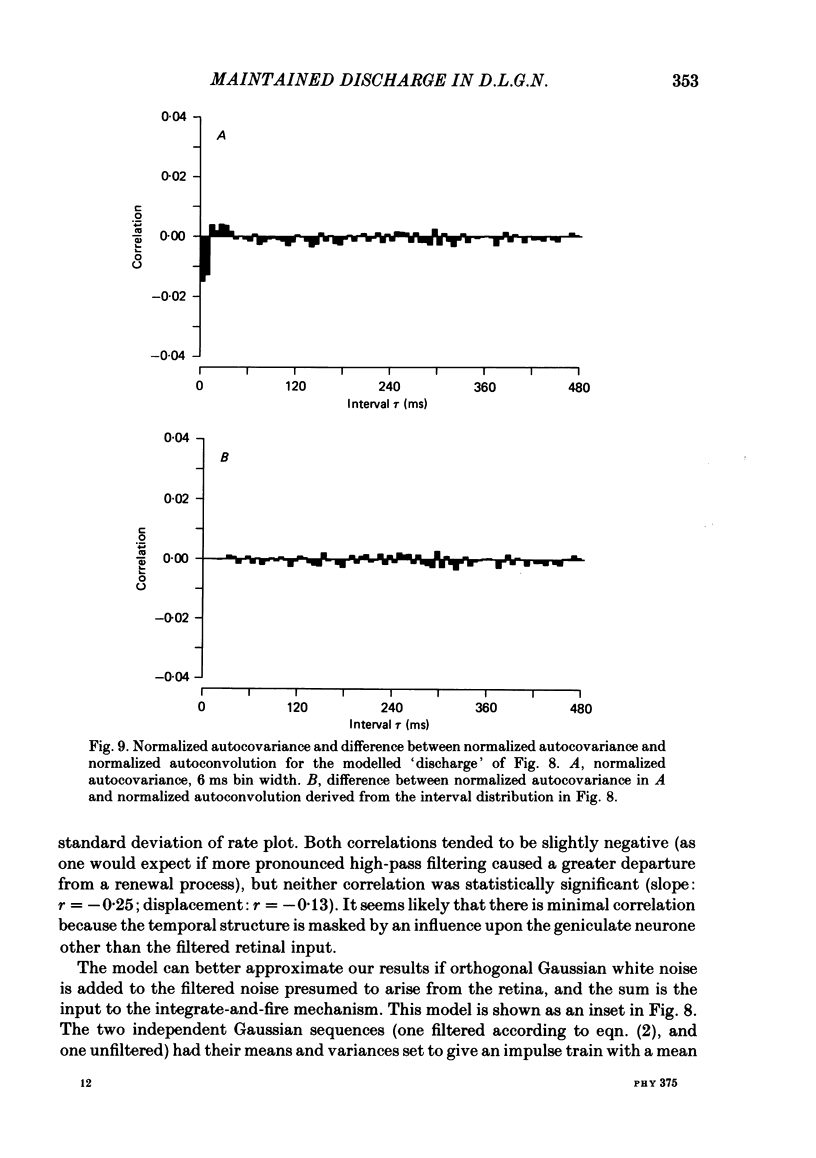
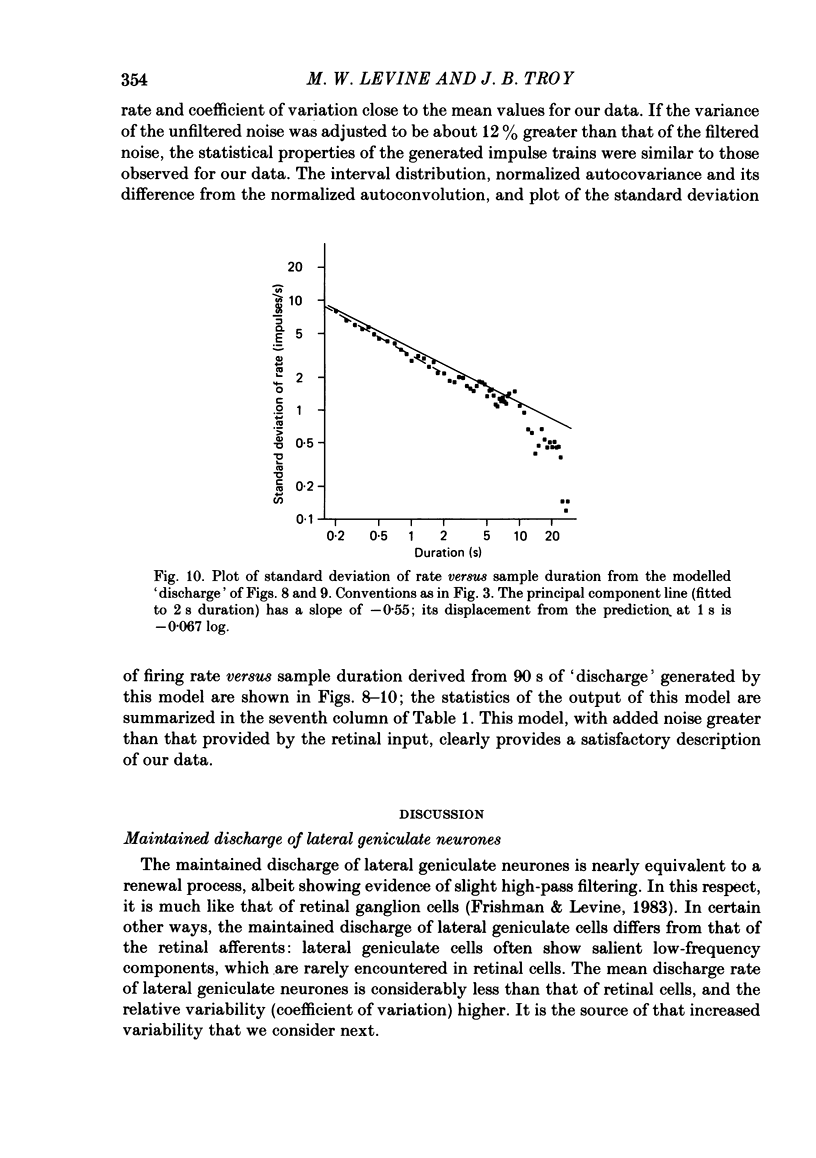
Maintained discharge in the presence of a steady background luminance was analysed from forty-eight cells in the A laminae of the dorsal lateral geniculate nucleus of cat. Cells were categorized as XG or YG and, in most cases, as on-or off-centre. The temporal contrast sensitivity function of twenty-seven of the cells was measured using drifting gratings of the optimal spatial frequency. The maintained discharge was characterized by several simple descriptors, including the interval distribution, mean firing rate, and coefficient of variability. Its temporal organization was revealed by two indicators of correlational properties, the normalized autocovariance and the serial correlogram, and more effectively, by the less familiar plot of the standard deviation of firing rate versus sample duration. The statistics revealing temporal organization of the maintained discharge indicated that the variability of firing was nearly, but not quite, derived from a renewal process. The maintained discharges of seven cells were studied for more than one luminance level. Mean luminance did not appear to have any consistent effect upon the statistics of the maintained discharge. The temporal filtering properties of lateral geniculate cells were deduced from a comparison of the temporal contrast sensitivities of geniculate neurones (Troy, 1983b) and retinal ganglion cells (Lennie, 1980). Analyses showed that the maintained discharge of retinal neurones passed through this filter could not account for the observed statistics of the maintained discharge of geniculate neurones. It is proposed that additional noise is added to the retinal signal at the level of the lateral geniculate. Models are presented to explain how the signals might be filtered in a way that does not also affect the added noise.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlsen G., Grant K., Lindström S. Monosynaptic excitation of principal cells in the lateral geniculate nucleus by corticofugal fibers. Brain Res. 1982 Feb 25;234(2):454–458. doi: 10.1016/0006-8993(82)90886-1. [DOI] [PubMed] [Google Scholar]
- Ahlsén G., Lindström S. Excitation of perigeniculate neurones via axon collaterals of principal cells. Brain Res. 1982 Mar 25;236(2):477–481. doi: 10.1016/0006-8993(82)90730-2. [DOI] [PubMed] [Google Scholar]
