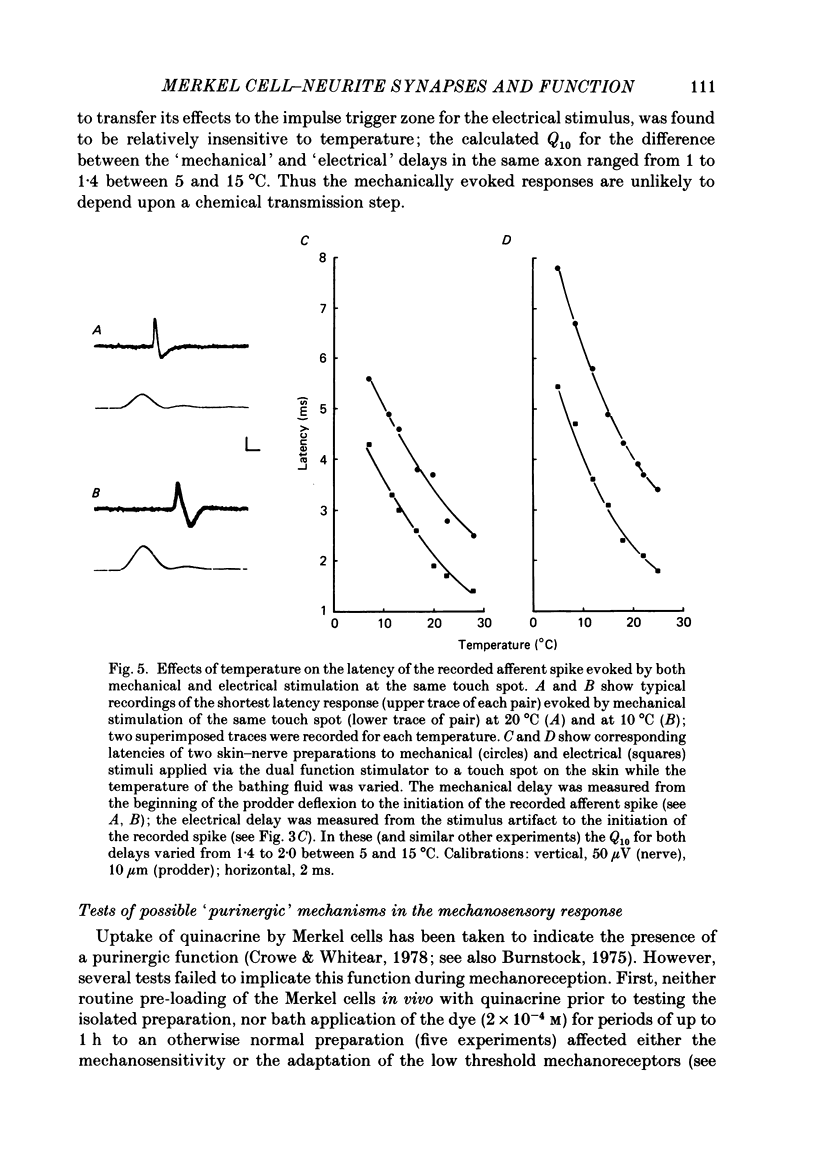
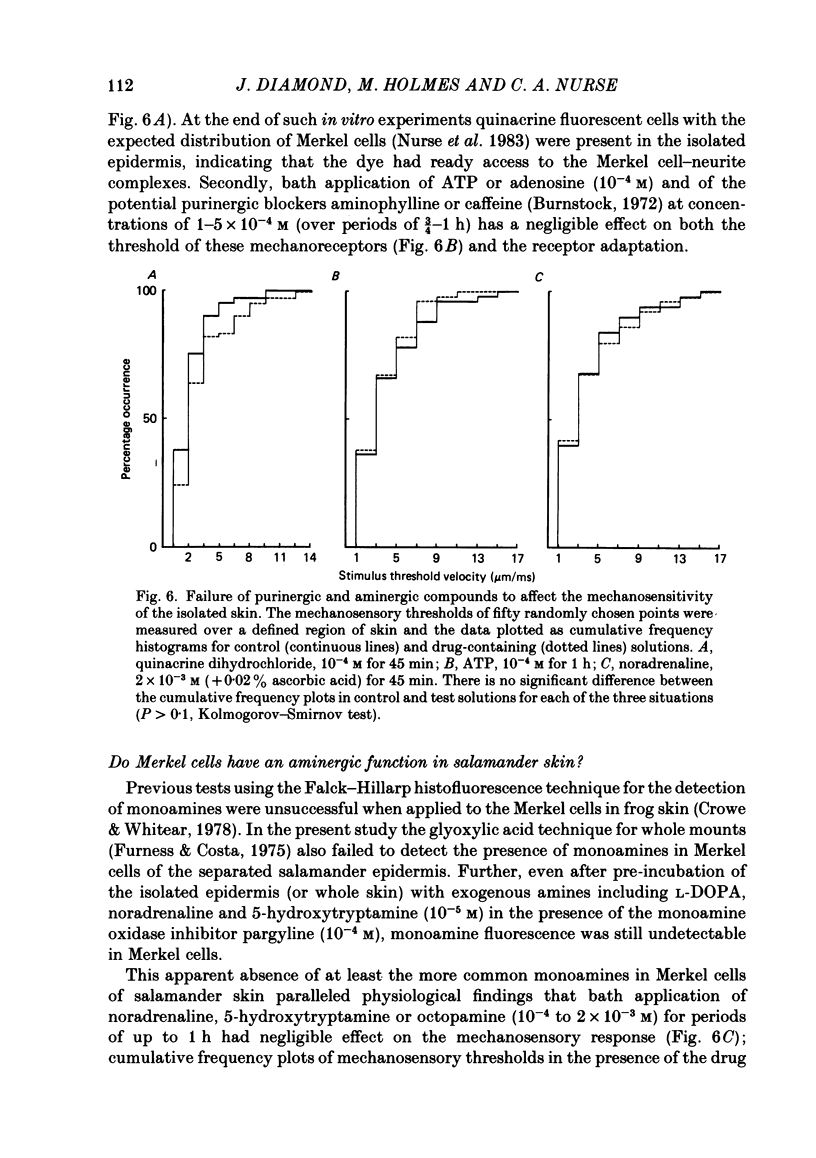
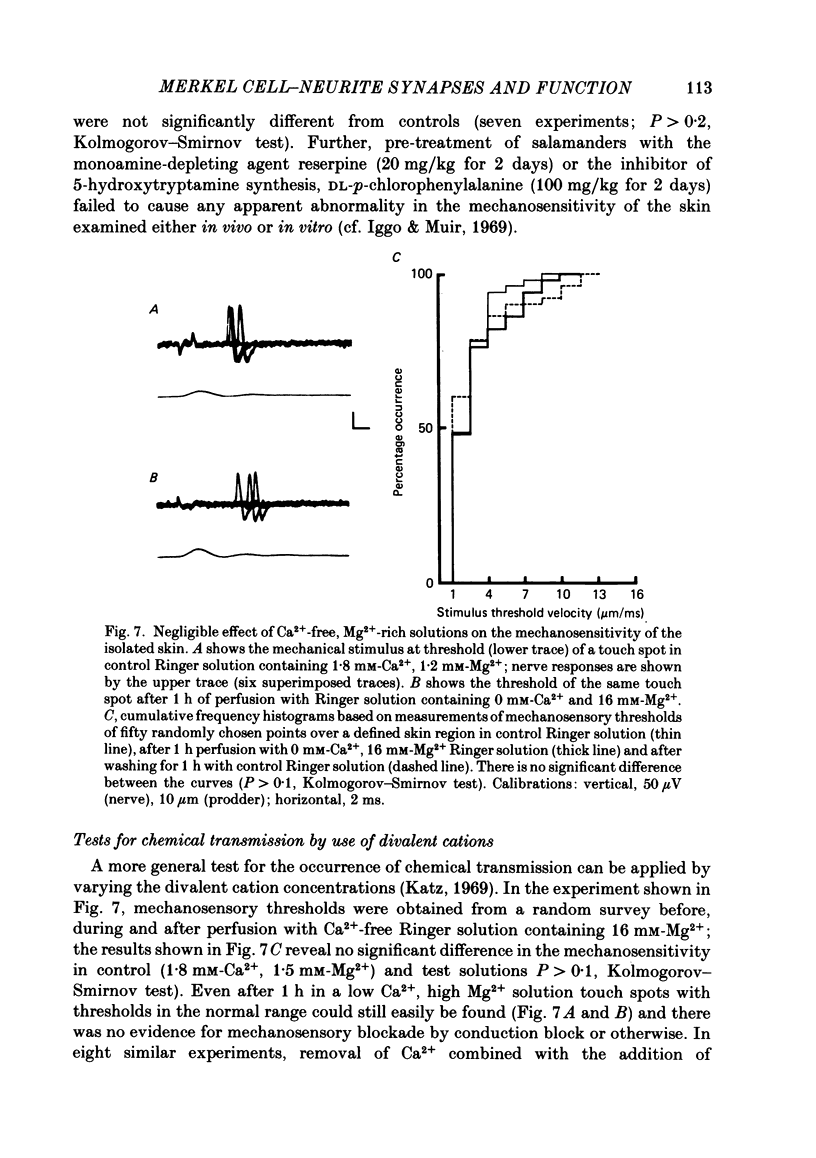
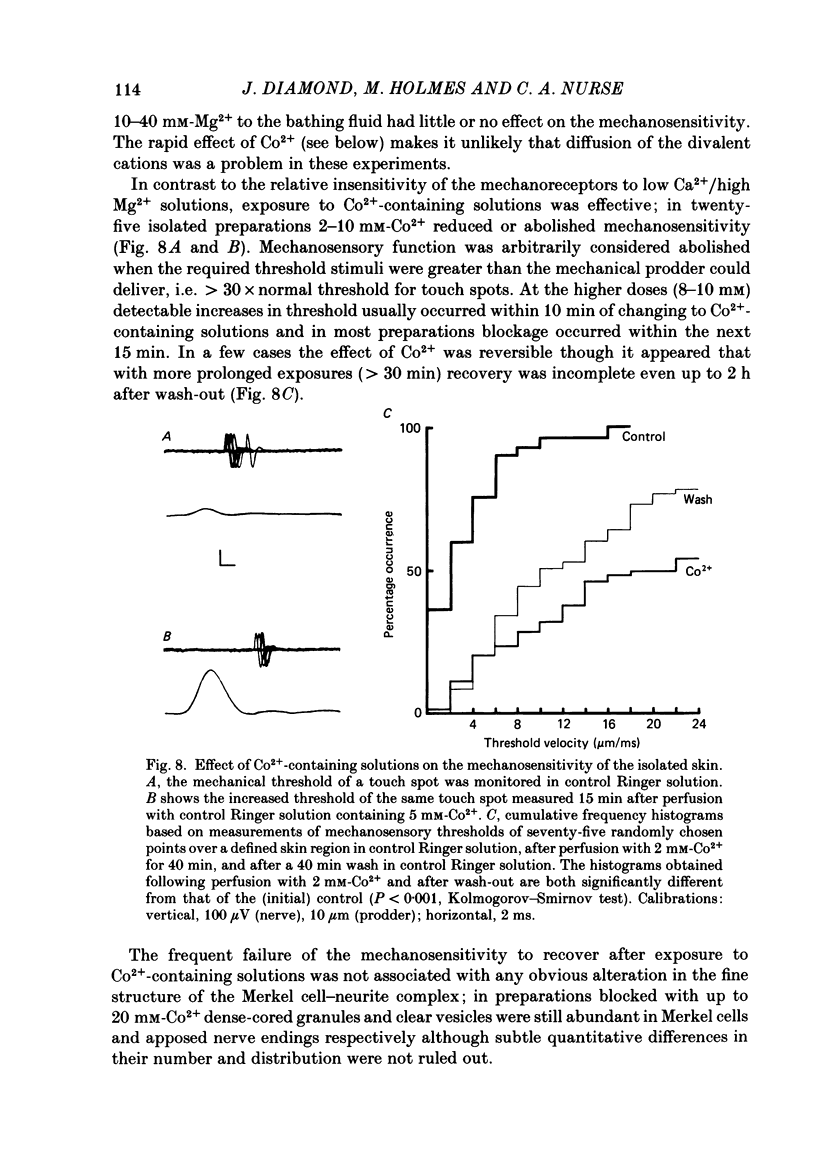
Abstract
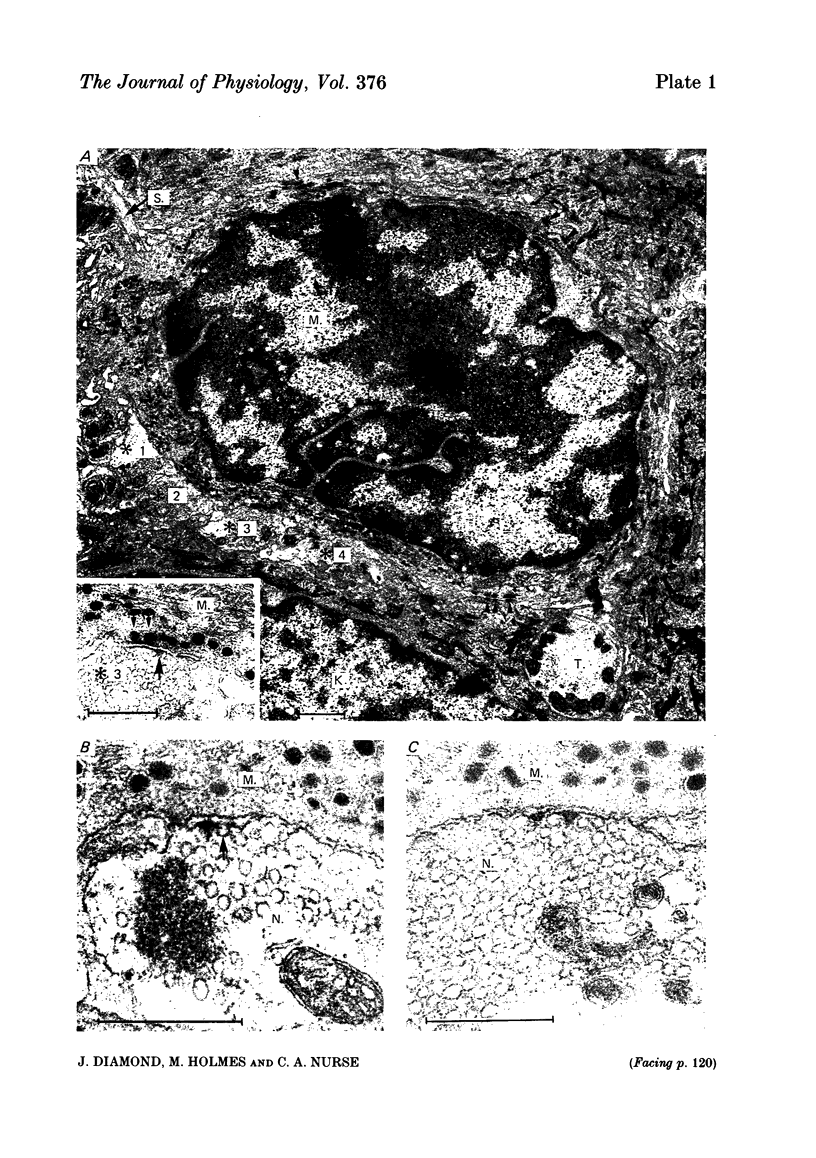
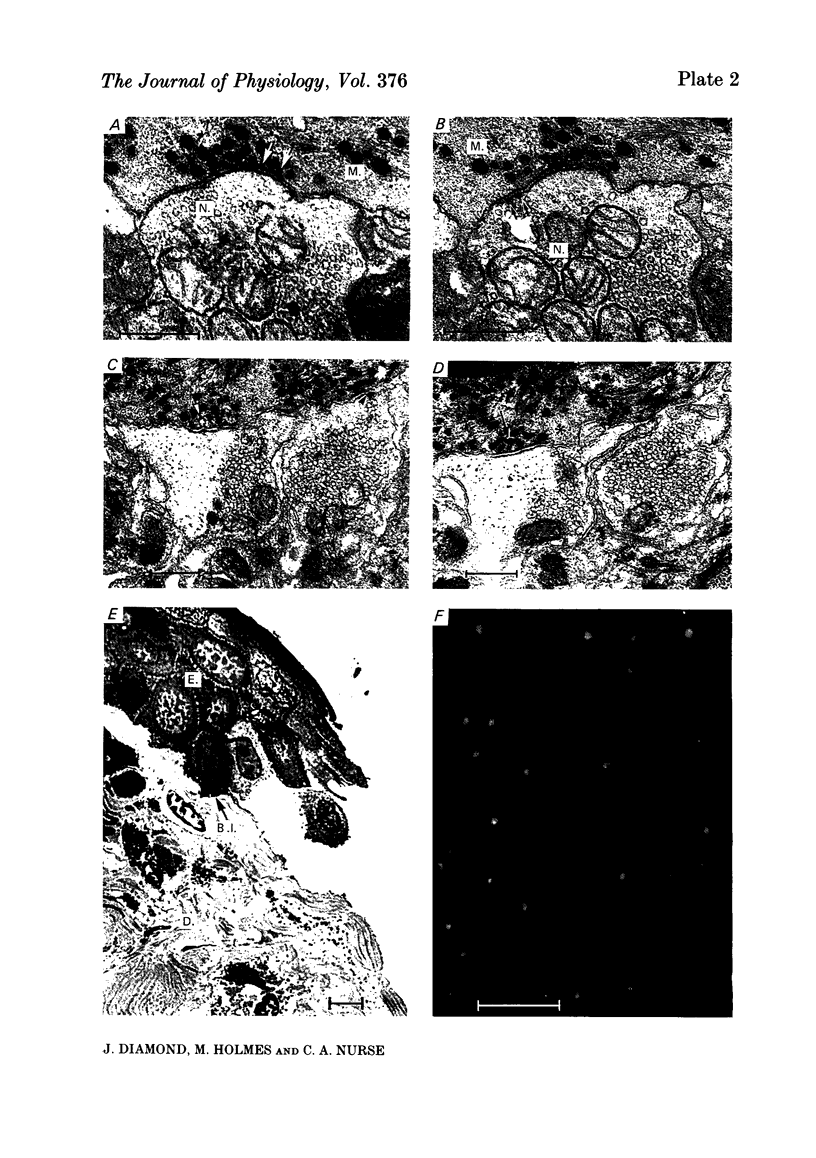
In salamander skin the Merkel cell-neurite complexes located near the base of the epidermis are the morphological correlates of the rapidly adapting touch receptors (Parducz, Leslie, Cooper, Turner & Diamond, 1977). The present electron microscopic studies revealed that these complexes contain reciprocal synapses polarized in the direction Merkel cell to neurite, and in the opposite direction, neurite to Merkel cell. The possible involvement of chemical transmission in the initiation of the mechanosensory response, was studied in vitro with the aid of a stable skin-nerve preparation in which single mechanoreceptors were activated under controlled conditions. Mechanosensitivity was measured with a calibrated prodder (tip diameter 10-30 