Abstract
Introduction
This study aims to investigate the therapeutic effect of paclitaxel temperature-responsive gel (PTRG) for interstitial chemotherapy on breast cancer, and to explore a new minimally invasive treatment for breast cancer.
Materials and methods
Breast cancer models were induced in rats using subcutaneous transplantation of tumor cells. The rats were then divided into control, paclitaxel injection, gel injection and paclitaxel-gel (PG) group. Following treatment, all animals were checked regularly by ultrasonography to observe changes in the tumors. Biopsy tumor tissues were processed for histopathological examination, and apoptotic index was determined by the terminal deoxynucleotidyl transferase dUTP nick end labeling method. In addition, blood cell count and liver transaminase activity were monitored, and the survival time of rats with cancer recorded.
Results
Rats in PG group exhibited liquefaction necrosis of tumors. Ninety days after the experiment, four rats exhibited complete extinction of tumors, indicating full recovery. Pathological examination revealed that the tumor tissues in these rats were mostly necrotic, and the apoptotic index of tumor cells increased markedly compared to PI group. Also, the red blood cell, hemoglobin and white blood cell levels declined significantly in the PI group compared with PG group, while glutamic-pyruvic transaminase and glutamic-oxalacetic transaminase activities significantly increased. Meanwhile, no toxicity due to treatment was observed in PG group.
Conclusion
Interstitial chemotherapy mediated by PTRG appeared to be a safe and effective treatment for breast cancer in rats. It might have clinical applications for treating human breast cancer.
Keywords: Ultrasonography, Paclitaxel, Gel, Breast cancer, Treatment
Introduction
Breast cancer (BC) is one of the most common malignant human tumors with the highest occurrence rate in women (Mc Pheson et al. 2000). Every year, 1.2 million women suffer from BC, and over 500,000 of them die of BC worldwide. In China, the incidence of BC is lower than in the western countries. However, there are increases in the areas where living conditions, lifestyle and diet are poor. In some large cities, BC has become one of the two most common diseases in women, making it a serious threat to human health (Ziegler et al. 2008). At present, BC treatment mainly involves combined therapies including surgery,chemo-, radio-, endocrine and bio-therapy. Modern imaging techniques provide reliable histological diagnostic means for BC through tissue biopsy. And, minimally invasive treatments for BC, such as, radio frequency ablation, laser ablation, focal ultrasound ablation and cryotherapy, have developed rapidly in the past decades (Garbay et al. 2008; Kontos et al. 2008; Haraldsdóttir et al. 2008; Wu et al. 2007; Sabel 2008).
In recent years, polymer materials have been widely applied in the field of medicine. Some sustained-release reagents, such as liposomes, microspheres and gels, are emerging (Ahmad et al. 2006; Ravivarapu et al. 2000; Jain et al. 2007a, b; Ghahremankhani et al. 2008). With the development of sustained-release techniques and renewable drug delivery systems, interstitial chemotherapy is gradually being considered an effective approach for tumor treatment. For instance, anti-cancer drugs can be incorporated into sustained-release delivery systems, which are then implanted into tumor tissues, interstitium of peri-tumor tissues or the tumor bed following tumor ablation. In this manner, a high drug concentration can be maintained in the tumor locally with a much reduced adverse reaction to the body (Ruel-Gariépy et al. 2004; Mok et al. 2001). In a clinical study on sustained-release interstitial chemotherapy, liver cancer patients were injected four times with cisplatin-adrenaline via percutaneous puncture in 6 weeks. As a result, 16 of the 56 liver cancer patients, who had tumors with diameters less than 7 cm, saw their tumors abolished. The patients’ 1-year, 2-year and 3-year survival rates were 79, 56 and 14%, respectively. And, there was no serious adverse reaction observed (Engelmann et al. 2000; Vogl et al. 2002). Furthermore, clinical studies on superficial tumors in human head and neck have also demonstrated a significant anti-cancer effect in response to the sustained-release drug delivery. Thus, it appears that injection of sustained-release chemotherapeutics on tumors is a safe, convenient and acceptable cancer treatment (Castro et al. 2003; Werner et al. 2002).