- Ahlsén G., Lindström S., Lo F. S. Inhibition from the brain stem of inhibitory interneurones of the cat's dorsal lateral geniculate nucleus. J Physiol. 1984 Feb;347:593–609. doi: 10.1113/jphysiol.1984.sp015085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Changes in the maintained discharge with adaptation level in the cat retina. J Physiol. 1969 Jun;202(3):699–718. doi: 10.1113/jphysiol.1969.sp008836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant H. L., Jr, Marcos A. R., Segundo J. P. Correlations of neuronal spike discharges produced by monosynaptic connections and by common inputs. J Neurophysiol. 1973 Mar;36(2):205–225. doi: 10.1152/jn.1973.36.2.205. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen A. M., Vendrik A. J. Determination of the transfer ratio of cat's geniculate neurons through quasi-intracellular recordings and the relation with the level of alertness. Exp Brain Res. 1972;14(3):227–242. doi: 10.1007/BF00816160. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. The influence of temporal frequency and adaptation level on receptive field organization of retinal ganglion cells in cat. J Physiol. 1982 Dec;333:343–366. doi: 10.1113/jphysiol.1982.sp014457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Lin C. S., Stanford L. R., Sherman S. M. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981 Jul;46(1):80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Frishman L. J., Levine M. W. Statistics of the maintained discharge of cat retinal ganglion cells. J Physiol. 1983 Jun;339:475–494. doi: 10.1113/jphysiol.1983.sp014728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight B. W. Dynamics of encoding in a population of neurons. J Gen Physiol. 1972 Jun;59(6):734–766. doi: 10.1085/jgp.59.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennie P. Perceptual signs of parallel pathways. Philos Trans R Soc Lond B Biol Sci. 1980 Jul 8;290(1038):23–37. doi: 10.1098/rstb.1980.0080. [DOI] [PubMed] [Google Scholar]
- Levine M. W. Firing rate of a retinal neuron are not predictable from interspike interval statistics. Biophys J. 1980 Apr;30(1):9–25. doi: 10.1016/S0006-3495(80)85073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. W. Retinal processing of intrinsic ad extrinsic noise. J Neurophysiol. 1982 Oct;48(4):992–1010. doi: 10.1152/jn.1982.48.4.992. [DOI] [PubMed] [Google Scholar]
- Levine M. W., Shefner J. M. A model for the variability of interspike intervals during sustained firing of a retinal neuron. Biophys J. 1977 Sep;19(3):241–252. doi: 10.1016/S0006-3495(77)85584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström S. Interneurons in the lateral geniculate nucleus with monosynaptic excitation from retinal ganglion cells. Acta Physiol Scand. 1983 Sep;119(1):101–103. doi: 10.1111/j.1748-1716.1983.tb07311.x. [DOI] [PubMed] [Google Scholar]