micron) applied to random or selected points on the surface of the skin while the afferent impulse was recorded in the attached nerve twig. In some experiments the (tungsten) prodder was also used as a surface electrode, allowing the same mechanosensory axon to be excited mechanically (i.e. physiologically), and/or electrically. When applied at a single 'touch spot', suitably timed subthreshold mechanical and subthreshold electrical stimuli could summate to produce a single action potential. The temperature coefficient (Q10) between 5 and 15 degrees C for the latency of the afferent spike was small, in the range 1.3-2, whether it was evoked by mechanical or electrical stimulation. The latency following the mechanical stimulus, which included the transduction step, was longer than that following the electrical stimulus by 0.5-2.5 ms, and this additional delay was also relatively insensitive to temperature. In several cases removal of the epidermis with its Merkel cells (and presumably the most distal portions of the afferent nerve terminations) did not render the remaining skin totally insensitive to mechanical stimulation; however, the remaining receptive elements, though still rapidly adapting, generally had increased mechanosensory thresholds. The mechanosensitivity of the skin was unaffected by bath application of several aminergic (e.g. noradrenaline, 5-hydroxytryptamine, octopamine) and purinergic (e.g. ATP, quinacrine) compounds at concentrations in the range 0.2-2 mM. Removal of extracellular Ca2+ combined with elevation of extracellular Mg2+ (10-40 mM) had relatively little effect on the mechanosensitivity over periods of up to 1 h. In contrast, application of Co2+ (2-10 mM) produced a decrease or blockade of the mechanosensitivity that was not associated with any obvious alterations in the ultrastructure of the Merkel cell-neurite complex.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

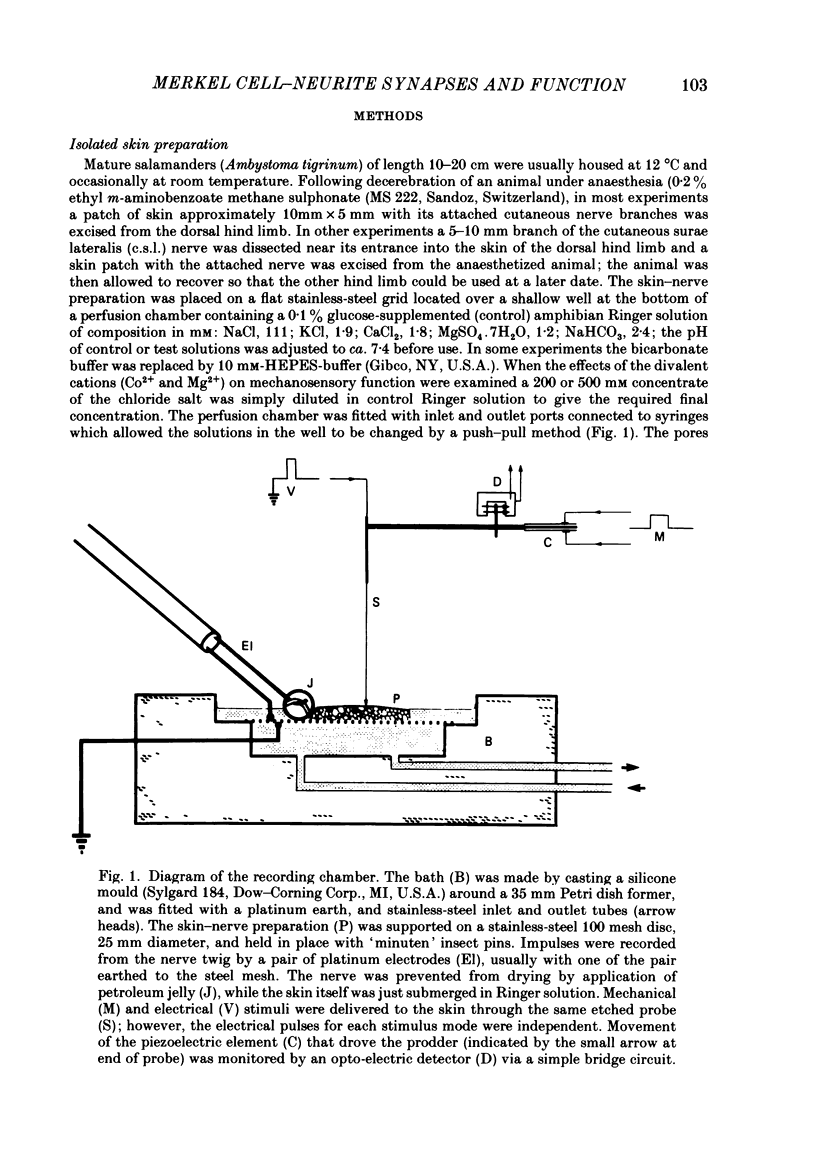



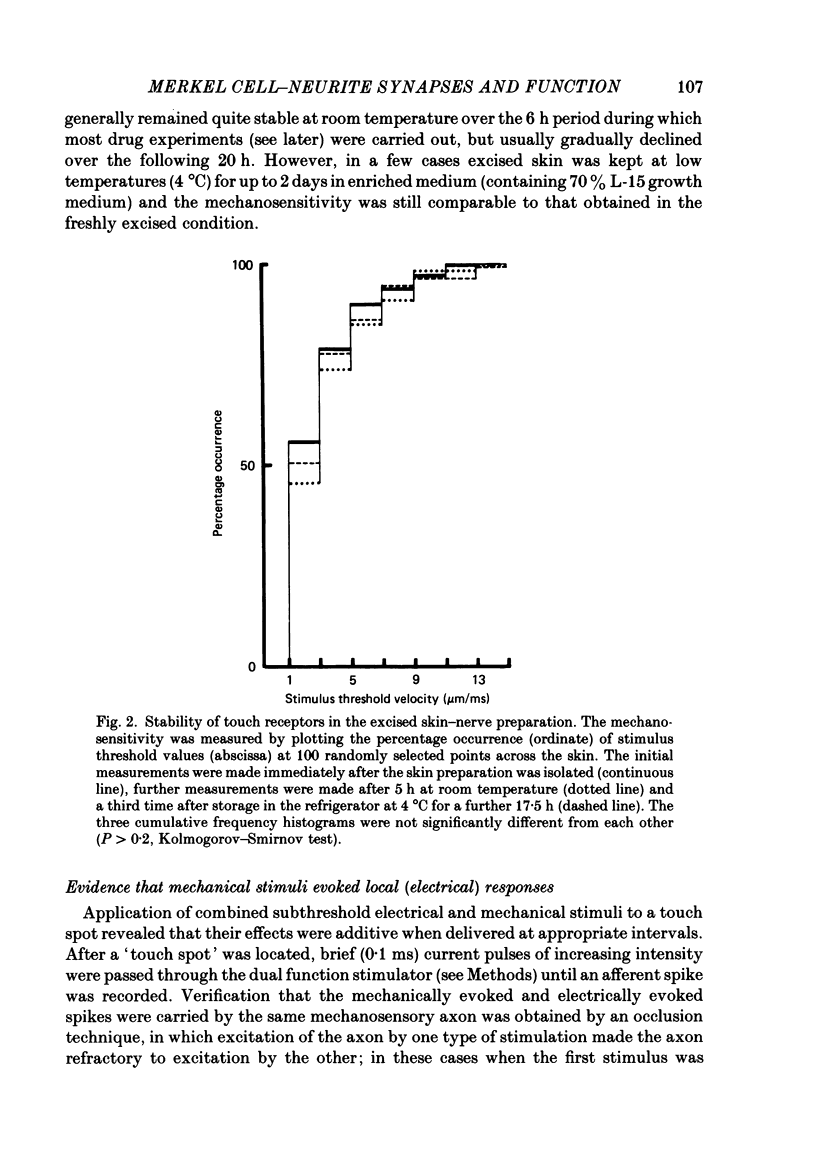