The PLGA–PEG–PLGA encapsulated drug system is injectable and biodegradable. The aqueous polymer solution is a low-viscosity liquid at room temperature, and a cross-linked gel when the temperature is higher than the critical gelation temperature. It is this gelation that is beneficial for fixed-point drug release. Drugs can be loaded into the gel simply by mixing them with the polymer. And, the relatively high loading volume and absence of organic solvent help maintain the drug’s lethality toward the targeted tumor. Once injected, the gel gradually degrades into non-toxic micromolecular products, and then is absorbed by the body. The gel can be easily administered, and needs no surgical removal afterward (Jeong et al. 1997; Qiao et al. 2005; Chen and Singh 2008).
Paclitaxel is a powerful anti-cancer drug extracted from the bark or needles and stems of the Pacific yew tree (Taxus brevifolia) and/or other Taxus species. It promotes microtubule assembly and inhibits cellular mitosis, and has been widely applied in treating breast, prostate, ovarian, head, neck and non-small cell lung cancers. Due to its poor water solubility (Cheon Lee et al. 2003), paclitaxel is frequently dissolved in polyoxyethylene castor oil, which often causes allergy and occasionally fatality in patients. Besides, when paclitaxel is administered in whole body chemotherapy, serious side effects, such as inhibition of bone marrow regeneration, as well as toxicity to the nerve, cardiovascular and liver systems, can result. By altering the administration route, it is hoped that these difficulties can be overcome, and the treatment effect can be enhanced as a result of the elevated local drug concentration. Therefore, the present study aims to develop a minimally invasive treatment for BC that involves the ultrasonically guided injection of PLGA–PEG–PLGA copolymer blocks loaded with paclitaxel into BC rats.
Materials and methods
Materials
Drugs and reagents
Paclitaxel crude drug (purity > 99.5%, Sichuan Taji Medicine Co., Ltd., China), paclitaxel injection (PI) (Haikou Pharmaceutical Factory Co., Ltd., China), polyethylene glycol and tin octoate were obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. d,l-lactide and l-glycolide were purchased from Glycolide Lactide Co., Ltd., Beijing, China. The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) in situ cell apoptosis detection kit was purchased from Nanjing KeyGen Biotech. Co., Ltd., China.
Tumor strains and animals
The Walker-256 strain BC rats were purchased from Shanghai Institute of Pharmaceutical Industry, China. Sixty female Wistar rats of clean grade, weighing 200 ± 20 g, were purchased from Shanghai Laboratory Animal Center, Chinese Academic Sciences, China. Rats were fed in pathogen-free animal rooms, and were allowed to eat and drink freely. All animal experiments were performed in accordance with guidelines approved by Fujian Medical University.
Synthesis of temperature-responsive gel
Polyethylene glycol, 3 g each of 1,000 and 1,500 dal molecular weight, were placed in a three-neck flask and mixed under vacuum for 3 h at 80°C. Next, 11.4 g lactide, 3 g glycolide and the catalyst, tin octoate, were added and incubated for 8 h at 150°C in a nitrogen atmosphere. Products were dissolved in water at 4°C and heated to 80°C. The resultant precipitates were dried to constant weight by freeze-drying. The sol–gel phase-transition temperature was determined using the turnover tube method (Yu et al. 2008; Qiao et al. 2008).
In vitro release behavior of paclitaxel temperature-responsive gel
Half a milliliter of aqueous PLGA–PEG–PLGA polymer solution containing 2 mg paclitaxel was placed in a 5 mL round-bottom centrifuge tube and incubated at 37°C with constant shaking. When the gel was formed, 2 mL of phosphate buffer (containing 0.02% sodium azide) were added, the suspension centrifuged, and the supernatant collected. The amount of released paclitaxel was determined by using a high pressure liquid chromatography apparatus (LC-10AT, Shimadu Device Corporation, Kyoto City, Japan).
Establishment of rat BC models
The Walker-256 rat BC cells were cultured from frozen stocks, and inoculated into the abdominal cavity of 4-week-old rats. One week later, malignant ascites were extracted. The trypan blue staining showed that the viable count was 95%. The cellular density was adjusted to 1 × 108 mL−1. Fifty rats were inoculated in the deep fascia of the mammary gland with 0.2 mL of the cell suspension to induce BC by subcutaneous transplantation. All animal handling and experimental procedures were conducted in accordance with the Guidelines of the Animal Care and Use Committee at Fujian Medical University.
