Abstract
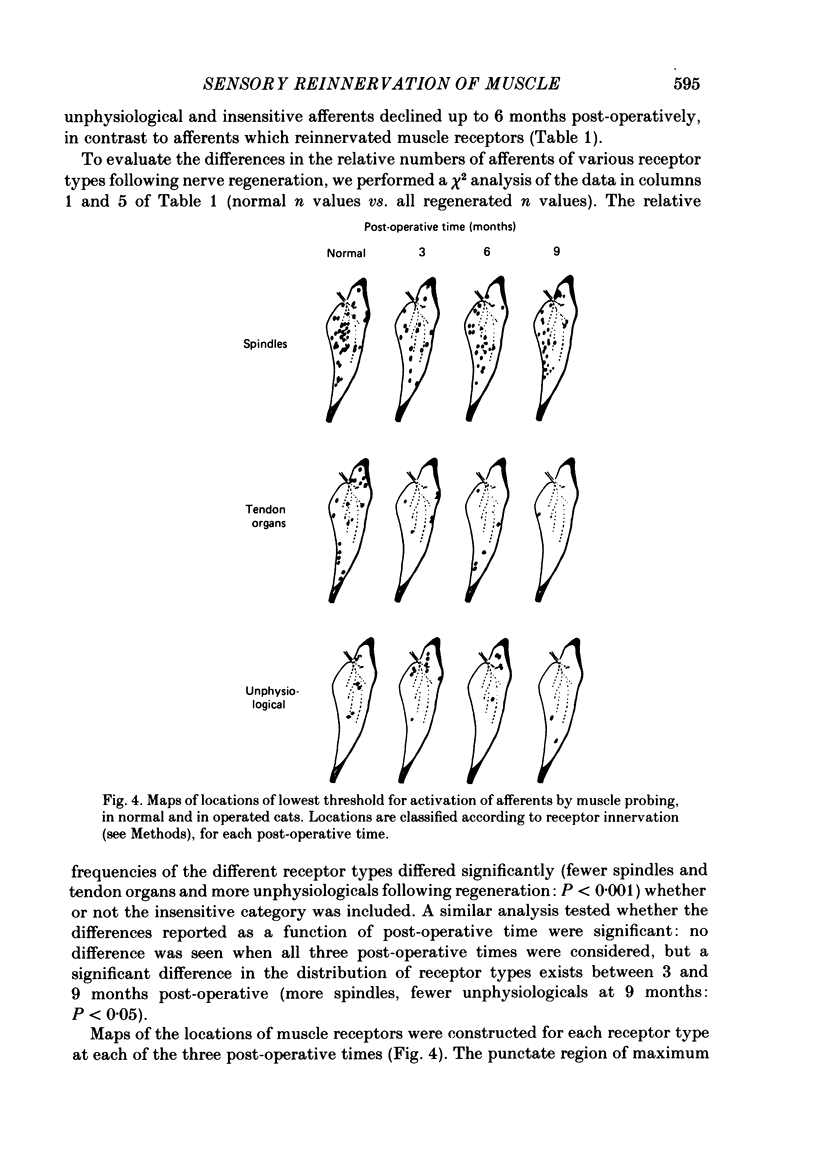
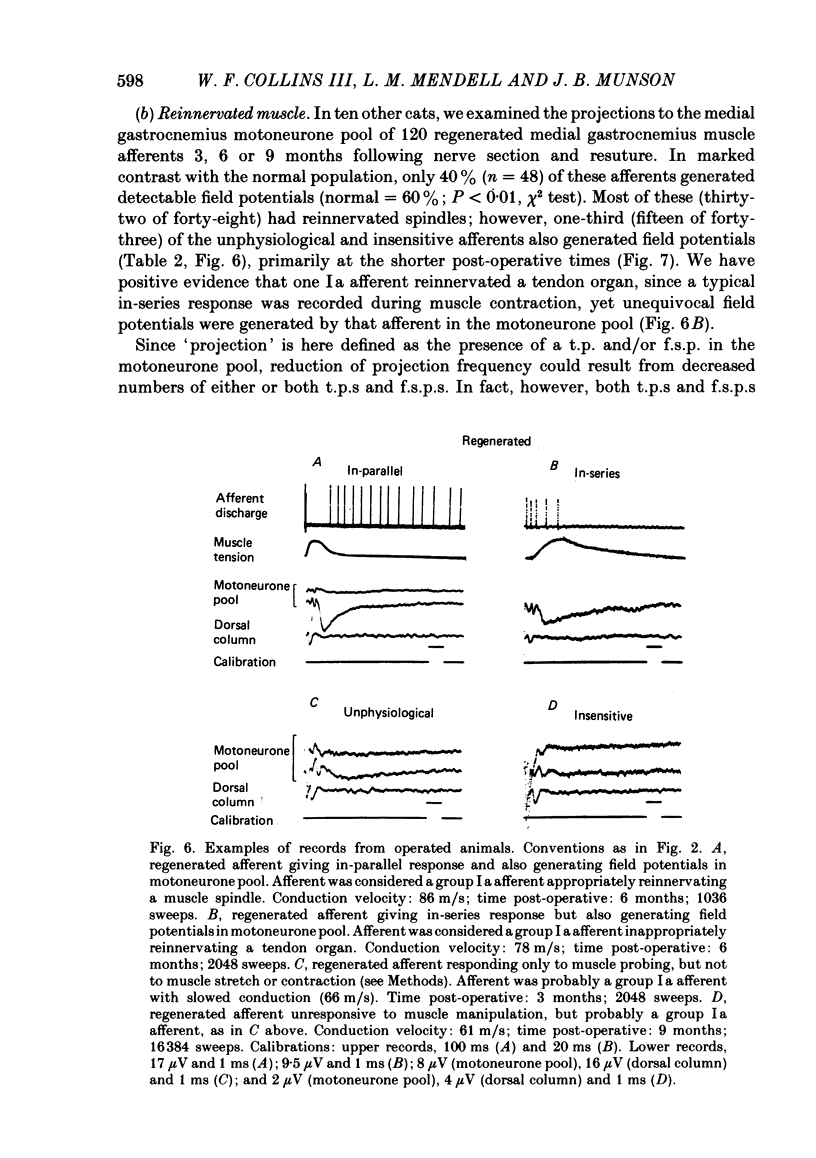
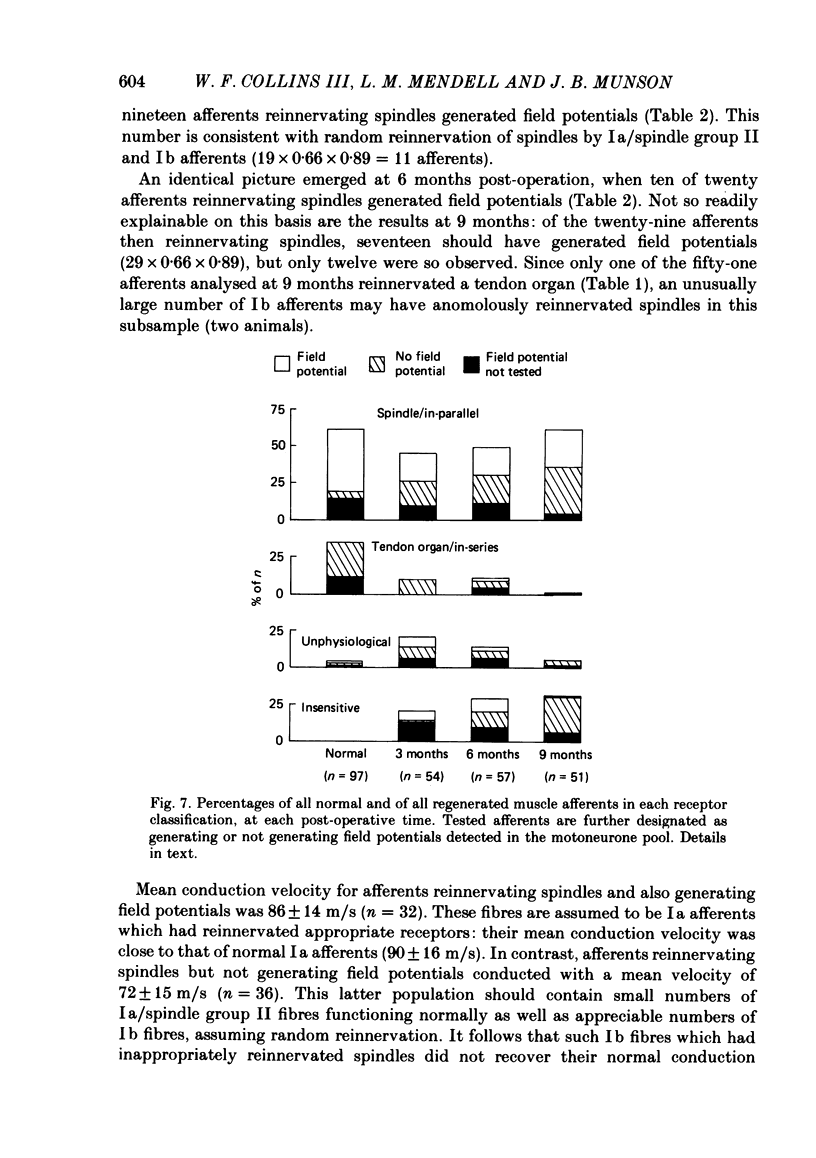
Experiments were addressed to the following questions: when a muscle nerve is sectioned and regenerates to what extent are muscle receptors (spindles and tendon organs) reinnervated? is the reinnervation specific? that is, do group Ia and spindle group II fibres preferentially reinnervate spindles and do group Ib fibres preferentially reinnervate tendon organs? what are the consequences to the afferent of failure to re-establish appropriate receptor innervation? In normal cats, and in cats 3, 6 or 9 months after section and resuture of the medial gastrocnemius muscle nerve, medial gastrocnemius afferent fibres in continuity were impaled in dorsal rootlets for recording and stimulation. Receptor innervation was determined electrophysiologically by manipulation of the medial gastrocnemius muscle. Afferent fibre type was determined by the presence (group Ia or spindle group II) or absence (group Ib) of field potentials in the homonymous motoneurone pool in response to activation of the afferent fibre. In normal cats, two-thirds of recorded afferents innervated spindles; 89% of these generated field potentials detected in the motoneurone pool. One-third of recorded afferents innervated tendon organs; none of these generated such field potentials. In operated cats, about half of the recorded afferents innervated spindles, about one-third responded abnormally or not at all to muscle manipulation, and fewer than one-tenth innervated tendon organs. Numbers of afferents which innervated spindles increased with time. The proportion of afferents generating field potentials was smaller in operated than in normal animals (40% vs. 60%) and declined progressively with time. Field potentials were generated by fibres in all categories of receptor reinnervation. This ability was lost at long post-operative intervals by fibres failing to reach the muscle. Conduction velocity of fibres fell in operated animals. Fibres reinnervating their original type of receptor (e.g. group Ia fibre----spindle) exhibited greater conduction velocity than fibres innervating an inappropriate receptor or no receptor. From