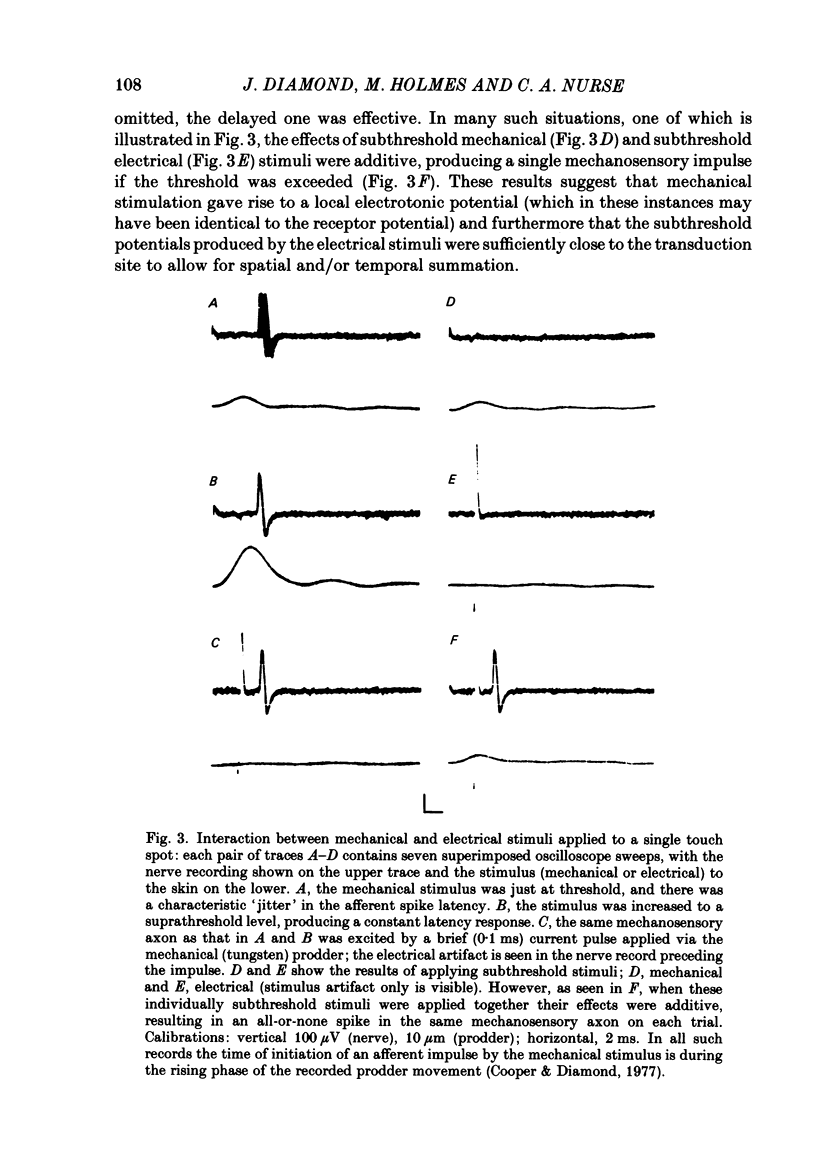

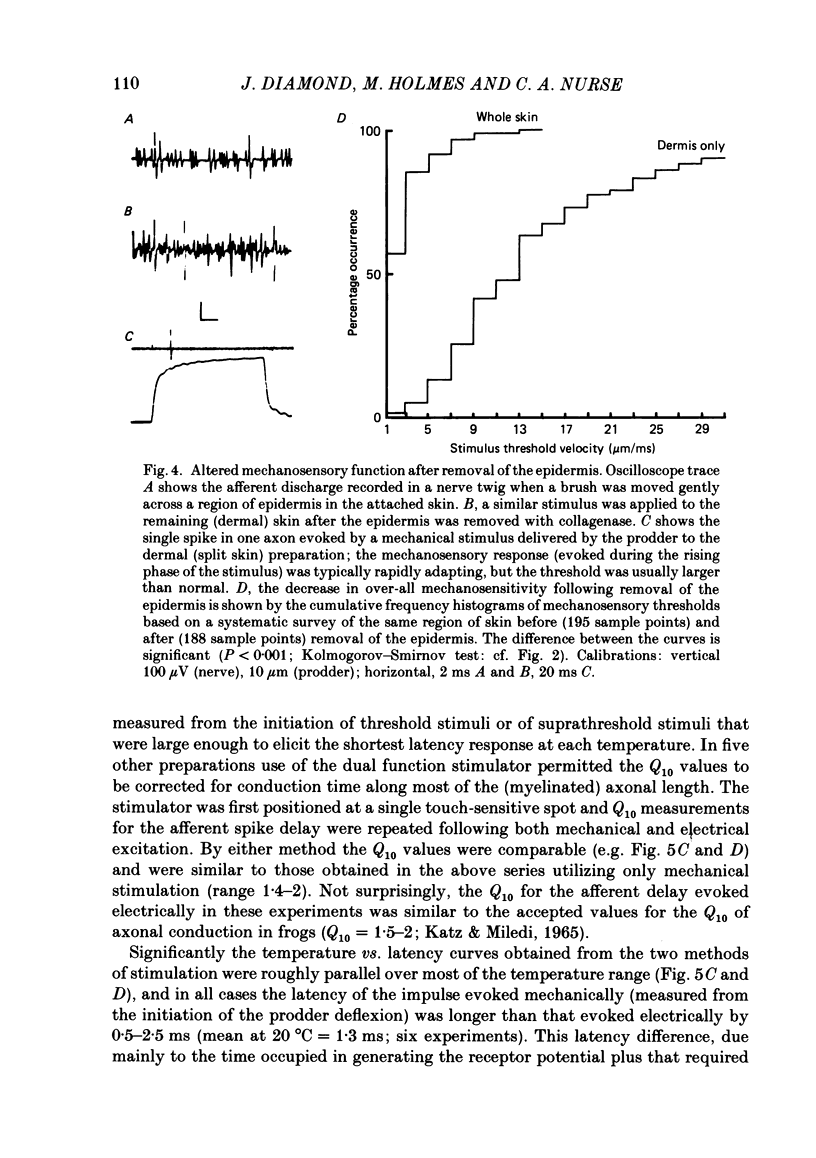






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Cattell M., Hoagland H. Sensory discharges in single cutaneous nerve fibres. J Physiol. 1931 Aug 14;72(4):377–391. doi: 10.1113/jphysiol.1931.sp002781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoev G. N. The effect of Mg2+ and Ca2+ on the excitability of Pacinian corpuscles. Brain Res. 1982 May 13;239(2):391–399. doi: 10.1016/0006-8993(82)90517-0. [DOI] [PubMed] [Google Scholar]
- Baba A., Ohta A., Iwata H. Inhibition by quinacrine of depolarization-induced acetylcholine release and calcium influx in rat brain cortical synaptosomes. J Neurochem. 1983 Jun;40(6):1758–1761. doi: 10.1111/j.1471-4159.1983.tb08152.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Comparative studies of purinergic nerves. J Exp Zool. 1975 Oct;194(1):103–133. doi: 10.1002/jez.1401940108. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Clarke J. D., Hayes B. P., Hunt S. P., Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984 Mar;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Diamond J. A quantitative study of the mechanosensory innervation of the salmander skin. J Physiol. 1977 Jan;264(3):695–723. doi: 10.1113/jphysiol.1977.sp011690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe R., Whitear M. Quinacrine fluorescence of Merkel cells in Xenopus laevis. Cell Tissue Res. 1978 Jul 5;190(2):273–283. doi: 10.1007/BF00218175. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Costa M. The use of glyoxylic acid for the fluorescence histochemical demonstration of peripheral stores of noradrenaline and 5-hydroxytryptamine in whole mounts. Histochemistry. 1975;41(4):335–352. doi: 10.1007/BF00490076. [DOI] [PubMed] [Google Scholar]
- GRAY J. A. B., MALCOLM J. L. The excitation of touch receptors in frog's skin. J Physiol. 1951 Sep;115(1):1–15. doi: 10.1113/jphysiol.1951.sp004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschaldt K. M., Vahle-Hinz C. Evidence against transmitter function of met-enkephalin and chemosynaptic impulse generation in "Merkel cell" mechanoreceptors. Exp Brain Res. 1982;45(3):459–463. doi: 10.1007/BF01208608. [DOI] [PubMed] [Google Scholar]
- Gottschaldt K. M., Vahle-Hinz C. Merkel cell receptors: structure and transducer function. Science. 1981 Oct 9;214(4517):183–186. doi: 10.1126/science.7280690. [DOI] [PubMed] [Google Scholar]
- Hartschuh W., Weihe E., Yanaihara N., Reinecke M. Immunohistochemical localization of vasoactive intestinal polypeptide (VIP) in Merkel cells of various mammals: evidence for a neuromodulator function of the Merkel cell. J Invest Dermatol. 1983 Oct;81(4):361–364. doi: 10.1111/1523-1747.ep12519966. [DOI] [PubMed] [Google Scholar]
- Iggo A., Muir A. R. The structure and function of a slowly adapting touch corpuscle in hairy skin. J Physiol. 1969 Feb;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzak H., Tapper D. N., Craig P. H. Functional development of the tactile pad receptor system. Exp Neurol. 