Ultrasound-guided gel injection
Ten days after the inoculation, tumor diameter was measured by using a high-frequency ultrasound device (Acuson Sequoia 512, Siemens AG, Munich, Germany). Forty rats with a tumor diameter of 2.0–2.5 cm were selected and randomly divided into four groups (n = 10): the control (C), PI, gel injection (GI) and paclitaxel-gel (PG) group. Rats in GI and PG groups were anesthetized and percutaneously injected with 5 mL of 23% TRG and paclitaxel temperature-responsive gel (PTRG) (paclitaxel concentration: 10 mg kg−1 in 23% gel solution), respectively. Injection was performed at several sites under an ultrasonic monitor to assure an even gel distribution throughout the entire tumor. For PI group, the injection was given through rat tail veins.
Blood test
Seven days after the administrations, blood samples were harvested from the rat femoral vein, and the red blood cell, hemoglobin, white blood cell and platelet counts determined. The alanine aminotransferase and aspartate amino transferase activities in the blood supernatants were also measured biochemically.
Histopathological examination of tumor tissues
Seven days after the administrations, tumor tissues were obtained by puncture with a biopsy needle under ultrasonic guidance. Tissues were processed by means of 10% formaldehyde fixation, conventional dehydration, paraffin embedment and serial sectioning. The slices were stained with hematoxylin–eosin for observation under a microscope.
Apoptotic index of tumor tissues
Apoptosis of tumor cells in tissue slices was determined using a TUNEL in situ detection kit. Subsequent to TUNEL staining, 500 tumor cells were observed. Apoptotic cells were identified by the presence of blue, fragmented nuclei. The apoptotic index was calculated as the percentage of positive staining: apoptotic index (%) = (number of apoptotic cells/total number of cells) × 100%. The blank controls were performed with PBS, instead of TdT enzyme.
Volume and survival time of rat tumors
Every week, the tumor echo and blood flow of the rats were monitored by using high-frequency ultrasound. The length (L), width (W) and thickness (T) of the tumor were measured. Tumor volume was calculated according to the formula: V (mm3) = 1/2(L × W×T). Data were plotted to analyze the tumor growth. Death time of cancer-bearing rats and the life-prolonging rate of each treatment were recorded. Life-prolonging rate (%) = (average survival time of treatment group/average survival time of C group-1) × 100%.
Statistical analysis
Measurement data were expressed as mean ± SD. One-factor analysis of variance was performed using SPSS 16.0 software. A P value of <0.05 was considered statistically significant. Survival curves were plotted according to the Kaplan–Meier method.
Results
Phase transition temperature of the synthetic polymer aqueous solution
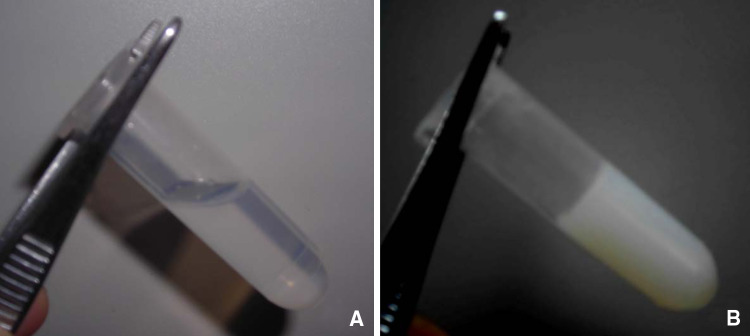
The PLGA–PEG–PLGA polymer solidified at concentration greater than 35%. At a concentration of 15–35%, it became liquid at below its gelation temperature (Fig. 1a). Conversely, it gelled at the temperature higher than the critical gelation temperature (Fig. 1b). The sol–gel phase transition temperature was defined as 28°C at the gel concentration of 23%.
Fig. 1.