these findings and other considerations (see Discussion) we conclude that following section and resuture of the medial gastrocnemius muscle nerve: about 75% of afferents regenerate into the medial gastrocnemius muscle, many more spindles than tendon organs become reinnervated, random populations of groups Ia and Ib (and probably spindle group II) fibres reinnervate spindles, fibres which fail to re-establish appropriate receptor innervation also fail to recover normal conduction velocity.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appenteng K., O'Donovan M. J., Somjen G., Stephens J. A., Taylor A. The projection of jaw elevator muscle spindle afferents to fifth nerve motoneurones in the cat. J Physiol. 1978 Jun;279:409–423. doi: 10.1113/jphysiol.1978.sp012353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Stuart D. G. Responses of Ia and spindle group II afferents to single motor-unit contractions. J Neurophysiol. 1980 Mar;43(3):621–629. doi: 10.1152/jn.1980.43.3.621. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Butler R. G. Regeneration of afferent and efferent fibres to muscle spindles after nerve injury in adults cats. J Physiol. 1976 Sep;260(2):253–266. doi: 10.1113/jphysiol.1976.sp011514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P. R., Horch K. W. Specific regeneration of cutaneous fibers in the cat. J Neurophysiol. 1973 Jan;36(1):101–114. doi: 10.1152/jn.1973.36.1.101. [DOI] [PubMed] [Google Scholar]
- CRAGG B. G., THOMAS P. K. Changes in conduction velocity and fibre size proximal to peripheral nerve lesions. J Physiol. 1961 Jul;157:315–327. doi: 10.1113/jphysiol.1961.sp006724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KRNJEVIC K., MILEDI R. Delayed effects of peripheral severance of afferent nerve fibres on the efficacy of their central synapses. J Physiol. 1959 Jan 28;145(1):204–220. doi: 10.1113/jphysiol.1959.sp006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Santini M., Schomburg E. D. Characteristics and distribution of spinal focal synaptic potentials generated by group II muscle afferents. Acta Physiol Scand. 1974 Jul;91(3):298–313. doi: 10.1111/j.1748-1716.1974.tb05686.x. [DOI] [PubMed] [Google Scholar]