- Lindström S. Synaptic organization of inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Brain Res. 1982 Feb 25;234(2):447–453. doi: 10.1016/0006-8993(82)90885-x. [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Hubel D. H. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981 Jun 18;291(5816):554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- Mastronarde D. N. Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X- and Y-cells. J Neurophysiol. 1983 Feb;49(2):303–324. doi: 10.1152/jn.1983.49.2.303. [DOI] [PubMed] [Google Scholar]
- McCarley R. W., Benoit O., Barrionuevo G. Lateral geniculate nucleus unitary discharge in sleep and waking: state- and rate-specific aspects. J Neurophysiol. 1983 Oct;50(4):798–818. doi: 10.1152/jn.1983.50.4.798. [DOI] [PubMed] [Google Scholar]
- Munemori J., Hara K., Kimura M., Sato R. Statistical features of impulse trains in cat's lateral geniculate neurons. Biol Cybern. 1984;50(3):167–172. doi: 10.1007/BF00340024. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J. 1967 Jul;7(4):391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHADE O. H., Sr Optical and photoelectric analog of the eye. J Opt Soc Am. 1956 Sep;46(9):721–739. doi: 10.1364/josa.46.000721. [DOI] [PubMed] [Google Scholar]
- Schmielau F., Singer W. The role of visual cortex for binocular interactions in the cat lateral geniculate nucleus. Brain Res. 1977 Jan 21;120(2):354–361. doi: 10.1016/0006-8993(77)90914-3. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Singer W., Pöppel E., Creutzfeldt O. Inhibitory interaction in the cat's lateral geniculate nucleus. Exp Brain Res. 1972;14(2):210–226. doi: 10.1007/BF00234800. [DOI] [PubMed] [Google Scholar]
- Smith C. E. A comment on a retinal neuron model. Biophys J. 1979 Feb;25(2 Pt 1):385–386. doi: 10.1016/s0006-3495(79)85301-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So Y. T., Shapley R. Spatial properties of X and Y cells in the lateral geniculate nucleus of the cat and conduction veolcities of their inputs. Exp Brain Res. 1979 Aug 1;36(3):533–550. doi: 10.1007/BF00238521. [DOI] [PubMed] [Google Scholar]
- So Y. T., Shapley R. Spatial tuning of cells in and around lateral geniculate nucleus of the cat: X and Y relay cells and perigeniculate interneurons. J Neurophysiol. 1981 Jan;45(1):107–120. doi: 10.1152/jn.1981.45.1.107. [DOI] [PubMed] [Google Scholar]
- Troy J. B. Spatial contrast sensitivities of X and Y type neurones in the cat's dorsal lateral geniculate nucleus. J Physiol. 1983 Nov;344:399–417. doi: 10.1113/jphysiol.1983.sp014948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy J. B. Spatio-temporal interaction in neurones of the cat's dorsal lateral geniculate nucleus. J Physiol. 1983 Nov;344:419–432. doi: 10.1113/jphysiol.1983.sp014949. [DOI] [PMC free article] [PubMed] [Google Scholar]