Temperature effect on aqueous solution of PLGA–PEG–PLGA polymer. The sol–gel phase transition temperature was determined using the turnover tube method. The polymer exists as a fluid, transparent liquid at temperature below its critical gelation temperature (a) The polymer transforms into a white, semi-solid gel at temperature higher than its critical gelation temperature (b)
In vitro release of PTRG
A drug release test was performed in a phosphate buffer. The results showed that about 50% of the paclitaxel were released in the first week. After that, the release occurred at a steady rate with the cumulative release reaching 86.9% after 18 days (Fig. 2).
Fig. 2.
In vitro release of PTRG. The amount of paclitaxel released in vitro was determined by using a high pressure liquid chromatography apparatus. About 50% of the paclitaxel are released in the first week. After that, the drug releases at a steady rate with the cumulative release reaching 86.9% in 18 days
Ultrasonic examination of BC rats
Three days after the rats were inoculated with the Walker-256 BC cells, subcutaneous tumor tubercles were palpable. As shown in Fig. 3, the tumor volume increased significantly after 10 days. And, a medium echo was detected by high-frequency ultrasound (Fig. 3a), and the affluent blood flow signals detected surrounding, as well as, inside the tumor (Fig. 3b). Under ultrasonic guidance, TRG was injected into the tumor tissues, resulting in an increase in the local echo (Fig. 3c). Seven days following the gel administration, rats in the PG group exhibited liquefaction necrosis of tumors. The gels that were not degraded produced a punctiform high echo (Fig. 3D). The tumor volumes of the cancer-bearing rats continued to increase in both C and GI groups. The tumors grew more slowly in the rats in PI group, suggesting that the growth was somewhat inhibited. On the other hand, in PG group, the tumor volume gradually reduced with time (Fig. 4).
Fig. 3.
Ultrasonic examination of rats with breast cancer. The tumor internal echo and blood flow signals were detected by high-frequency ultrasound. Two-dimensional ultrasonography before treatment exhibits an even, medium echo (a). The color Doppler ultrasonography before treatment shows detectable affluent blood flow signals surrounding and inside the tumor (b). A high echo surrounding the injection needle (arrow) appears when the tumor is injected with gel under ultrasonic guidance (c). Tumors show liquefaction necrosis one week following the treatment, while the undegraded gels exhibit a punctiform high echo (arrow) (d)
Fig. 4.
Tumor growth in cancer-bearing rats. The tumor volume was measured by using high-frequency ultrasound, and calculated according to the formula: V (mm3) = 1/2(L × W×T). The tumor size in the cancer-bearing rats continues to increase in both C and GI groups. The tumor growth is somewhat inhibited in the rats in PI group, whereas a gradual reduction with time in PG group is evident
One week after the PG treatment, the ultrasonic examination showed that tumor tissues had undergone liquefaction and necrosis. With increased time, some necrotic tissues were found to be gradually absorbed. At the 90th day, four rats achieved complete extinction of the tumor with a full recovery. In PI group, paclitaxel rapidly metabolized in vivo and exhibited a low drug concentration and a poor anti-cancer effect. Thus, the survival time of cancer-bearing rats in PI group was only 31.8 ± 4.13 days.
Routine blood tests and examination of liver function in rats
In PI group, the red blood cells, hemoglobin and white blood cells of the rats all decreased significantly as compared to C group (P < 0.05). In PG group, the red blood cell and hemoglobin counts were reduced; however, no significant differences were found between it and C group (P > 0.05) (Table 1). Blood biochemical tests revealed that the glutamic-pyruvic transaminase and aspartate aminotransferase activities were threefold higher in PI group than in C group. The enzymatic activities were also significantly increased in PG group as compared to C group (P < 0.05), but lower than those in PI group (P < 0.05) (Table 2).
Table 1.
Blood cell counts in each group ( )
)
| Group | Red blood cells (×106 mm−3) | Hemoglobin [g (100 mL)−1] | White blood cells (×103 mm−3) | Platelet (×103 mm−3) |
|---|---|---|---|---|
| C | 7.15 ± 1.61 | 13.19 ± 3.48 | 8.27 ± 1.73 | 311.24 ± 65.30 |
| PI | 4.97 ± 1.07# | 8.05 ± 2.11# | 5.26 ± 1.67# | 300.28 ± 57.34 |
| GI | 6.89 ± 1.54 | 12.38 ± 3.27 | 7.87 ± 1.55 | 348.27 ± 60.57 |
| PG | 6.57 ± 1.28 | 11.86 ± 3.10 | 7.62 ± 1.37 | 309.25 ± 56.27 |
#P < 0.05, C versus PI group
Table 2.