1970 Mar;26(3):439–446. doi: 10.1016/0014-4886(70)90140-8. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of temperature on the synaptic delay at the neuromuscular junction. J Physiol. 1965 Dec;181(3):656–670. doi: 10.1113/jphysiol.1965.sp007790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M., Hashimoto K., Ueda K., Kumakiri M. The specialized junctions between Merkel cell and neurite: an electron microscopic study. J Invest Dermatol. 1979 Nov;73(5):325–334. doi: 10.1111/1523-1747.ep12550322. [DOI] [PubMed] [Google Scholar]
- Munger B. L. Neural-epithelial interactions in sensory receptors. J Invest Dermatol. 1977 Jul;69(1):27–40. doi: 10.1111/1523-1747.ep12497861. [DOI] [PubMed] [Google Scholar]
- Nurse C. A., Macintyre L., Diamond J. A quantitative study of the time course of the reduction in Merkel cell number within denervated rat touch domes. Neuroscience. 1984 Feb;11(2):521–533. doi: 10.1016/0306-4522(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Nurse C. A., Mearow K. M., Holmes M., Visheau B., Diamond J. Merkel cell distribution in the epidermis as determined by quinacrine fluorescence. Cell Tissue Res. 1983;228(3):511–524. doi: 10.1007/BF00211472. [DOI] [PubMed] [Google Scholar]
- Parducz A., Leslie R. A., Cooper E., Turner C. J., Diamond J. The Merkel cells and the rapidly adapting mechanoreceptors of the salamander skin. Neuroscience. 1977;2(4):511–521. doi: 10.1016/0306-4522(77)90048-3. [DOI] [PubMed] [Google Scholar]
- Rall W., Shepherd G. M., Reese T. S., Brightman M. W. Dendrodendritic synaptic pathway for inhibition in the olfactory bulb. Exp Neurol. 1966 Jan;14(1):44–56. doi: 10.1016/0014-4886(66)90023-9. [DOI] [PubMed] [Google Scholar]
- Scott S. A., Cooper E., Diamond J. Merkel cells as targets of the mechanosensory nerves in salamander skin. Proc R Soc Lond B Biol Sci. 1981 Mar 27;211(1185):455–470. doi: 10.1098/rspb.1981.0017. [DOI] [PubMed] [Google Scholar]