- Gallego R., Kuno M., Núez R., Snider W. D. Disuse enhances synaptic efficacy in spinal mononeurones. J Physiol. 1979 Jun;291:191–205. doi: 10.1113/jphysiol.1979.sp012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego R., Kuno M., Núez R., Snider W. D. Enhancement of synaptic function in cat motoneurones during peripheral sensory regeneration. J Physiol. 1980 Sep;306:205–218. doi: 10.1113/jphysiol.1980.sp013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie M. J., Stein R. B. The relationship between axon diameter, myelin thickness and conduction velocity during atrophy of mammalian peripheral nerves. Brain Res. 1983 Jan 17;259(1):41–56. doi: 10.1016/0006-8993(83)91065-x. [DOI] [PubMed] [Google Scholar]
- Gregory J. E., Luff A. R., Proske U. Muscle receptors in the cross-reinnervated soleus muscle of the cat. J Physiol. 1982 Oct;331:367–383. doi: 10.1113/jphysiol.1982.sp014377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig M. G., Collins W. F., 3rd, Mendell L. M. Alpha-motoneuron EPSPs exhibit different frequency sensitivities to single Ia-afferent fiber stimulation. J Neurophysiol. 1983 Apr;49(4):886–901. doi: 10.1152/jn.1983.49.4.886. [DOI] [PubMed] [Google Scholar]
- Hyde D., Scott J. J. Responses of cat peroneus brevis muscle spindle afferents during recovery from nerve-crush injury. J Neurophysiol. 1983 Aug;50(2):344–357. doi: 10.1152/jn.1983.50.2.344. [DOI] [PubMed] [Google Scholar]
- Ip M. C., Vrbová G. Motor and sensory reinnervation of fast and slow mammalian muscles. Z Zellforsch Mikrosk Anat. 1973 Dec 31;146(2):261–279. doi: 10.1007/BF00307351. [DOI] [PubMed] [Google Scholar]
- Ip M. C., Vrbová G., Westbury D. R. The sensory reinnervation of hind limb muscles of the cat following denervation and de-efferentation. Neuroscience. 1977;2(3):423–434. doi: 10.1016/0306-4522(77)90007-0. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Johannisson T., Lipski J. Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol. 1981 Jan;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Mendell L. M. Modifiability of spinal synapses. Physiol Rev. 1984 Jan;64(1):260–324. doi: 10.1152/physrev.1984.64.1.260. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Scott J. G. The effect of peripheral nerve cross-union on connections of single Ia fibers to motoneurons. Exp Brain Res. 1975 Mar 27;22(3):221–234. doi: 10.1007/BF00234765. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Fleshman J. W., Sypert G. W. Properties of single-fiber spindle group II EPSPs in triceps surae motoneurons. J Neurophysiol. 1980 Oct;44(4):713–725. doi: 10.1152/jn.1980.44.4.713. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Fleshman J. W., Zengel J. E., Sypert G. W. Synaptic and mechanical coupling between type-identified motor units and individual spindle afferents of medial gastrocnemius muscle of the cat. J Neurophysiol. 1984 Jun;51(6):1268–1283. doi: 10.1152/jn.1984.51.6.1268. [DOI] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W. Properties of single central Ia afferent fibres projecting to motoneurones. J Physiol. 1979 Nov;296:315–327. doi: 10.1113/jphysiol.1979.sp013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J. B., Sypert G. W., Zengel J. E., Lofton S. A., Fleshman J. W. Monosynaptic projections of individual spindle group II afferents to type-identified medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1982 Nov;48(5):1164–1174. doi: 10.1152/jn.1982.48.5.1164. [DOI] [PubMed] [Google Scholar]
- SWETT J. E., ELDRED E. Distribution and numbers of stretch rceptors in medial gastrocnemius and soleus muscles of the cat. Anat Rec. 1960 Aug;137:453–460. doi: 10.1002/ar.1091370405. [DOI] [PubMed] [Google Scholar]
- Stacey M. J. Free nerve endings in skeletal muscle of the cat. J Anat. 1969 Sep;105(Pt 2):231–254. [PMC free article] [PubMed] [Google Scholar]
- Stauffer E. K., Watt D. G., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol. 1976 Nov;39(6):1393–1402. doi: 10.1152/jn.1976.39.6.1393. [DOI] [PubMed] [Google Scholar]
- Taylor A., Stephens J. A., Somjen G., Appenteng K., O'Donovan M. J. Extracellular spike triggered averaging for plotting synaptic projections. Brain Res. 1978 Jan 27;140(2):344–348. doi: 10.1016/0006-8993(78)90466-3. [DOI] [PubMed] [Google Scholar]
- Watt D. G., Stauffer E. K., Taylor A., Reinking R. M., Stuart D. G. Analysis of muscle receptor connections by spike-triggered averaging. 1. Spindle primary and tendon organ afferents. J Neurophysiol. 1976 Nov;39(6):1375–1392. doi: 10.1152/jn.1976.39.6.1375. [DOI] [PubMed] [Google Scholar]