Activities of blood transaminases in each group (n = 10,  )
)
| Group | Glutamic-pyruvic transaminase (mmol mL−1) | Aspartate aminotransferase (mmol mL−1) |
|---|---|---|
| C | 1.77 ± 0.29 | 3.95 ± 0.69 |
| PI | 4.23 ± 1.07# | 8.51 ± 2.96# |
| GI | 1.52 ± 0.33 | 3.28 ± 0.66 |
| PG | 2.23 ± 0.49# * | 4.57 ± 0.84# * |
#P < 0.05, C versus PI group or PG
* P < 0.05, PG versus PI group
Histopathological examination of tumor tissue in rats
The rat’s tumor tissues in C group exhibited intensely stained polygonal cells with large nuclei (Fig. 5a). In PI group, some tumor tissues started to show necrotic effect (Fig. 5b). Whereas, following the injection of PG, a large amount of tumor tissue in the cancer-bearing rats began to denature and become necrotic, and no intact cells could be found (Fig. 5c–d).
Fig. 5.
Histopathological examination of tumor tissue in rats. Biopsy tumor tissues were processed to prepare the slices, which were stained with hematoxylin–eosin for observation. In C group, tumor tissue cells are polygonal in shape, with large nuclei and intense stain (a). In PI group, some tumor tissues appear necrotic (b). In PG group, a large amount of tumor tissues begin to denature and undergo necrosis (c). In PG group, intact cells disappear (d)
Apoptotic index of tumor cells
The apoptotic indices in C and GI groups were 2.36 ± 0.41% and 2.09 ± 0.44%, respectively. In PG group, on the other hand, the apoptotic cells significantly increased, and the apoptotic index reached 39.22 ± 5.69%, which was significantly higher than that in PI group (i.e., 14.28 ± 2.37%) (Fig. 6).
Fig. 6.
Apoptotic indices of tumor tissues in rats of different treatment groups. Apoptotic index was determined by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method. In PG group, the apoptotic index reached 39.22 ± 5.69%, which was significantly higher than that in PI group (i.e., 14.28 ± 2.37%) (**P < 0.01)
Survival time of cancer-bearing rats
The survival times of cancer-bearing rats in C group were 25.7 ± 5.01, 23.8 ± 4.34 days in GI group, and 31.8 ± 4.13 days in PI group. Life-prolonging rate of the animals in PI group was 23.74%. The rat’s survival time was also extended in PG group. Moreover, complete extinction of tumors was evident in four rats in 90 days, indicating a full recovery (Fig. 7).
Fig. 7.
Survival times of cancer-bearing rats. The survival time data were plotted by means of the Kaplan–Meier product-limit method. The survival time of rat in C group is 25.7 ± 5.01 days, 23.8 ± 4.34 days in GI group, and 31.8 ± 4.13 days in PI group. Four rats survived after 90 days in PG group
Discussion
Because of the low water solubility and short half-life in plasma of paclitaxel, researchers tried to overcome the disadvantages by altering the dosage forms. Lee et al. (2007) employed paclitaxel-loaded sterically stabilized solid lipid nanoparticles (200 nm diameter) in an in vitro experimentation with success in the delayed drug release effect. The nanoparticulation improved the water solubility and the sustaining time of the drug, but the treatment was, nonetheless, whole body therapy that carried a substantial toxic side effect. Subsequently, Sun et al. (2008) prepared a poly(d,l-lactide-co-glycolide)/montmorillonite nanoparticles (312.3 ± 8.2 nm in diameter), which was further decorated by human epidermal growth factor receptor-2 (HER2) antibody, trastuzumab, for a BC targeted therapy. Their in vitro anti-tumor effect was 13.1 times of that of paclitaxel. These nanoparticles could be guided by the specified antibody for targeted chemotherapy, which were similar to immuno-toxin. However, the in vivo effect of immuno-toxin was not as good as expected due to various reasons. Fundamentally, application of nanoparticles remains questionable as to whether the particle size, e.g., 312 nm, could allow their passing through the capillary wall into the tumor tissue.
Interstitial chemotherapy is a recently developed approach for cancer treatments. It injects the sustained release drug directly into the tumor tissue through imaging technologies. By delayed and continual releasing of the drug, the anti-cancer effect is improved and side effect considerably reduced. The environmentally sensitive carrier gels are being studied for such applications. The chitosan gel consisted of chitosan and glyceryl monooleate is one of these materials. Jauhari and Dash (2006) used chitosan gel to encapsulate paclitaxel. However, its in vitro release time was relatively short. In the presence of surfactant (i.e., 0.05% wt/vol) in the dissolution medium, the percentage of paclitaxel released was 97.1 ± 1.22% within 4 h. Furthermore, since paclitaxel does not dissolve in the chitosan solution, the drug distribution in the gel was not even, and the long gelation time of chitosan gel might negatively affect the steady drug release in vivo.
The PLGA–PEG–PLGA block copolymer hydrogel system has a hydrophilic coating and a hydrophobic substrate to form the encapsulated spheres. Paclitaxel, or other insoluble drug materials, can thus be carried for a delayed release when the external coating is dissolved in an aqueous environment or at different temperature. Through the use of ultrasonic guidance, PTRG could be injected into tumor tissues of BC rats. The results suggested that such treatment produces an apparent anti-cancer effect. The punctiform high-echo observed was indicative of an even distribution of the drug-carrying gel in tumor tissues. One injection allowed drug release for 3 weeks providing a persistent anti-cancer effect.
The critical gelation temperature of a TRG is important for its successful application. It should fall within the range between the room and human body temperatures. Therefore, a solution injected at room temperature becomes a gel following the injection. If the critical gelation temperature is lower than room temperature, injection needles are easily blocked. On the other hand, if the critical gelation temperature is higher than human body temperature, a gel cannot form in the body and no sustained-release effect can be realized. The relative molecular weight of polyethylene glycol directly affects the critical gelation temperature: the greater the molecular weight, the higher the critical gelation temperature (Zentner et al. 2001). In the present study, TRG was synthesized using polyethylene glycols of molecular weight 1,000 and 1,500 dal at a molar ratio of 1:1. The critical gelation temperature of the resulting gel was 28°C at a concentration of 23%, which was adequate for injection. And, a desirable sustained-release effect could be obtained over 3 weeks in vitro.
Animal models of BC include spontaneous BC, induced BC, transplantable BC and transgenic or gene knock-out BC models (Nakamura et al. 2008; Al-Dhaheri et al. 2008). Transplantable BC is a commonly applied for animal model. It can be divided into allotransplantation and xenotransplantation. Allotransplantation BC models involve a short preparation cycle. They are less expensive, and can provide good distribution of tumor tissue leading to a high success rate. The Walker-256 tumor cells, obtained from a rat breast cancer by Walker in 1928, produced large tumors when transplanted into rats, which could be observed ultrasonically. In preparing animal models, the tumor cell suspension is injected into deep fascia close to the pectoral muscle, which has an affluent blood flow and a high rate of tumor formation. Data from the present study suggested that tumors produced in this way had a regular morphology, as observed by ultrasonic examination 10 days after inoculation. The tumor boundary was clear, and the tumor exhibited medium echo by ultrasound. There were affluent blood flow signals both surrounding and inside the tumor tissues. The survival time of the cancer-bearing rats was 25 days, which is sufficient for ultrasonic investigation.
Injection of a sustained-release preparation of paclitaxel into tumor tissue could not only increase the local drug concentration, but also reduce general toxicity. By means of intra-tumor injection, PTRG could sustain drug release with a low drug concentration in blood resulting in minimal damage to non-tumor organs. In addition, no skin ulcers or infections were found following intra-tumor PTRG injection. In contrast, the intravenous injection of paclitaxel reduced the blood cell counts and increased liver transaminase activity. With the ultrasonic guidance, the interstitial chemotherapy was performed in BC rats by PTRG injection directly into the tumor tissues. This appeared to be an effective, safe and convenient minimally-invasive method for treating BC.
Acknowledgments
This study was financially supported by the Key Laboratory Fund at the Affiliated Union Hospital, Fujian Medical University and the Fujian Natural Science Foundation Grant provided by Fujian Provincial Department of Science and Technology (2009J05066).
References
- Ahmad N, Arif K, Faisal SM, Neyaz MK, Tayyab S, Owais M (2006) PLGA-microsphere mediated clearance of bilirubin in temporarily hyperbilirubinemic rats: an alternate strategy for the treatment of experimental jaundice. Biochim Biophys Acta 1760:227–232 [DOI] [PubMed] [Google Scholar]
- Al-Dhaheri WS, Hassouna I, Al-Salam S, Karam SM (2008) Characterization of breast cancer progression in the rat. Ann N Y Acad Sci 1138:121–131 [DOI] [PubMed] [Google Scholar]
- Castro DJ, Sridhar KS, Garewal HS, Mills GM, Wenig BL, Dunphy FR 2nd, Costantino PD, Leavitt RD, Stewart ME, Orenberg EK (2003) Intratumoral cisplatin/epinephrine gel in advanced head and neck cancer: a multicenter, randomized, double-blind, phase III study in North America. Head Neck 25:717–731 [DOI] [PubMed] [Google Scholar]
- Chen S, Singh J (2008) Controlled release of growth hormone from thermosensitive triblock copolymer systems: In vitro and in vivo evaluation. Int J Pharm 352:58–65 [DOI] [PubMed] [Google Scholar]
- Cheon Lee S, Kim C, Chan Kwon I, Chung H, Young Jeong S (2003) Polymeric micelles of poly(2-ethyl-2-oxazoline)-block-poly (epsilon-caprolactone) copolymer as a carrier for paclitaxel. J Control Release 89:437–446 [DOI] [PubMed] [Google Scholar]
- Engelmann K, Mack MG, Straub R, Eichler K, Zangos S, Orenberg E, Vogl TJ (2000) CT-guided percutaneous intratumoral chemotherapy with a novel cisplatin/epinephrine injectable gel for the treatment of inoperable malignant liver tumors. Rofo 172:1020–1027 [DOI] [PubMed] [Google Scholar]
- Garbay JR, Mathieu MC, Lamuraglia M, Lassau N, Balleyguier C, Rouzier R (2008) Radiofrequency thermal ablation of breast cancer local recurrence: a phase II clinical trial. Ann Surg Oncol 15:3222–3226 [DOI] [PubMed] [Google Scholar]
- Ghahremankhani AA, Dorkoosh F, Dinarvand R (2008) PLGA-PEG-PLGA tri-block copolymers as in situ gel-forming peptide delivery system: effect of formulation properties on peptide release. Pharm Dev Technol 13:49–55 [DOI] [PubMed] [Google Scholar]
- Haraldsdóttir KH, Ivarsson K, Götberg S, Ingvar C, Stenram U, Tranberg KG (2008) Interstitial laser thermotherapy (ILT) of breast cancer. Eur J Surg Oncol 34:739–745 [DOI] [PubMed] [Google Scholar]
- Jain AK, Chalasani KB, Khar RK, Ahmed FJ, Diwan PV (2007a) Muco-adhesive multivesicular liposomes as an effective carrier for transmucosal insulin delivery. J Drug Target 15:417–427 [DOI] [PubMed] [Google Scholar]
- Jain SK, Gupta Y, Jain A, Bhola M (2007b) Multivesicular liposomes bearing celecoxib-beta-cyclodextrin complex for transdermal delivery. Drug Deliv 14:327–335 [DOI] [PubMed] [Google Scholar]
- Jauhari S, Dash AK (2006) A mucoadhesive in situ gel delivery system for paclitaxel. AAPS Pharm Sci Technol 7:E154–E159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong B, Bae YH, Lee DS, Kim SW (1997) Biodegradable block copolymers as injectable drug-delivery system. Nature 388:860–862 [DOI] [PubMed] [Google Scholar]
- Kontos M, Felekouras E, Fentiman IS (2008) Radiofrequency ablation in the treatment of primary breast cancer: no surgical redundancies yet. Int J Clin Pract 62:816–820 [DOI] [PubMed] [Google Scholar]
- Lee M-K, Lim S-J, Kim C-K (2007) Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials 28:2137–2146 [DOI] [PubMed] [Google Scholar]
- Mc Pheson K, Steel CM, Dixon JM (2000) ABC of breast disease. Breast cancer epidemiology risk factors, and genetics. BMJ 321:624–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok TS, Kanekal S, Lin XR, Leung TW, Chan AT, Yeo W, Yu S, Chak K, Leavitt R, Johnson P (2001) Pharmacokinetic study of intralesional cisplatin for the treatment of hepatocellular carcinoma. Cancer 91:2369–2377 [PubMed] [Google Scholar]
- Nakamura H, Hiraga T, Ninomiya T, Hosoya A, Fujisaki N, Yoneda T, Ozawa H (2008) Involvement of cell–cell and cell–matrix interactions in bone destruction induced by metastatic MDA-MB-231 human breast cancer cells in nude mice. J Bone Miner Metab 26:642–647 [DOI] [PubMed] [Google Scholar]
- Qiao M, Chen D, Ma X, Liu Y (2005) Injectable biodegradable temperature-responsive PLGA-PEG-PLGA copolymers: synthesis and effect of copolymer composition on the drug release from the copolymer-based hydrogels. Int J Pharm 294:103–112 [DOI] [PubMed] [Google Scholar]
- Qiao M, Chen D, Hao T, Zhao X, Hu H, Ma X (2008) Injectable thermosensitive PLGA-PEG-PLGA triblock copolymers-based hydrogels as carriers for interleukin-2. Pharmazie 63(1):27–30 [PubMed] [Google Scholar]
- Ravivarapu HB, Burton K, DeLuca PP (2000) Polymer and microsphere blending to alter the release of a peptide from PLGA microspheres. Eur J Pharm Biopharm 50(2):263–270 [DOI] [PubMed] [Google Scholar]
- Ruel-Gariépy E, Shive M, Bichara A, Berrada M, Le Garrec D, Chenite A, Leroux JC (2004) A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur J Pharm Biopharm 57:53–63 [DOI] [PubMed] [Google Scholar]
- Sabel MS (2008) Cryoablation for breast cancer: no need to turn a cold shoulder. J Surg Oncol 97(6):485–486 [DOI] [PubMed] [Google Scholar]
- Sun B, Ranganathan B, Feng S-S (2008) Multifunctional poly (d, l-lactide-co-glycolide)/montmorillonite (PLGA/MMT) nanoparticles decorated by Trastuzumab for targeted chemotherapy of breast cancer. Biomaterials 29:475–486 [DOI] [PubMed] [Google Scholar]
- Vogl TJ, Engelmann K, Mack MG, Straub R, Zangos S, Eichler K, Hochmuth K, Orenberg E (2002) CT-guided intratumoural administration of cisplatin/epinephrine gel for treatment of malignant liver tumours. Br J Cancer 86(4):524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner JA, Kehrl W, Pluzanska A, Arndt O, Lavery KM, Glaholm J, Dietz A, Dyckhoff G, Maune S, Stewart ME, Orenberg EK, Leavitt RD (2002) A phase III placebo-controlled study in advanced head and neck cancer using intratumoural cisplatin/epinephrine gel. Br J Cancer 87(9):938–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, ter Haar G, Chen WR (2007) High-intensity focused ultrasound ablation of breast cancer. Expert Rev Anticancer Ther 7(6):823–831 [DOI] [PubMed] [Google Scholar]
- Yu L, Chang GT, Zhang H, Ding JD (2008) Injectable block copolymer hydrogels for sustained release of a PEGylated drug. Int J Pharm 348(1–2):95–106 [DOI] [PubMed] [Google Scholar]
- Ziegler RG, Anderson WF, Gail MH (2008) Increasing breast cancer incidence in China: the numbers add up. J Natl Cancer Inst. 100(19):1339–1341 [DOI] [PubMed] [Google Scholar]